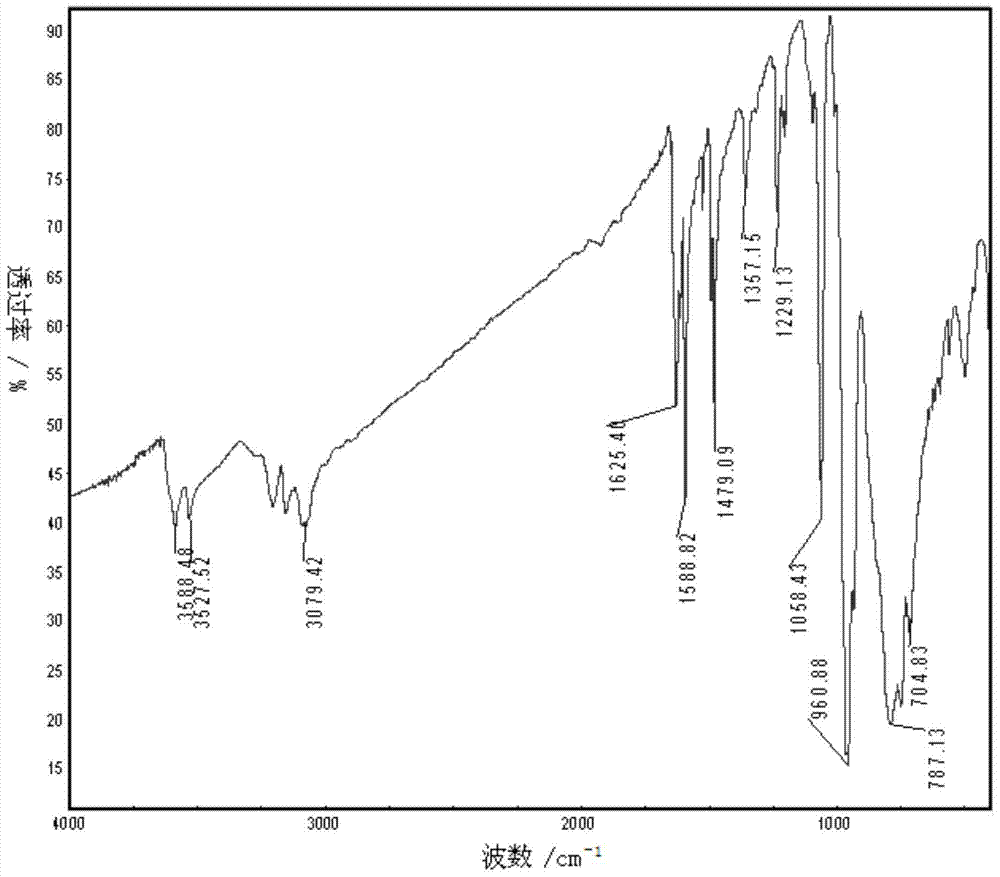
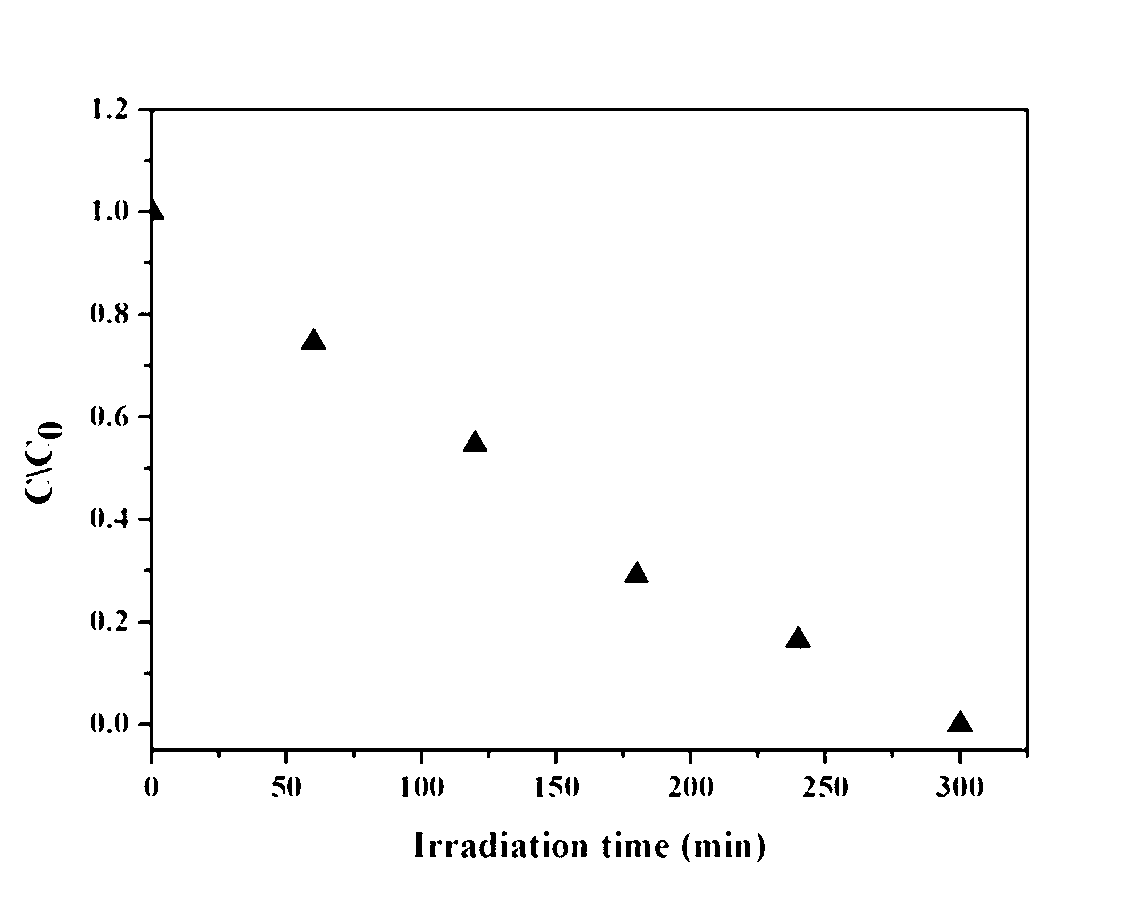
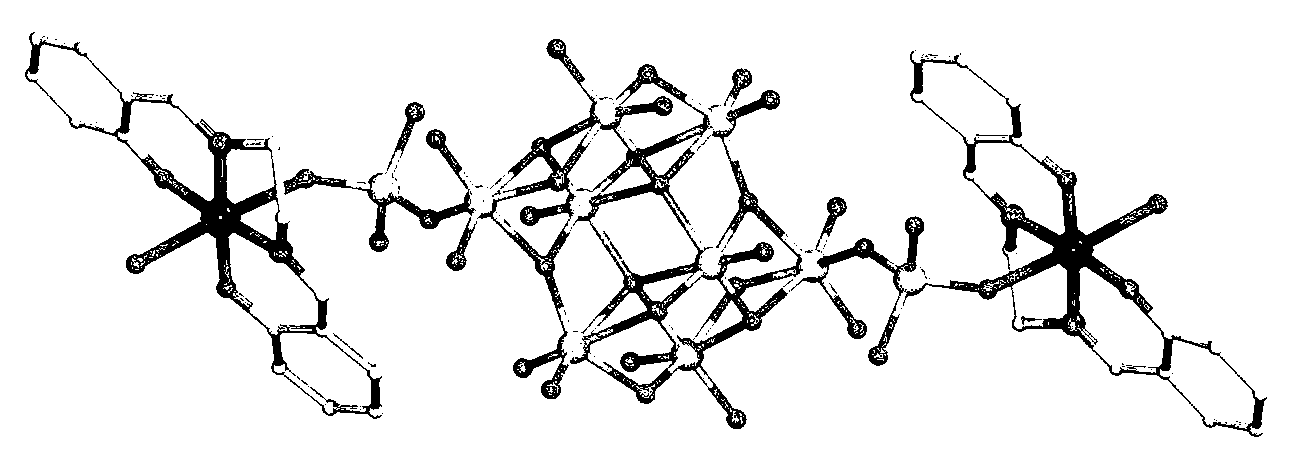
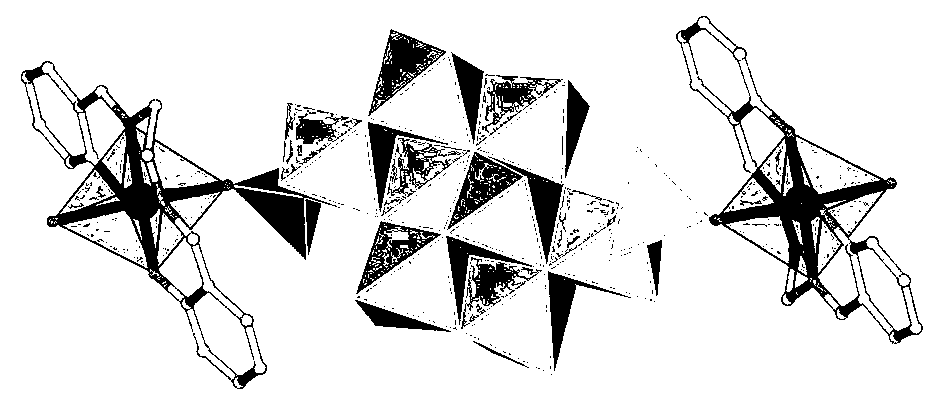
Patents
Literature
128 results about "Triclinic crystal system" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
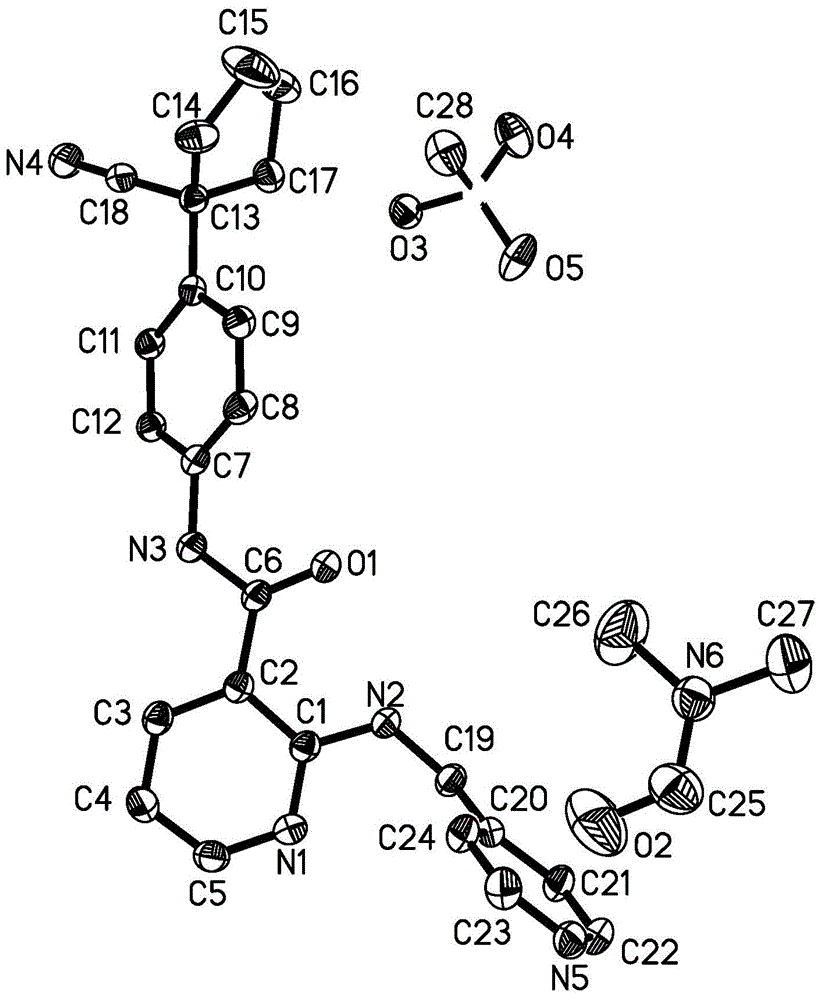
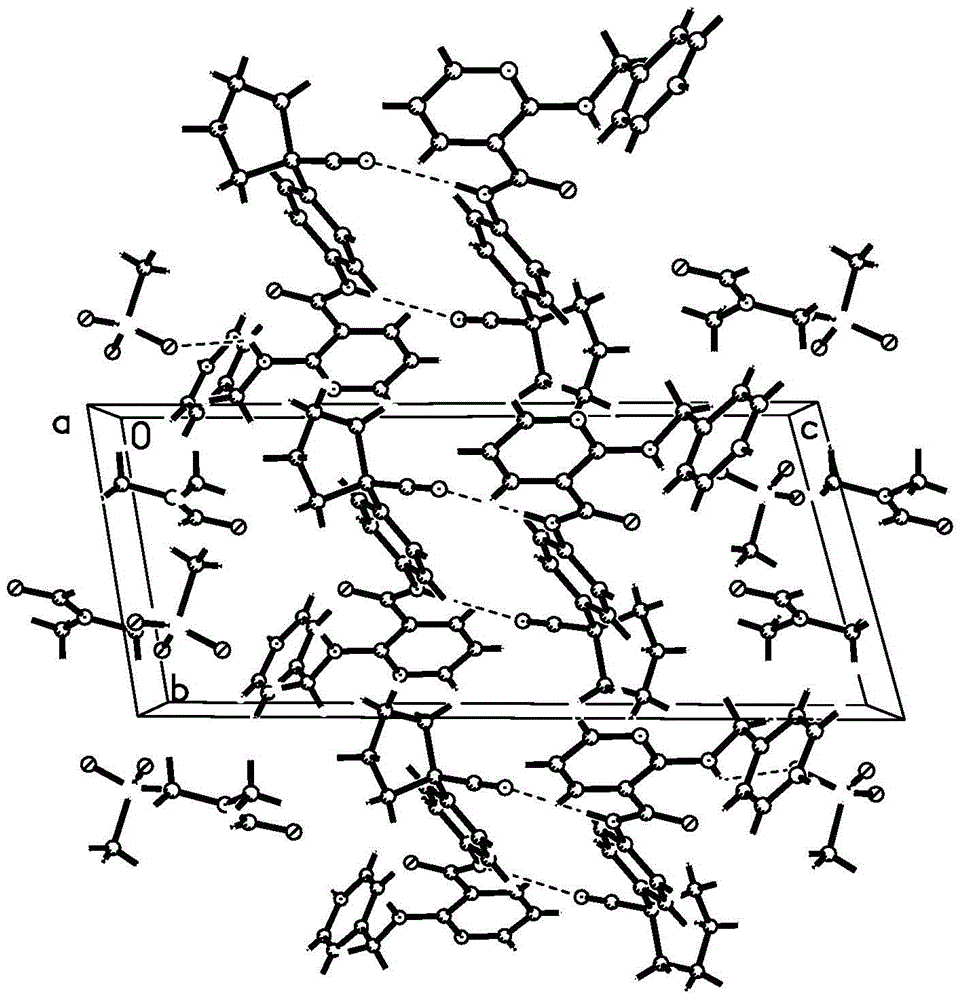
In crystallography, the triclinic (or anorthic) crystal system is one of the 7 crystal systems. A crystal system is described by three basis vectors. In the triclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. In addition, the angles between these vectors must all be different and may include 90°.
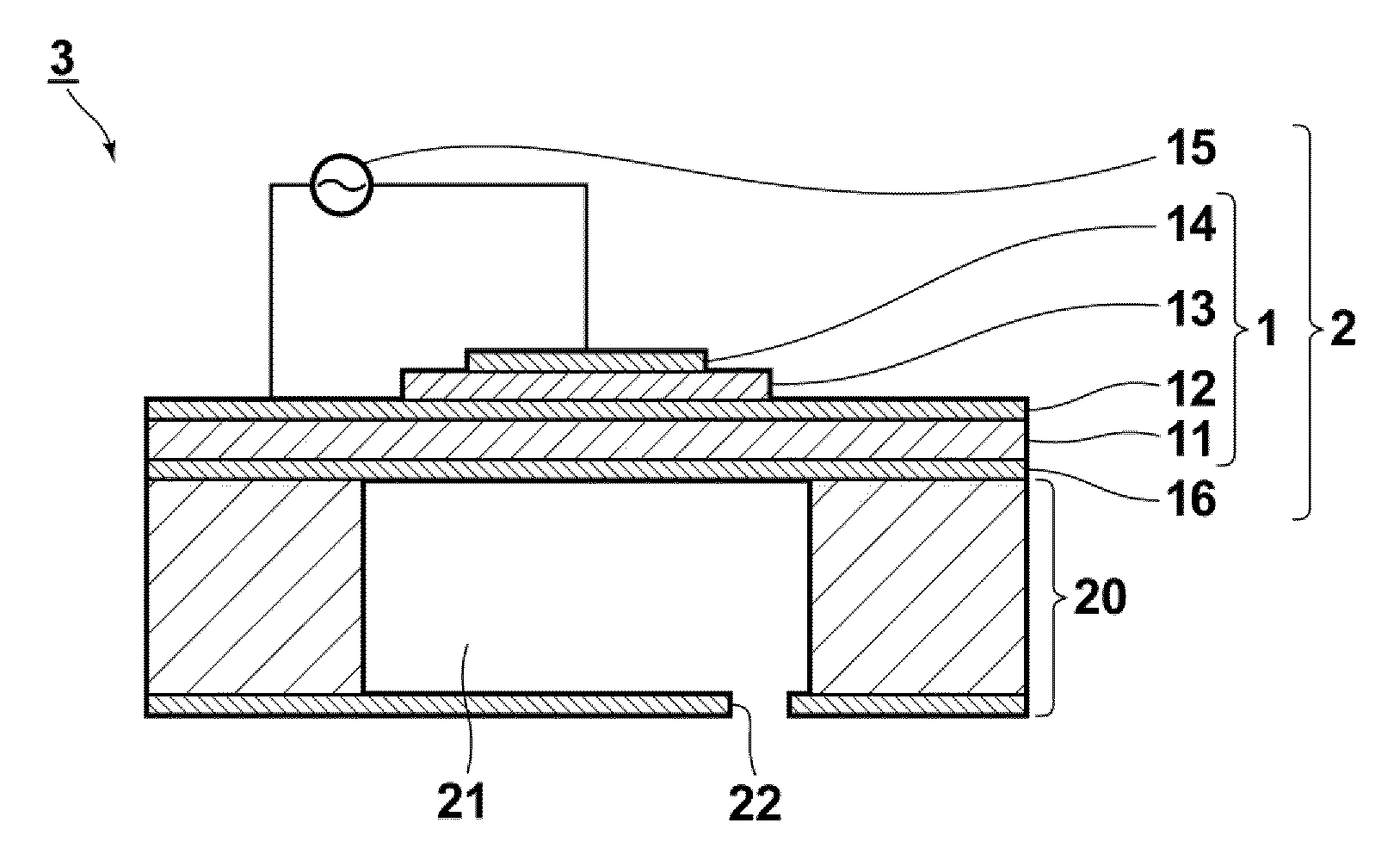
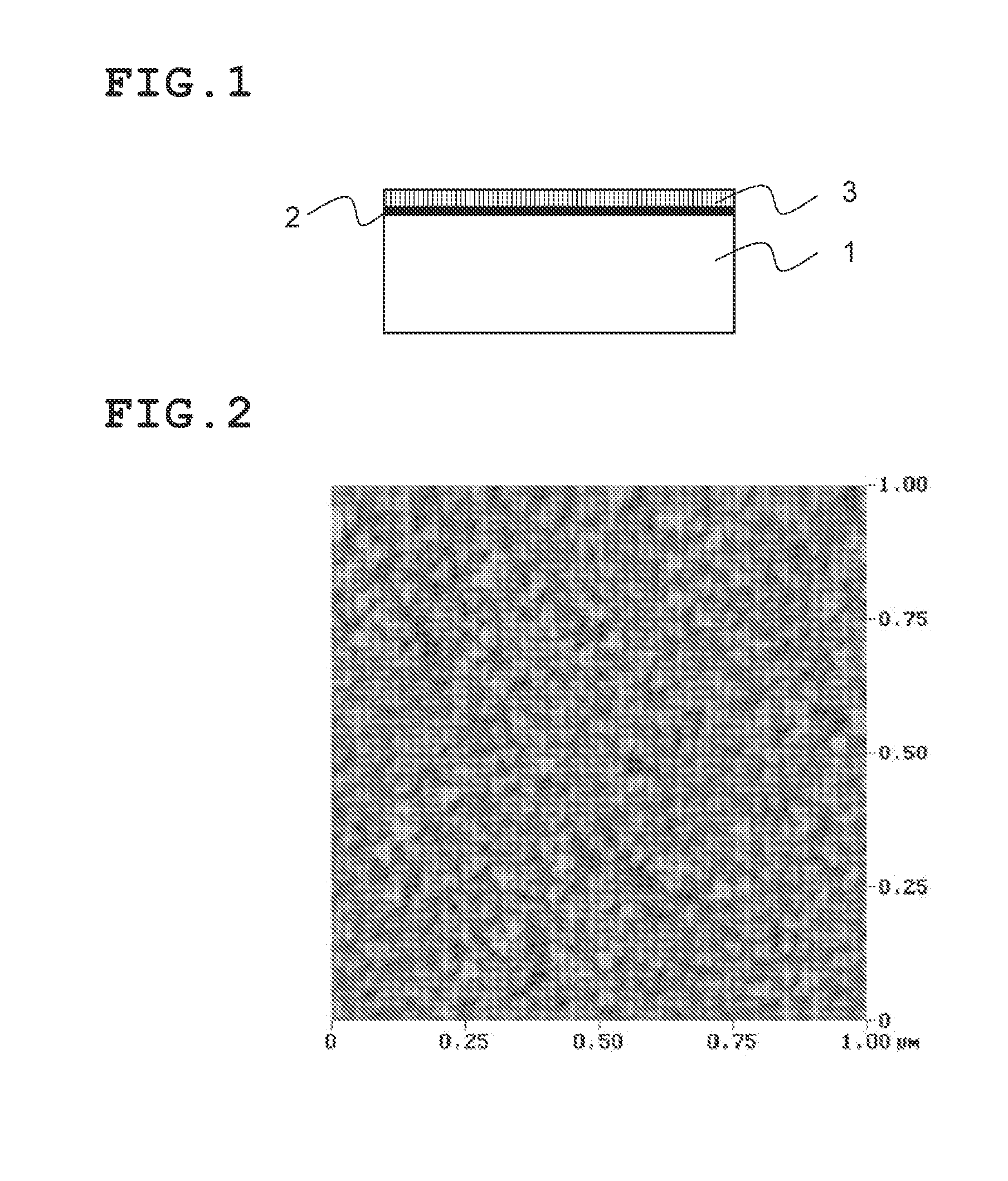
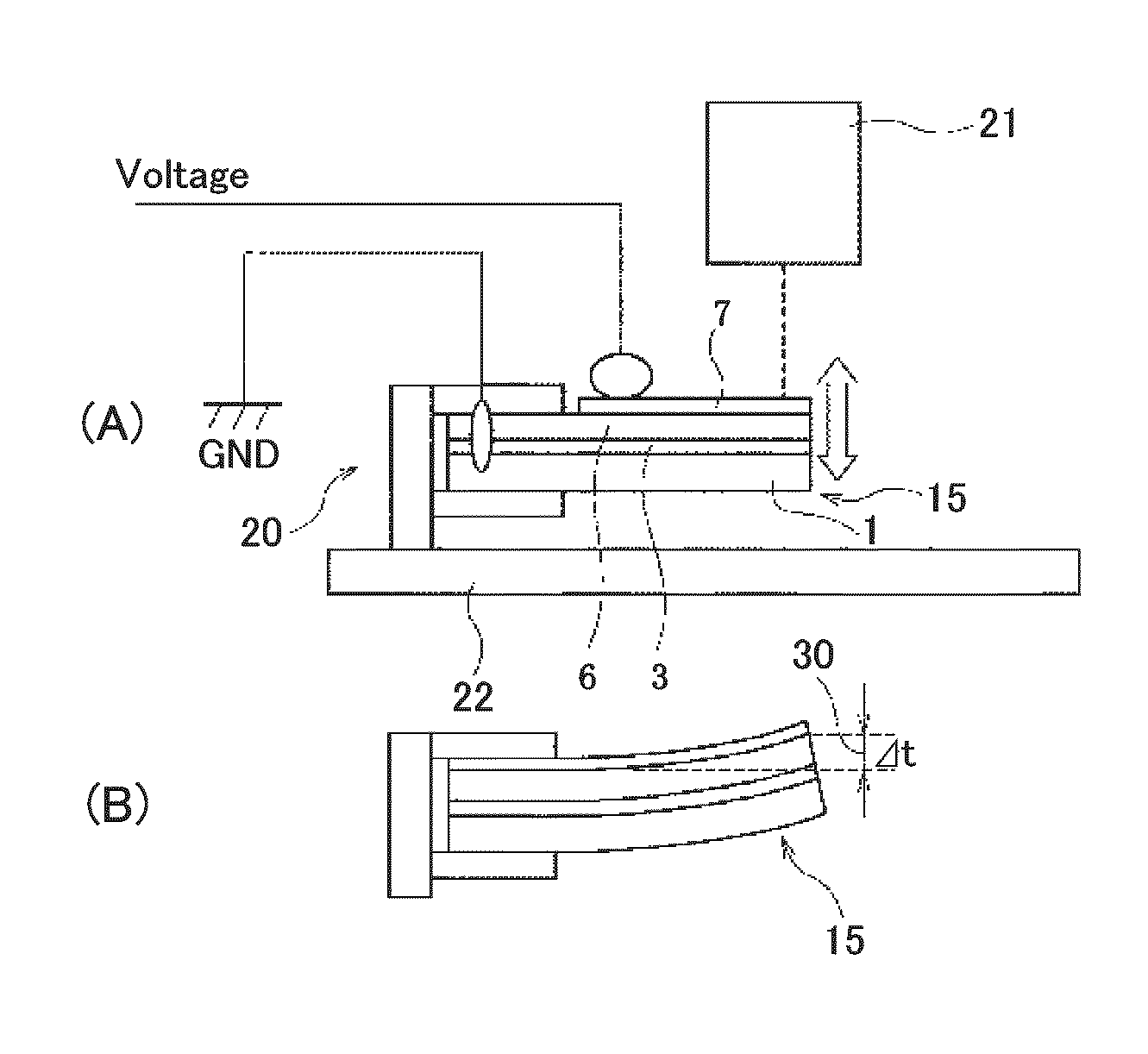
Perovskite oxide material, ferroelectric compound, piezoelectric body, piezoelectric device, and liquid discharge device
ActiveUS20110007115A1Reduce contentSimple compositionAlkaline earth titanatesPiezoelectric/electrostriction/magnetostriction machinesOxygenFerric
