Novel piracetam drug co-crystal and preparation method thereof
A piracetam and drug technology, applied in the field of novel piracetam co-crystal and its preparation, to achieve the effect of improving stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
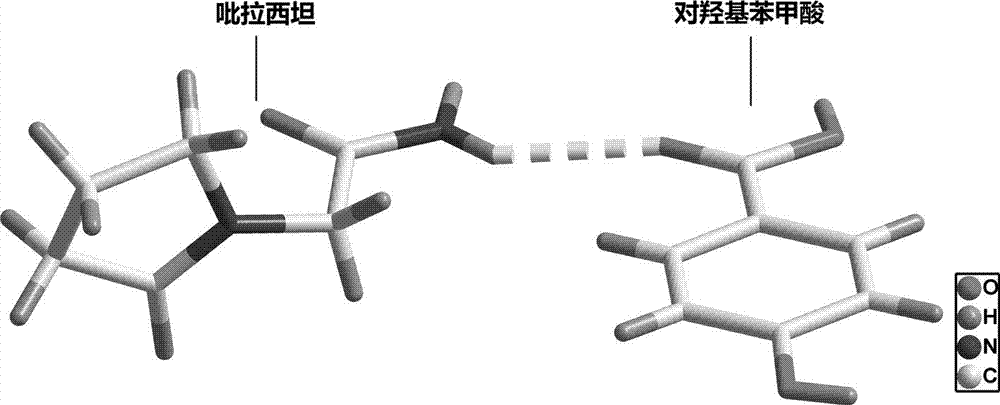
[0024] Example 1: Synthesis of co-crystals using piracetam and p-hydroxybenzoic acid:
[0025] Weighing: The reactant is fed with 20.00 mg of piracetam and 20.00 mg of p-hydroxybenzoic acid. Accurately weigh 20.00 mg of piracetam and 20.00 mg of p-hydroxybenzoic acid with an analytical balance.
[0026] Dissolution of the raw material drug: Use a 5ml pipette to accurately measure 4ml of methanol into a 20ml transparent glass vial container, stir for 1 hour to dissolve all the solids, and the solution becomes a colorless clear liquid.
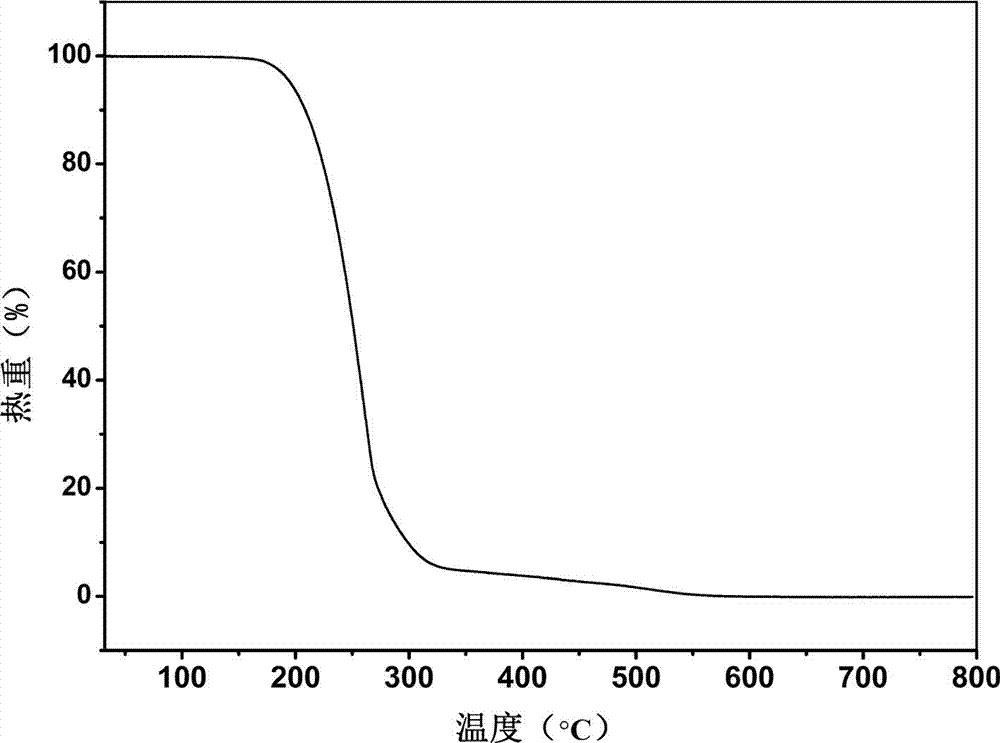
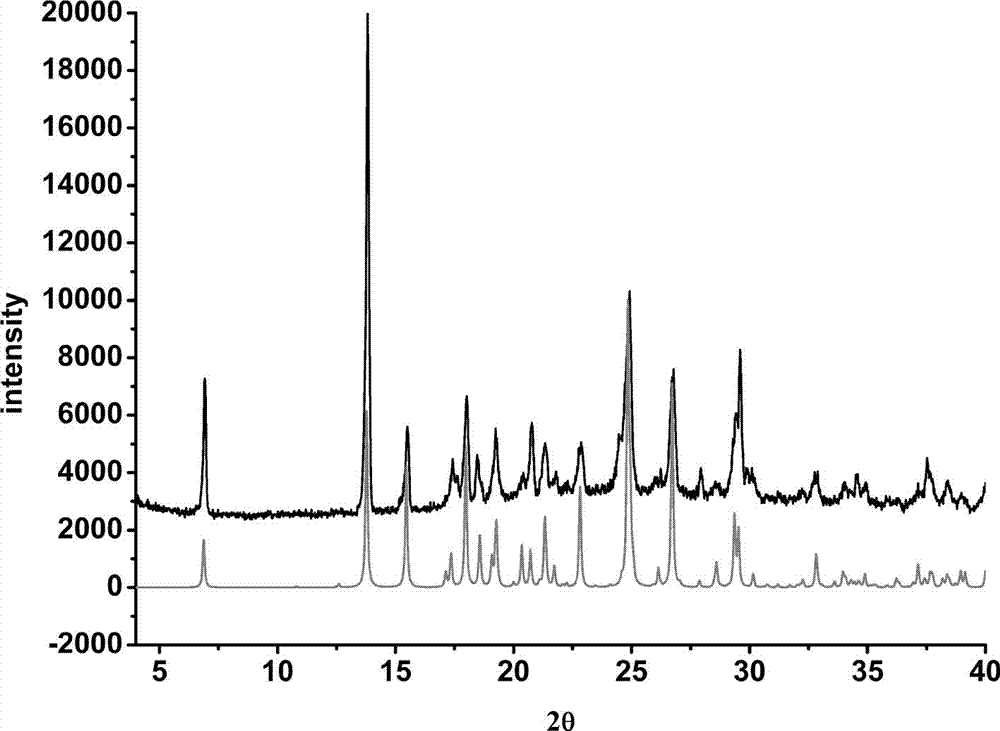
[0027] Solvent room temperature volatilization heating method: After the solid is completely dissolved, take out the stirring bar, seal the bottle mouth with tin foil, prick a few small holes with a needle, and let it stand for volatilization. After about 6 days, colorless transparent block crystals precipitated in the bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com