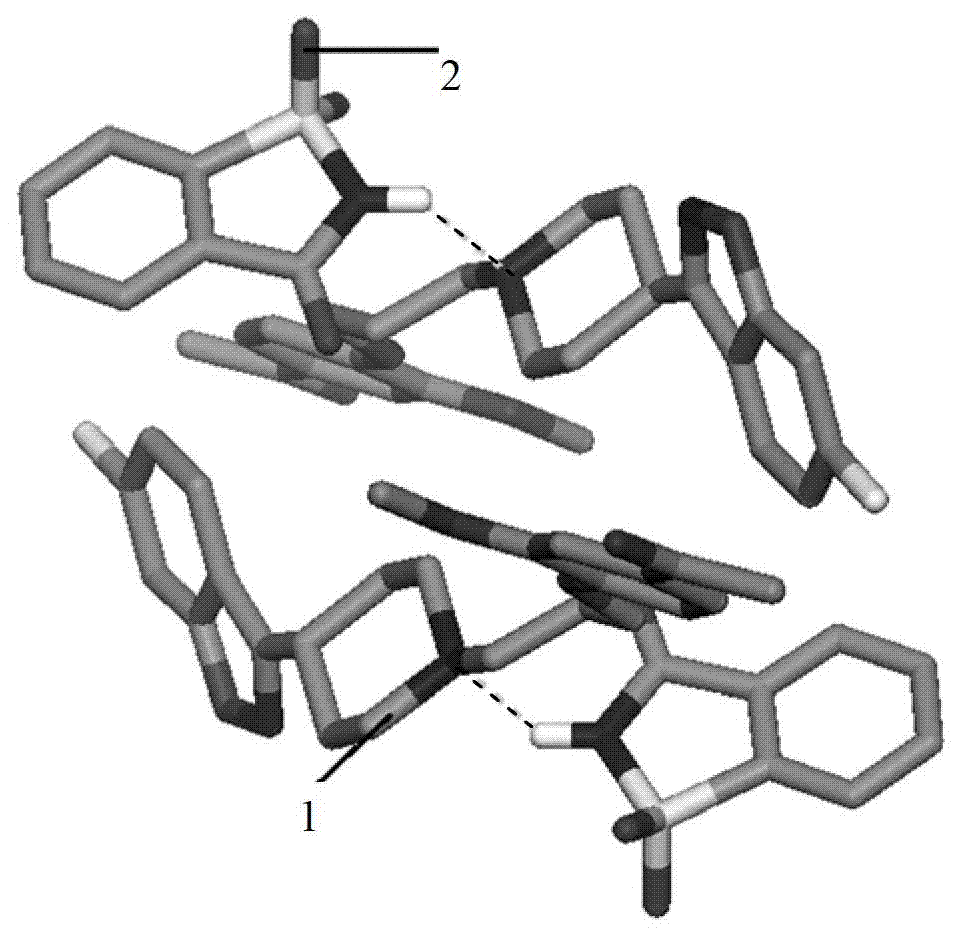
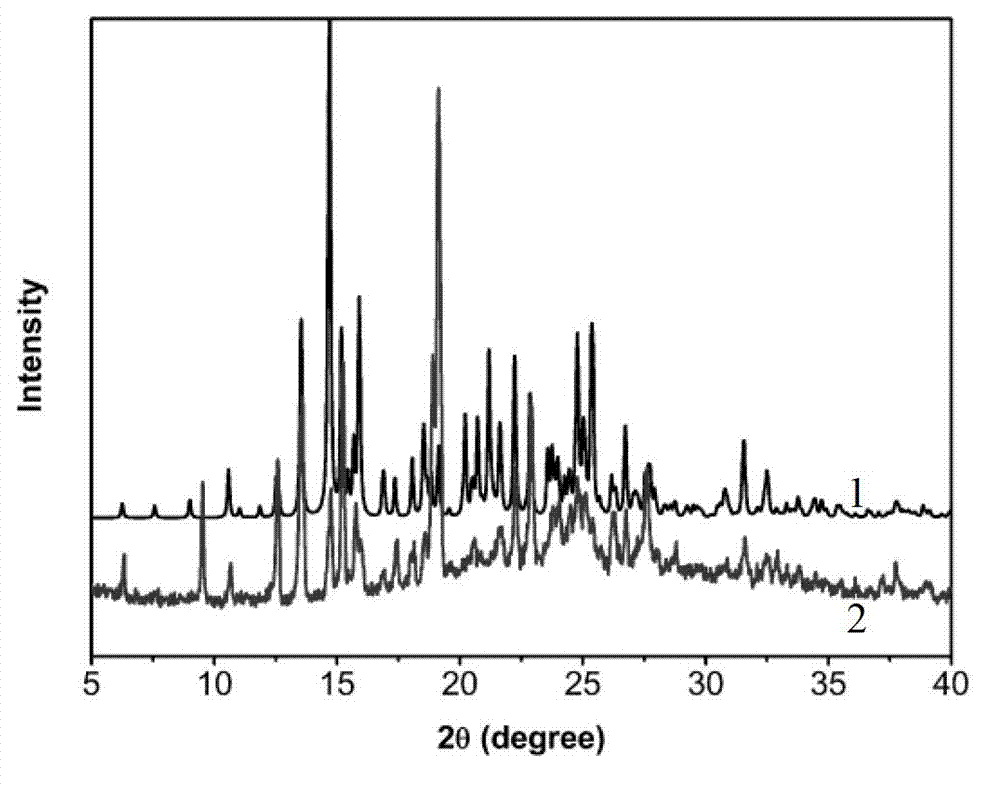
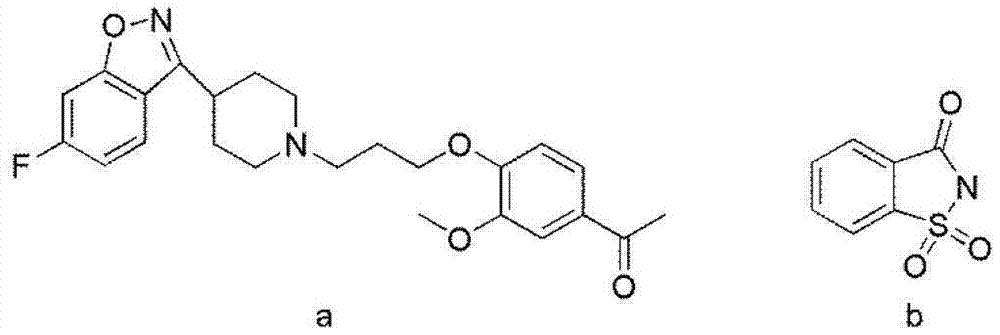
Iloperidone-saccharin organic pharmaceutical co-crystal and preparation method thereof
A technology of iloperidone and organic medicine is applied in the field of iloperidone-saccharin organic medicine co-crystal and preparation thereof, and the effects of improved bioavailability and improved stability are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of new crystalline forms of drugs using iloperidone and saccharin:
[0030] Weighing:
[0031] Use an analytical balance to weigh 20 mg each of iloperidone and saccharin into a 25 ml round bottom flask.
[0032] Dissolution of API:
[0033] Use a 5ml pipette to measure 5ml of ethanol into the round bottom flask.
[0034] Room temperature evaporation method:
[0035] (1) Set up the reflux condensing device, turn on the condensed water and heating and stirring settings, and the reflux time is 2 hours;
[0036] (2) After stopping stirring, filter while hot;
[0037] (3) The filtrate is placed in a 25mL beaker, and the mouth of the cup is sealed with tin foil and placed in a quiet place. After 20 hours, it is found that there are colorless and transparent massive crystals at the bottom of the beaker. After 48 hours, the crystals are completely separated out;
[0038] (5) After drying the obtained crystals in vacuum, 25 mg of iloperidone-saccharin organic drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com