A perovskite oxide material containing: BiFeO3 as a first component; a second component containing at least one perovskite oxide which is constituted by A-site atoms having an average ionic valence of two and has a tendency to form a tetragonal structure; and a third component containing at least one perovskite oxide which has a tendency to form one of monoclinic, triclinic, and orthorhombic structures; where each perovskite oxide in the first component, the second component, and the third component contains A-site atoms, B-site atoms, and oxygen atoms substantially in a molar ratio of 1:1:3, and the molar ratio can deviate from 1:1:3 within a range
Owner:FUJIFILM CORP
Piezoelectric film element, and manufacturing method of the same and piezoelectric film device
ActiveUS20110187237A1Piezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostriction/magnetostriction machinesTetragonal crystal systemHexagonal crystal system
A piezoelectric film element is provided, which is capable of improving piezoelectric properties, having on a substrate at least a lower electrode, a lead-free piezoelectric film, and an upper electrode, wherein at least the lower electrode out of the lower electrode and the upper electrode has a crystal structure of a cubic crystal system, a tetragonal crystal system, an orthorhombic crystal system, a hexagonal crystal system, a monoclinic crystal system, a triclinic crystal system, a trigonal crystal system, or has a composition in which one of these crystals exists or two or more of them coexist, and crystal axes of the crystal structure are preferentially oriented to a specific axis smaller than or equal to two axes of these crystals, and a ratio c / a′ is set in a range of 0.992 or more and 0.999 or less, which is the ratio of a crystal lattice spacing c in a direction of a normal line to the substrate surface, with respect to a crystal lattice spacing a′ whose inclination angle from the substrate surface is in a range of 10° or more and 30° or less.
