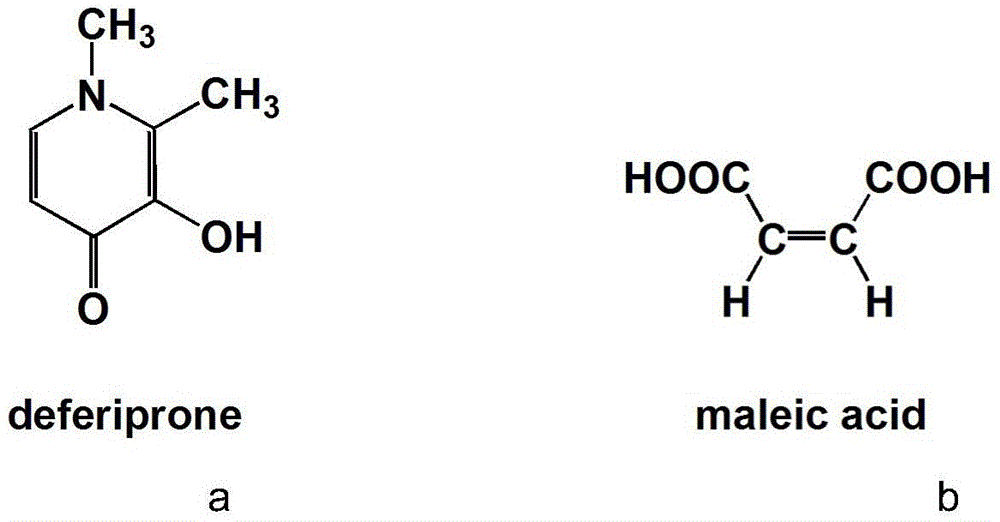Pharmaceutical cocrystal of deferiprone with maleic acid as precursor, and preparation method thereof
A technology of maleic acid and deferiprone, applied in the field of deferiprone drug co-crystal and its preparation, can solve the problems of blood transfusion iron overload, death, etc., and achieve the effect of improving stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
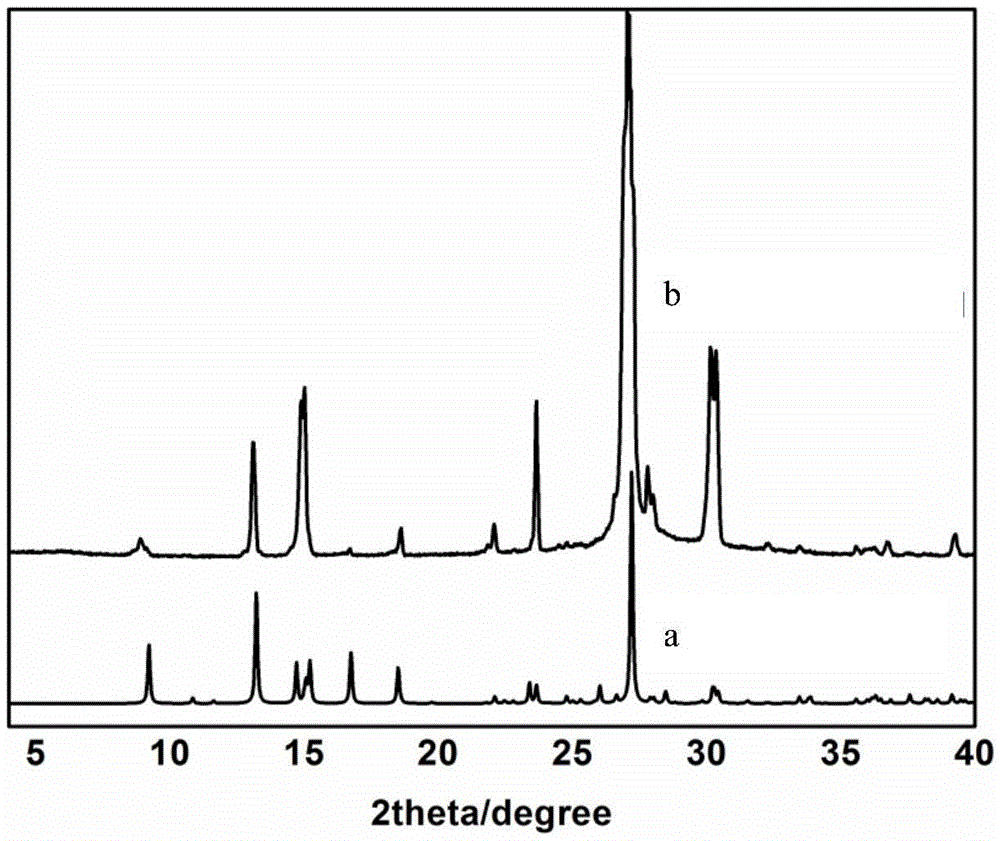
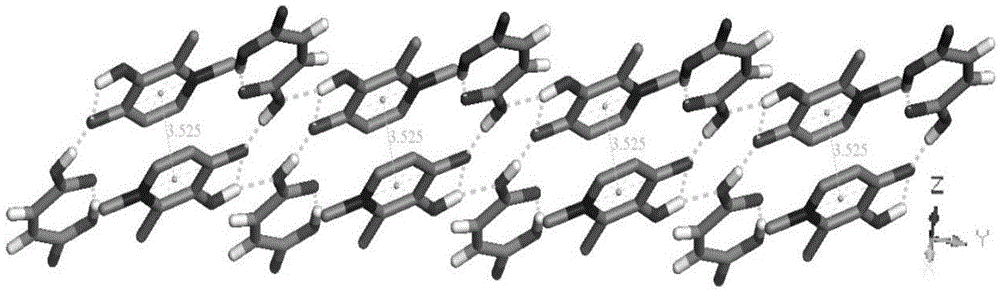
Image
Examples
Embodiment 1
[0027] (1) Feed deferiprone and maleic acid at a molar ratio of 0.75:1, accurately weigh 42.50mg of deferiprone and 51.20mg of maleic acid, mix them in a glass vial, add 3mL ethanol and 6mL acetone, and put Put a 1cm magnetic stirrer, spread a layer of tin foil on the mouth of the glass vial, and tighten the cap to seal the vial.
[0028] (2) Place the above glass vial on a magnetic stirrer at 50°C, stir to completely dissolve the raw materials in the solvent, and after stirring at constant temperature for 120 minutes, quickly take out the stir bar, and cap and seal the glass vial.
[0029] (3) The glass vial is sealed and placed at room temperature for 24 hours, and light yellow flake crystals are formed, which is the co-crystal of deferiprone-maleic acid drug. The dry weight of the product was 61.20 mg.
Embodiment 2
