Novel iloperidone pharmaceutical cocrystal and preparation method thereof
A technology of iloperidone and drugs, applied in the field of new iloperidone drug co-crystal and its preparation, to achieve the effect of improved stability and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
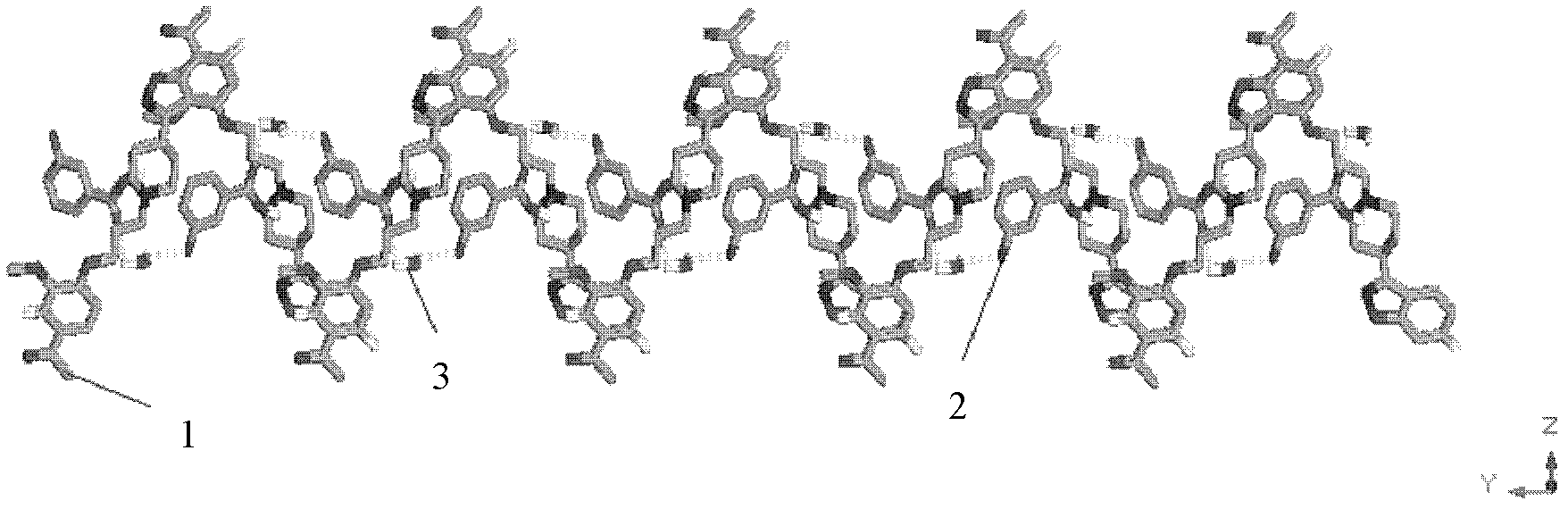
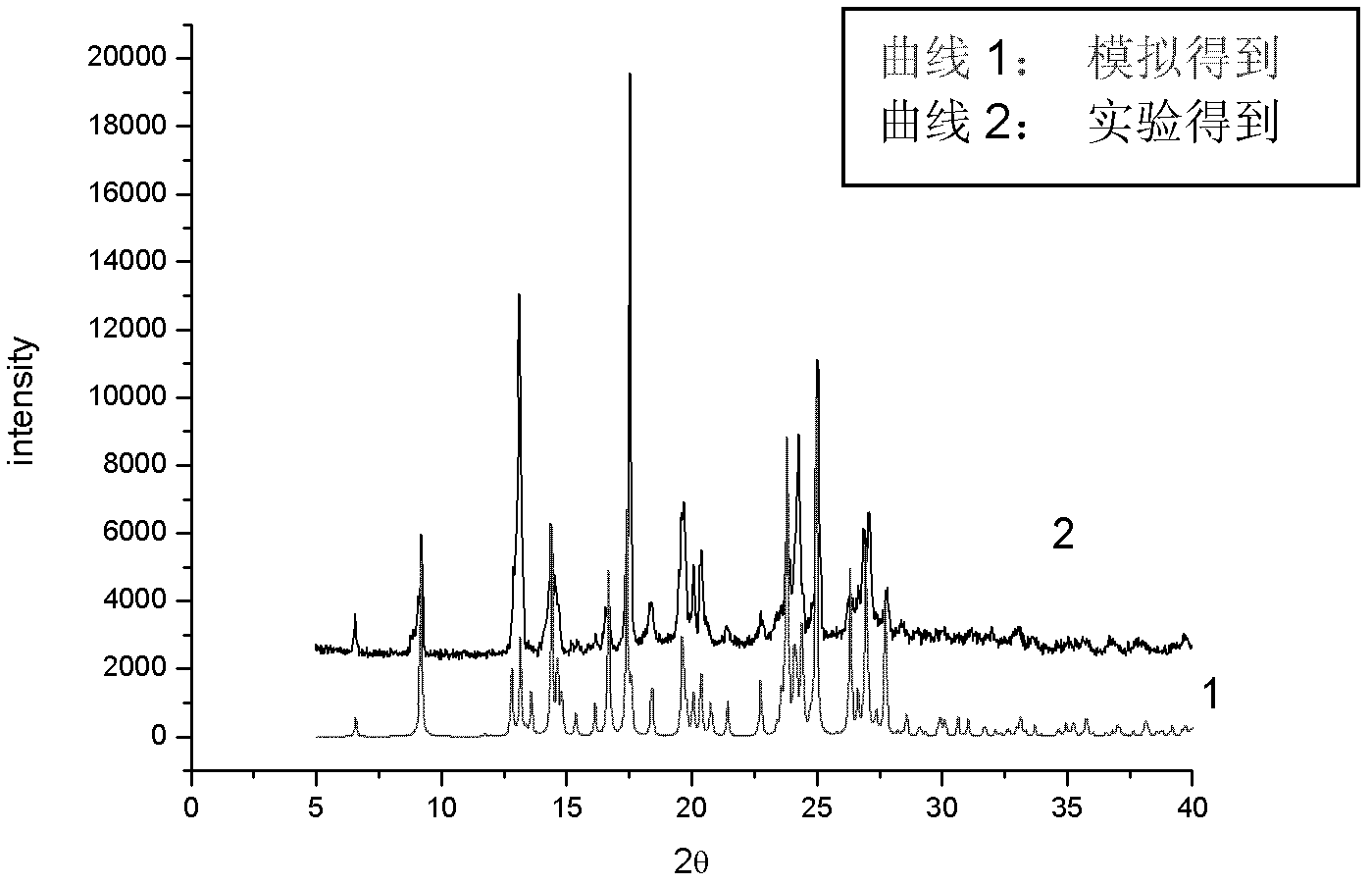
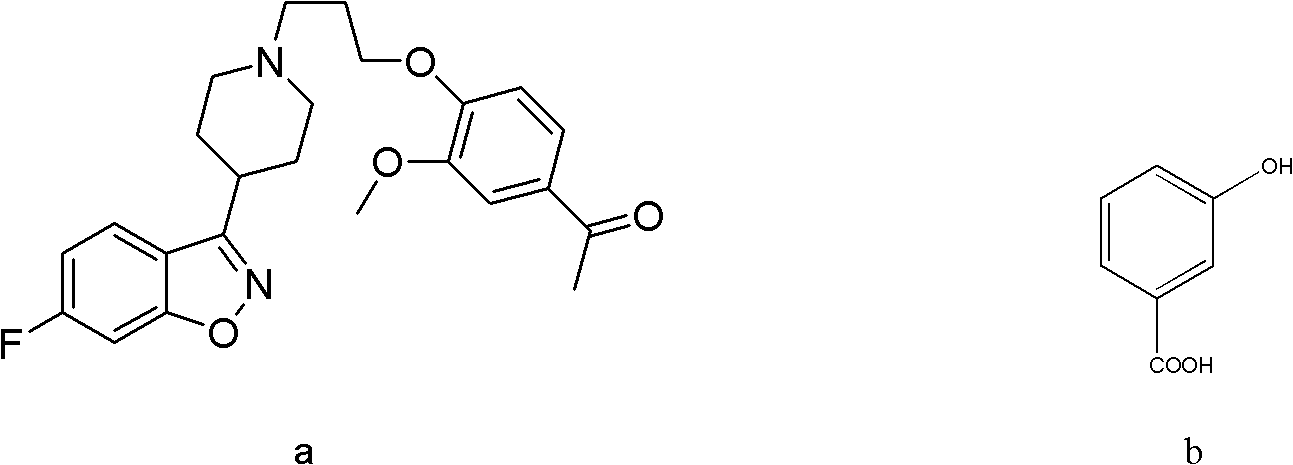
Image
Examples
Embodiment 1
[0030] Synthesis of co-crystals using iloperidone and 3-hydroxybenzoic acid:
[0031] Weighing:
[0032] The reactant is fed according to iloperidone: 3-hydroxybenzoic acid=1:1 mass ratio. Accurately weigh 20.00 mg each of iloperidone and 3-hydroxybenzoic acid with an analytical balance.
[0033] Dissolution of API:
[0034] Use a 5ml pipette to accurately measure 5ml of ethanol into a 25ml single-necked round bottom flask.
[0035] Reflux-room temperature volatilization heat method:
[0036] Put a magnetic stirrer in the above-mentioned round-bottomed flask of uniformly dissolved medicine, set up a reflux device, set the reflux time to 2 hours, and turn on the magnetic stirrer and condensate at a temperature of 90°C.
[0037] After reflux, the reaction solution was filtered, and the filtrate was placed in a 25ml transparent glass vial and placed at room temperature. After the solvent was slowly volatilized for 3 hours, transparent massive crystals were formed, and the weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com