Owner:SUMITOMO CHEM CO LTD
High nuclear gadolinium cluster complex with large magnetocaloric effect and preparation method thereof
InactiveCN102964368ALarge magnetocaloric effectRaw materials are simple and cheapOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsSpace groupGadolinium oxide
The invention relates to a high nuclear gadolinium cluster complex with a large magnetocaloric effect. The chemical formula is C110H82Gd10O56S22 which belongs to the triclinic system, and the space group is P-1. The preparation method comprises the steps of: placing gadolinium oxide, thiophene-3-carboxylic acid and water into a stainless steel reaction kettle and mixing uniformly; then, reacting for 72 hours at 150-170 DEG G; cooling to 20 DEG C to obtain block crystals; collecting the crystals and washing the crystals with water and ethanol; and vacuum drying to obtain the target product: high nuclear gadolinium cluster complex. The invention has the advantages that according to the preparation method based on large self-rotating ground state of gadolinium ions, weaker magnetic interaction among metals and larger metal ligand proportion, the high nuclear gadolinium cluster complex with the large magnetocaloric effect is prepared; the raw materials are simple and cheap to the benefit of large-scale production; and the complex has the large magnetocaloric effect, and has a huge potential application value in the aspect of magnetic refrigeration.
Owner:NANKAI UNIV
Preparation method for three-dimensional layered CoV2O6 micro-nano powder
InactiveCN102730769AHigh purityPorous pore size distribution is uniformOther chemical processesCobalt compoundsMicro nanoCOBALTOUS NITRATE HEXAHYDRATE
The present invention discloses a preparation method for three-dimensional layered CoV2O6 micro-nano powder. According to the present invention, cobaltous nitrate hexahydrate or cobalt chloride hexahydrate, sodium hydroxide or hydrochloric acid, and vanadium pentoxide as raw materials; and steps of mixing, hydrothermal reaction, separation, washing, drying and the like are performed to obtain the three-dimensional layered CoV2O6 micro-nano powder. The three-dimensional layered CoV2O6 product prepared by the method of the present invention is black powder, wherein the three-dimensional layered CoV2O6 product belongs to a triclinic crystal system, and has advantages of large specific surface area (86.8 m<2> / g), high purity and good product quality. In addition, the three-dimensional layered CoV2O6 micro-nano powder prepared by the method of the present invention provides characteristics of fast adsorption rate and large saturated adsorption capacity for methylene blue trihydrate in solutions, wherein the saturated adsorption capacity is up to165.6 mg / g when the pH value of the solution is 1.0.
Owner:ANHUI NORMAL UNIV
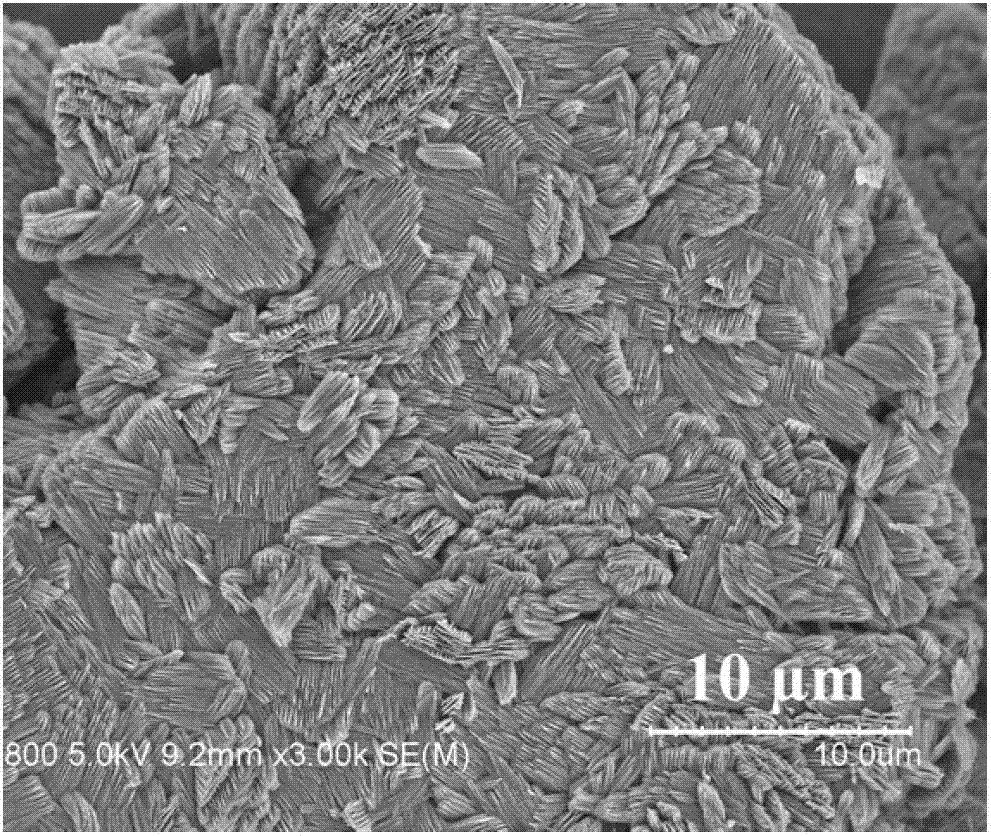
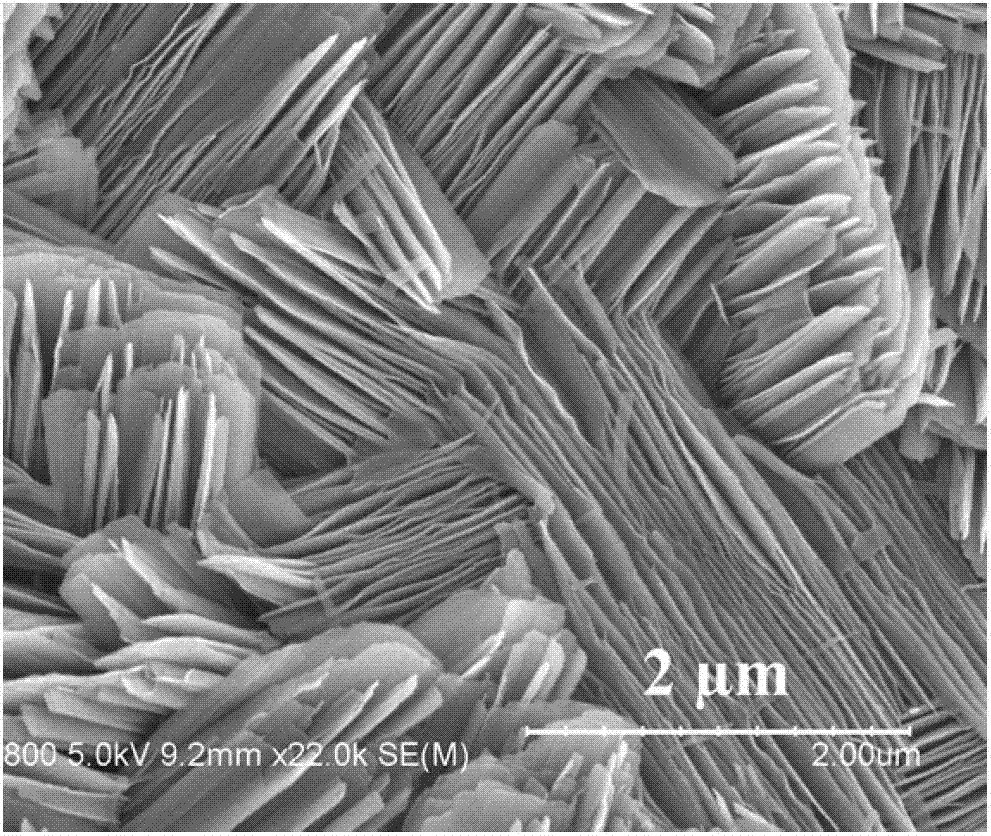
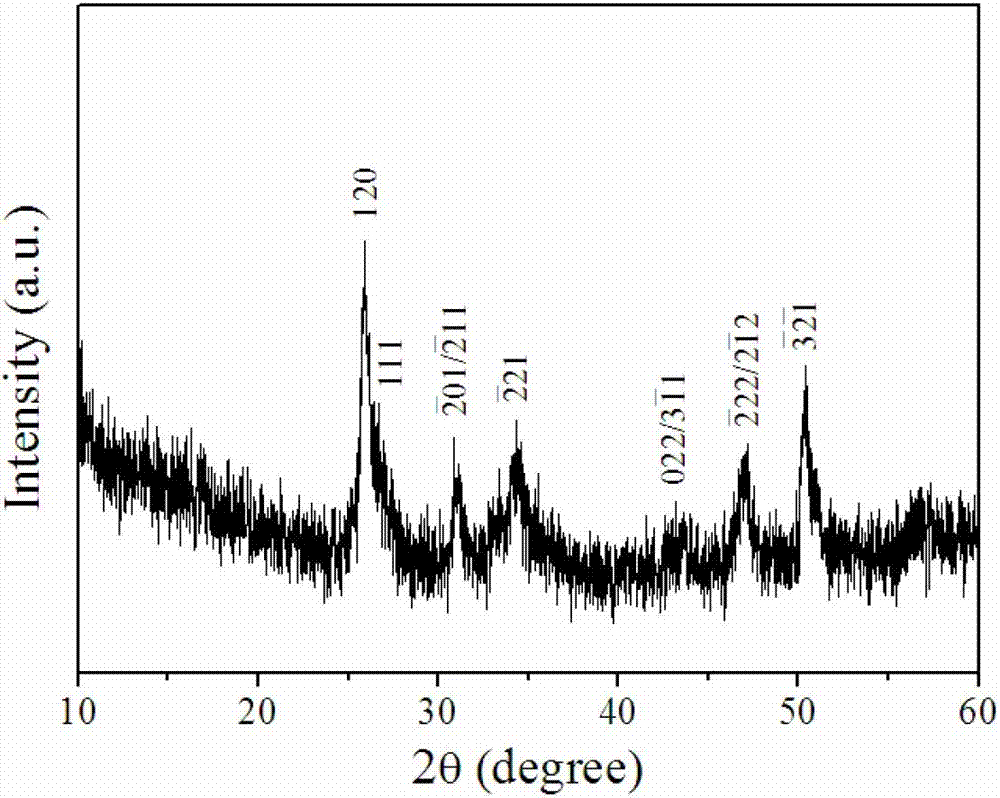
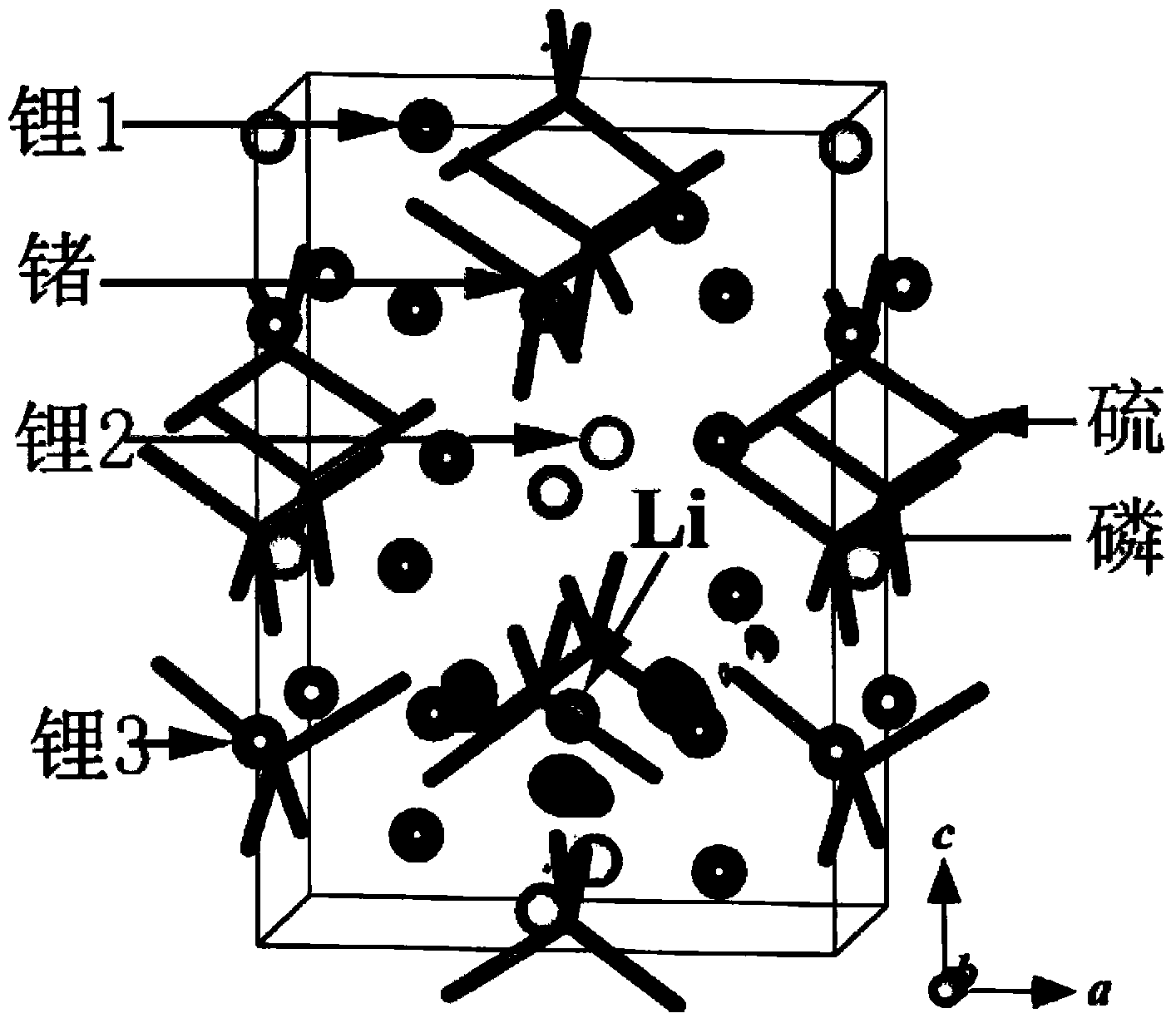