[0031] (1) Feed deferiprone and maleic acid at a molar ratio of 1:1, accurately weigh 41.70 mg of deferiprone and 34.80 mg of maleic acid, mix them in a glass vial, add 4 mL of ethanol and 4 mL of acetone, and put Put a 1cm magnetic stirrer, spread a layer of tin foil on the mouth of the glass vial, and tighten the cap to seal the vial.
[0032] (2) Place the above glass vial on a magnetic stirrer at 40°C, stir to completely dissolve the raw materials in the solvent, and after constant temperature stirring for 110 minutes, quickly take out the stir bar, and seal the glass vial.
[0033] (3) The glass vial is sealed and placed at room temperature for 72 hours, and light yellow flake crystals are formed, which is the co-crystal of deferiprone-maleic acid drug. The dry weight of the product was 56.10 mg.
Embodiment 3
[0035] (1) Feed deferiprone and maleic acid at a molar ratio of 1:2, accurately weigh 28.00mg of deferiprone and 23.20mg of maleic acid, mix them in a glass vial, add 6mL ethanol and 3mL acetone, and put Put a 1cm magnetic stirrer, spread a layer of tin foil on the mouth of the glass vial, and tighten the cap to seal the vial.
[0036] (2) Place the above-mentioned glass vial on a magnetic stirrer at 50°C, stir to completely dissolve the raw materials in the solvent, and then quickly take out the stir bar after stirring at constant temperature for 115 minutes, and seal the glass vial.
[0037] (3) The glass vial is sealed and placed at room temperature for 48 hours, and light yellow flake crystals are formed, which is the co-crystal of deferiprone-maleic acid drug. The dry weight of the product was 39.50 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com