Solid-state electrolyte material of lithium ion battery
ActiveCN103401018AImprove structural stabilityIncrease attractivenessSecondary cellsSolid state electrolyteSpace group
The invention provides a solid-state electrolyte material of a lithium ion battery. The general formula of the solid-state electrolyte material is (LimZn)MP2X12, and the solid-state electrolyte belongs to a triclinic crystal system and a P1 space group, wherein Z is a high-valence metal element, the cationic valence is more than +1 valence and less than or equal to +3 valence, and the high-valence metal element Z is at least one of Mg, Al, Ca, Ti, Cu, Zn, Ga, In, Sr, Ru, Rh, Pd, Ag, Cd, Ba, Os, Ir, Pt or Hg; M is at least one of Ge, Si, Sn, Al or P; and X is at least one of O, S or Se. The research on the micro structure characteristics of the solid-state electrolyte material (LimZn)MP2X12 indicates that the micro interaction mechanisms such as Coulomb force, Van der Waals force and the like have important effect on the structure stability of the solid electrolyte material; and by enhancing the Coulomb attraction effect among ions and enhancing the shielding of the Coulomb repulsion effect among the ions, the total energy of the material system is greatly reduced, thereby enhancing the structure stability of the solid electrolyte material.
Owner:CONTEMPORARY AMPEREX TECH CO
Organic-inorganic hybrid polyoxomolybdate crystal material and preparation method thereof
InactiveCN103924302AAcidity can be adjustedThe synthesis method is simplePolycrystalline material growthFrom normal temperature solutionsSpace groupFluorescence
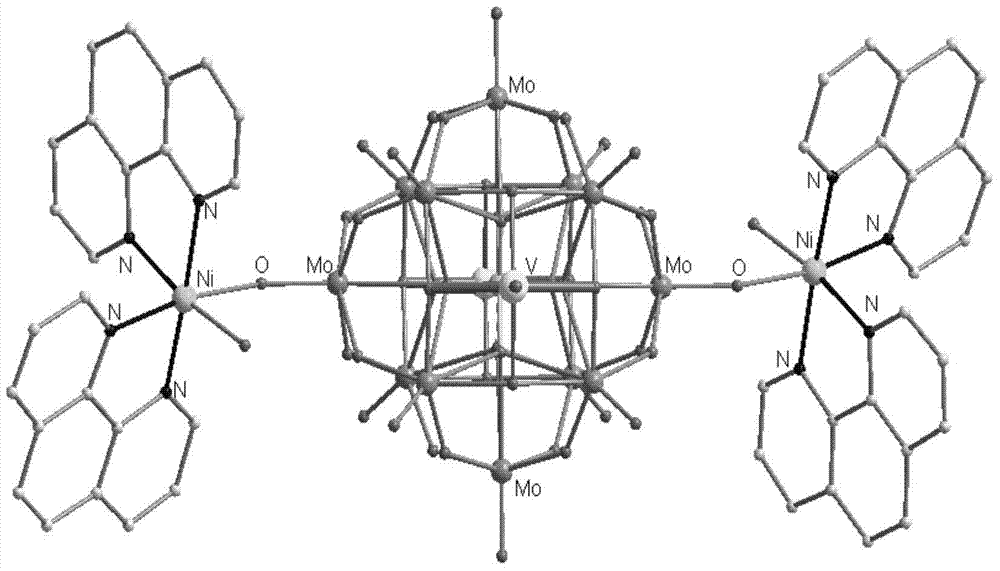
The invention discloses an organic-inorganic hybrid polyoxomolybdate crystal material and a preparation method thereof. The organic-inorganic hybrid polyoxomolybdate crystal material comprises a polyoxomolybdate crystal, and the chemical formula of the polyoxomolybdate crystal is [Ni(H2O)(C12H8N2)2]2(PMo12O40(VO)2].2H2O, the polyoxomolybdate crystal belongs to a triclinic system, and the space group of the polyoxomolybdate crystal is P-1. The organic-inorganic hybrid polyoxomolybdate crystal material is prepared by adopting a hydrothermal synthesis method. The preparation method of the organic-inorganic hybrid polyoxomolybdate crystal material comprises the following steps: and stirring and mixing molybdenum oxide, ammonium metavanadate, 1, 10-phenanthroline, nickel acetate, phosphoric acid and water, and then putting the mixture in a closed container, reacting for at least 1 hour at least 160 DEG C, and carrying out solid-liquid separation, thus obtaining the organic-inorganic hybrid polyoxomolybdate crystal material. When being excited by ultraviolet light with the wavelength of 310nm, the organic-inorganic hybrid polyoxomolybdate crystal material emits relatively strong fluorescence at a place with the central wavelength of 410nm, which shows that the crystal material has good fluorescence characteristic.
Owner:HEFEI UNIV
Multi-metal hybrid compound formed by transplanting metal-Schiff base fragment on isopolymolybdate, preparation method and applications thereof
InactiveCN103254237AGood for performance characterizationPromote Applied ResearchWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsCrystal systemMolybdate
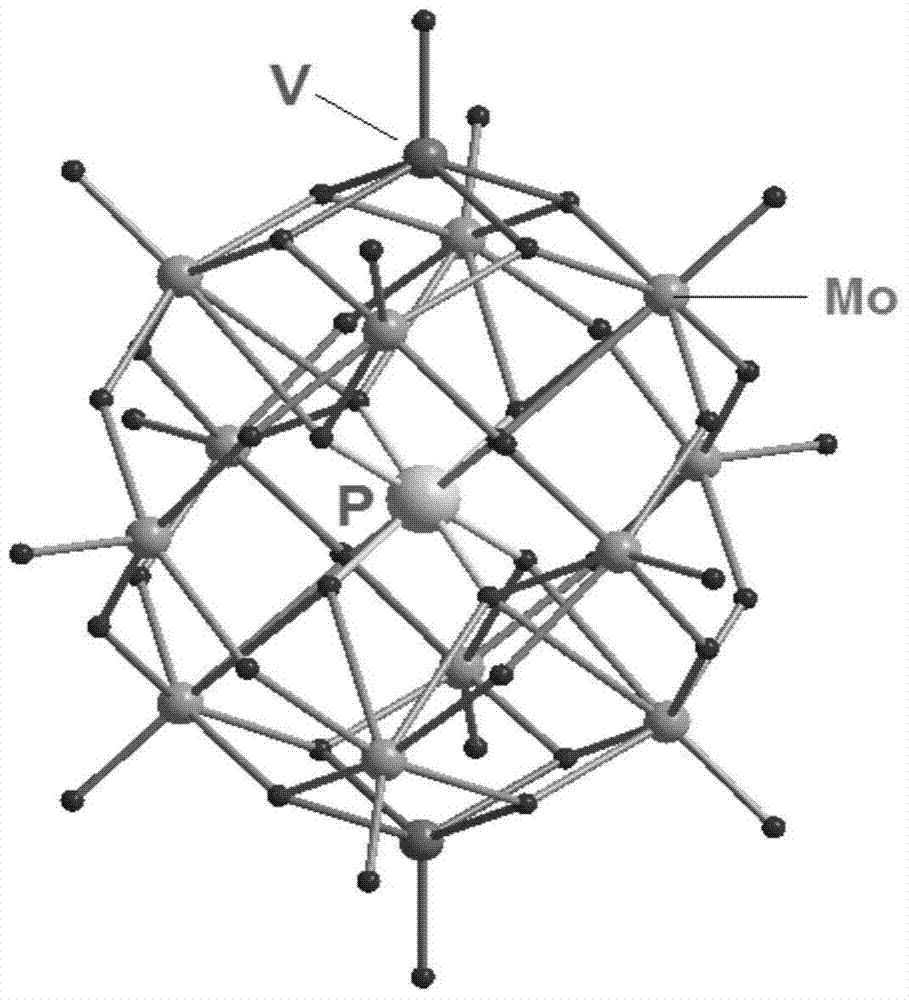
The present invention discloses a multi-metal hybrid compound formed by transplanting a metal-Schiff base fragment on an isopolymolybdate, a preparation method and applications thereof. The compound comprises an isopolymolybdate and a metal Schiff base fragment, has a molecular formula [NH4]2{[Mn(salen)(H2O)]2[Mo8O26(MoO4)2]}(H2en)2.9H2O, and is a triclinic system, wherein a space group is P-1, crystal cell parameters comprise that a is 10.507(2)angstrom, b is 13.708(3)angstrom, c is 14.701(3)angstrom, alpha is 108.94(3), beta is 110.16(3), gamma is 93.98(3), V is 1840.5(6)angstrom<3>, and Z is 2, and the crystal cell has a structure and a composition represented by a formula 1, and is represented by a Figure 2. The preparation method comprises: adding a [Mn(salen)(H2O)]2(ClO4)2.H2O-containing methanol solution in a dropwise manner, continuously stirring for 11-13 h at a temperature of 55-65 DEG C to obtain a brown clear solution, filtering, adopting a film with fine holes to carry out sealing storage, slowly evaporating the filtrate at a room temperature to obtain a sheet-like crystal product after 21 days, washing with methanol, and drying in air to obtain the multi-metal hybrid compound formed by transplanting the metal-Schiff base fragment on the isopolymolybdate, wherein the compound has characteristics of excellent stability and reusability, and can be used for catalytic degradation of pollutants in water in the photocatalysis field.
Owner:KUNMING UNIV
Method for synthesizing rare-earth organic-inorganic hybrid fluorescent material
The invention provides a novel method for synthesizing rare-earth organic phosphotungstate hybrid compound (Ln(PW11O39)(H2O)2)(H2bpy)2.6.5H2O(Ln=Eu(1), Gd(2); bpy=4,4'-bipyridyl). The hybrid compound belongs to a triclinic system, has a space group of P(-1) and is synthesized by Ln2Cl3.6H2O, 4, 4'-bipyridyl, (NH4)3PW12O40.3H2O and deionized water by a hydro-thermal method. A fluorescence spectrum displays that the compound has the obvious fluorescent characteristics.
Owner:BEIJING UNIV OF CHEM TECH
Mesylate solvate crystal of nicotinamide derivatives and preparation method and application of mesylate solvate crystal
ActiveCN104086484AImprove high temperature stabilityImprove stabilityOrganic active ingredientsOrganic chemistrySpace groupCell parameter
The invention discloses a mesylate solvate crystal of nicotinamide derivatives and a preparation method and application of the mesylate solvate crystal. The mesylate solvate crystal of nicotinamide derivatives is a mesylate dimethylformamide solvate crystal and has the characteristic parameters that the crystal system is a triclinic system; the space group is P-1; the cell parameters are as follows: a is 8.9418(2) angstrom, b is 9.4513(2) angstrom, c is 19.5111(4) angstrom, alpha is 76.489(1), beta is 84.199(1), and gamma is 61.952(1); the cell volume V is 1414.91(5) cubic angstrom; the number Z of asymmetry units in cells is equal to 2. The mesylate solvate crystal of nicotinamide derivatives provided by the invention has high high-temperature stability, high humidity stability and illumination stability, is applied to medicines for treating advanced non-small cell lung cancer, gastric carcinoma, liver cancer or breast cancer and is obvious in bioavailability, and the provided qualitative and quantitative information has significance for further researching the curative effect of the solid drugs.
Owner:SHANGHAI SUNTRONG BIOTECH
Structure, preparation method and use of diethylenetriamine-based cantharidimide dimer derivative
InactiveCN105601650AEasy to synthesizeOrganic chemistry methodsAntineoplastic agentsEpoxySpace group
The invention relates to a structure, a preparation / crystallization method and partial characters of a cantharidin derivative. The compound is of a colorless rod crystal in appearance, the melting point is 126.0-127.2 DEG C, the molecular formula is C20H21N3O6, the chemical name is 2,2'-(3-azapentane-1,5-diyl)bi(3a,4,7,7a-tetrahydro-4,7-epoxy-1,3-dihydro-isoindole-1,3-dione), and the structure formula is shown in the specification. The structure is of a triclinic system, the space group is P-1, a is equal to 8.765(3) angstroms, b is equal to 9.709(3) angstroms, c is equal to 11.940(4) angstroms, alpha is equal to 76.723(4) degrees, beta is equal to 78.423(4) degrees, gamma is equal to 74.682(4) degrees, V is equal to 943.1(5) cubic angstroms, and Z is equal to 2. The preparation method of the compound is simple, and the compound has a mild inhibition effect on lung carcinoma cells and breast cancer cells.
Owner:QILU UNIV OF TECH
Low-temperature reversible thermochromic crystal material as well as preparation method and application thereof
InactiveCN108103582AChange color quicklyHas reversible thermochromic propertiesPolycrystalline material growthFrom normal temperature solutionsCrystal cellPrepared Material
Owner:JIANGNAN UNIV
Piezoelectric film element using (Na,K,Li)NbO3
ActiveUS8581477B2Piezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostriction/magnetostriction machinesTetragonal crystal systemHexagonal crystal system
A piezoelectric film element is provided, which is capable of improving piezoelectric properties, having on a substrate at least a lower electrode, a lead-free piezoelectric film, and an upper electrode, wherein at least the lower electrode out of the lower electrode and the upper electrode has a crystal structure of a cubic crystal system, a tetragonal crystal system, an orthorhombic crystal system, a hexagonal crystal system, a monoclinic crystal system, a triclinic crystal system, a trigonal crystal system, or has a composition in which one of these crystals exists or two or more of them coexist, and crystal axes of the crystal structure are preferentially oriented to a specific axis smaller than or equal to two axes of these crystals, and a ratio c / a′ is set in a range of 0.992 or more and 0.999 or less, which is the ratio of a crystal lattice spacing c in a direction of a normal line to the substrate surface, with respect to a crystal lattice spacing a′ whose inclination angle from the substrate surface is in a range of 10° or more and 30° or less.
Owner:SUMITOMO CHEM CO LTD
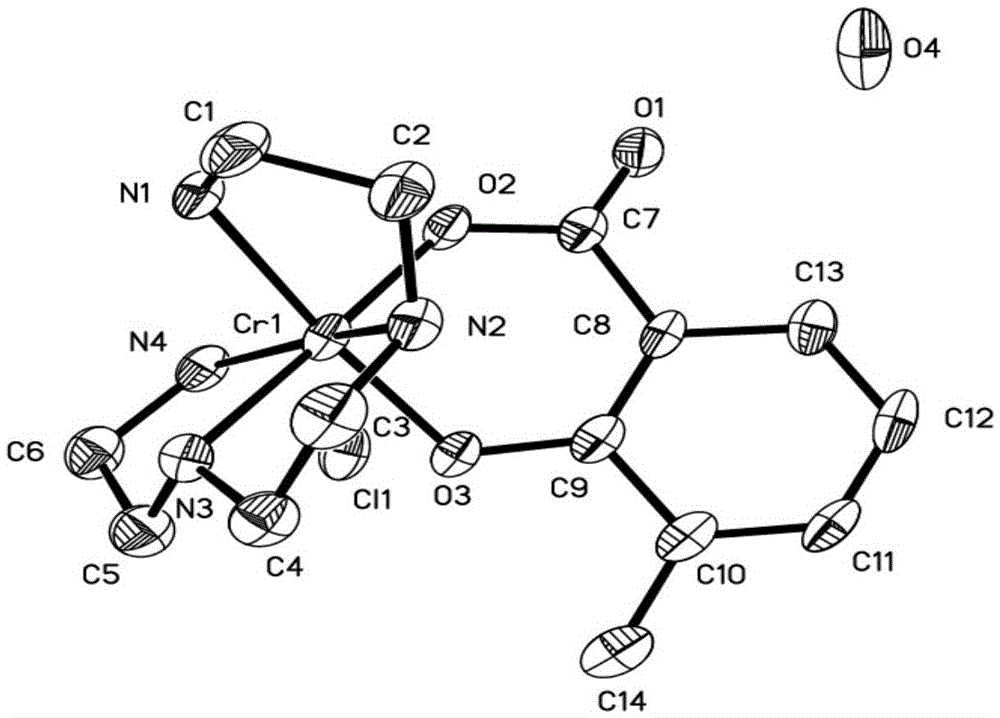
Chromium (III) complex as well as preparation method and application thereof
InactiveCN105601670AGood water solubilityModerate stabilityMetabolism disorderOrganic chemistry methodsRefluxOrganic solvent
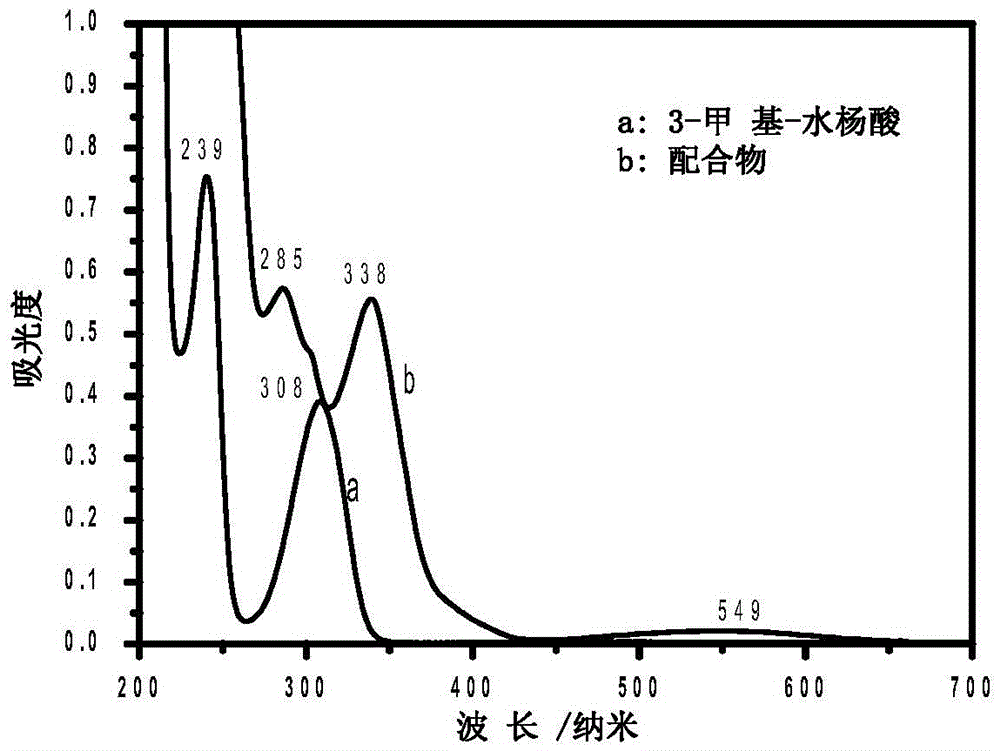
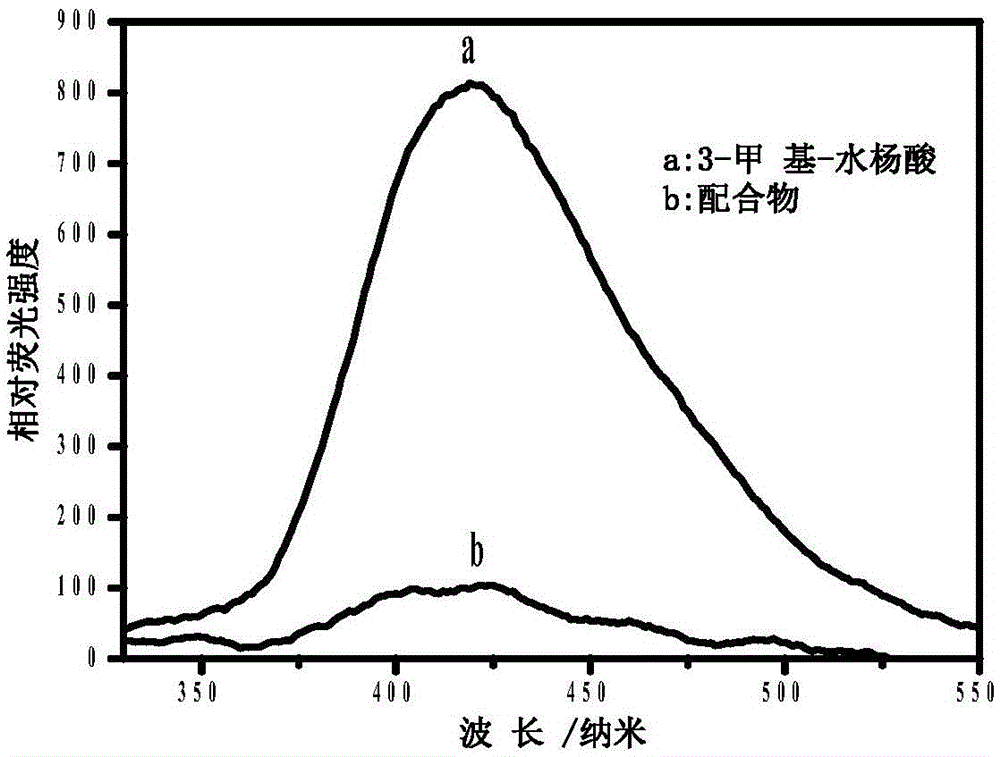
The invention provides a chromium (III) complex as well as a preparation method and application thereof. According to the chromium (III) complex, the molecular formula is CrC14H26N4O4Cl, the molecular weight is 402, the crystal structure belongs to a triclinic crystal system, the spatial point group is P-1, a is equal to 7.8880(6), b is equal to 9.8380(7), c is equal to 14.4490(12) angstroms, alpha is equal to 70.4550(10), beta is equal to 85.729(2), gamma is equal to 82.0880(10) degrees, V is equal to 1046.12(14) cubic angstroms, and Z is equal to 2. The preparation method comprises the steps of dissolving chromium (III) and 3-methyl-salicylic acid by virtue of an organic solvent, feeding a small amount of zinc granules, carrying out heating reflux, then dropwise adding triethylene tetramine, continuing to carry out reflux for a period of time, stopping reaction, cooling, filtering, standing obtained filtrate, and naturally volatilizing at the normal temperature, so as to obtain purple red crystals. The chromium (III) complex can be applied to the preparation of sugar-reducing medicines.
Owner:SHANXI UNIV
Er(III) luminescent material containing mixed ligand of phenanthroline, modified imidazolecarboxylic acid and pyridine carboxylic acid and preparation method
PendingCN110041351ALow costHigh crystallinityGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsElectron donorPhenanthroline
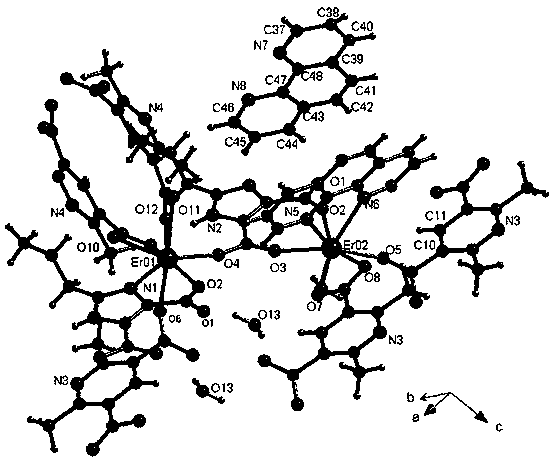
The invention relates to an Er(III) luminescent material containing a mixed ligand of phenanthroline, modified imidazolecarboxylic acid and pyridine carboxylic acid and a preparation method. The molecular formula of the luminescent material is {Er2(dpda)2(Hpimda)phen.H2O].0.5phen}n, n is greater than 1, wherein a ligand H2dpda is 2,6-dimethyl pyridine-3,5-dioctyl phthalate, H3pimda is 1-H-2-propyl-4,5-imidazole dicarboxylic acid, phen is phenanthroline, a complex crystal belongs to a triclinic system, and a space group is p-1; in cell parameters, a is equal to 8.5811(3) angstroms, b is equal to 15.7987(8) angstroms, c is equal to 18.0740(9) angstroms, alpha is equal to 113.357(5) degrees, beta is equal to 97.903(4) degrees, gamma is equal to 104.086(3) degrees, V is equal to 2181.61(19) angstrom<3>; the luminescent material can be excited by near ultraviolet to emit near infrared fluorescence. The preparation method of the luminescent material involved in the invention is simple, the conditions are mild, and the product yield is relatively high; by introduction of multiple conjugate ligands, electron transfer among electron donors and receptors is adjusted and enhanced; the crystallinity is relatively good, no toxicity and pollution are caused, the heat stability is high, the luminescent property is excellent, the raw material cost is low, a preparation process and equipment are simple, and the operation is convenient.
Owner:LUOYANG NORMAL UNIV
Anti-deliquesce terahertz non-linear optical crystal 4-(4-dimethyl amino styryl)methylpyridine 2-amino-5-toluenesulfonate
InactiveCN104389023AThe synthetic route is simpleObvious deliquescence resistancePolycrystalline material growthFrom normal temperature solutionsNonlinear optical crystalTerahertz radiation
The invention discloses a non-linear optical crystal material 4-(4-dimethyl amino styryl)methylpyridine 2-amino-5-toluenesulfonate, as well as preparation and application thereof, and belongs to the field of function materials. The non-linear optical crystal material has molecular formula of C23H27N3O3S, and belongs to a triclinic system and a P1 space group. The cell parameters of the non-linear optical crystal material are as follows: a=7.2651(6) angstroms, b=8.983(2) angstroms, c=10.1090(15) angstroms, alpha=97.82(8) degrees, beta=110.42(5) degrees, gamma=112.30(6) degrees, V=543.92(54) angstrom<3>, and Z=4. The nonlinear frequency multiplication coefficient of the crystal is about 0.83 times that of DAST. Above all, the crystal material does not generate crystal water after being crystallized in water so as to have the excellent anti-deliquesce performance and overcome the defects that DAST crystals are easy to deliquesce; the crystal material has a potential use value when being used as a terahertz radiation source crystal material.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Berberine hydrochloride eutectic crystal and preparation method thereof
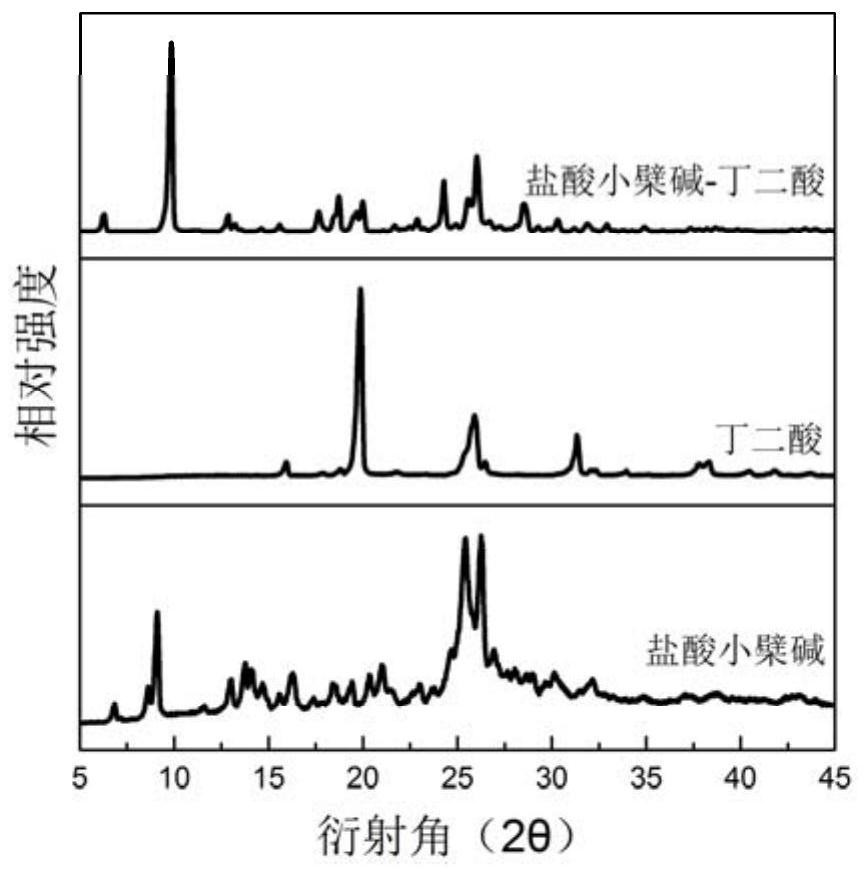
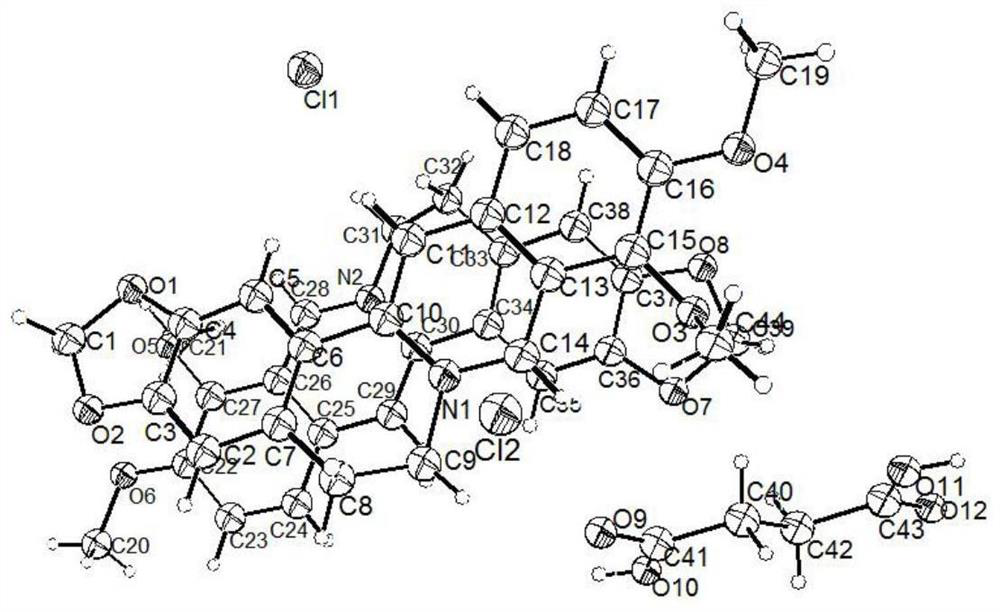
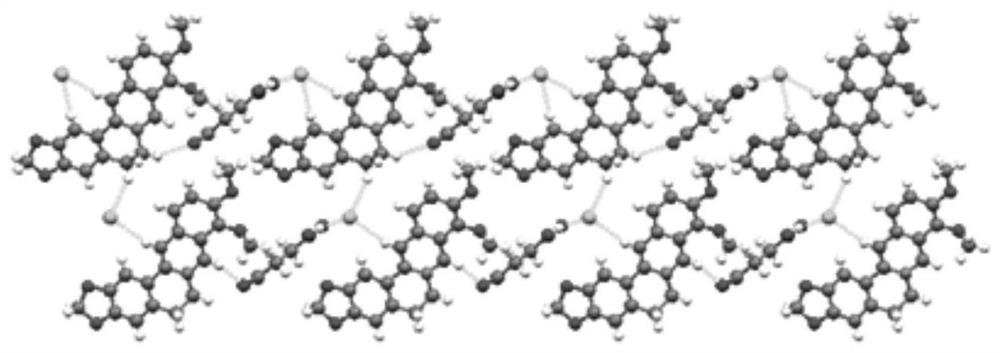
ActiveCN112521385AHigh dissolution rateIncrease dissolution rateOrganic chemistry methodsCarboxylic compound separation/purificationCrystal systemBerberine
The invention relates to a berberine hydrochloride eutectic crystal and a preparation method thereof. The method comprises the steps that: berberine hydrochloride and diacid are dissolved in a solvent, then cooling crystallization or standing crystallization is adopted, and a separated crystal is the berberine hydrochloride eutectic crystal; the berberine hydrochloride eutectic crystal is a berberine hydrochloride-diacid eutectic crystal of a basic structural unit formed by taking the berberine hydrochloride as an API and the diacid as a CCF; and the berberine hydrochloride-diacid eutectic crystal is a triclinic system and a P1 space group. According to the berberine hydrochloride eutectic crystal and the preparation method thereof of the invention, by means of the eutectic crystal mode, the powder dissolution rate and the inherent dissolution rate of the berberine hydrochloride are improved, and the hygroscopicity of the berberine hydrochloride is reduced, the berberine hydrochloridehas better stability, the bioavailability of the berberine hydrochloride can be greatly improved, and the curative effect of the berberine hydrochloride can be better exerted. In addition, the preparation method of the eutectic crystal is simple and easy to implement, mild in condition, easy to control, good in reproducibility and low in production cost, and has a great commercial application value; and the large-scale industrial production of the eutectic crystal is easy to achieve.
Owner:SHANGHAI UNIV OF ENG SCI
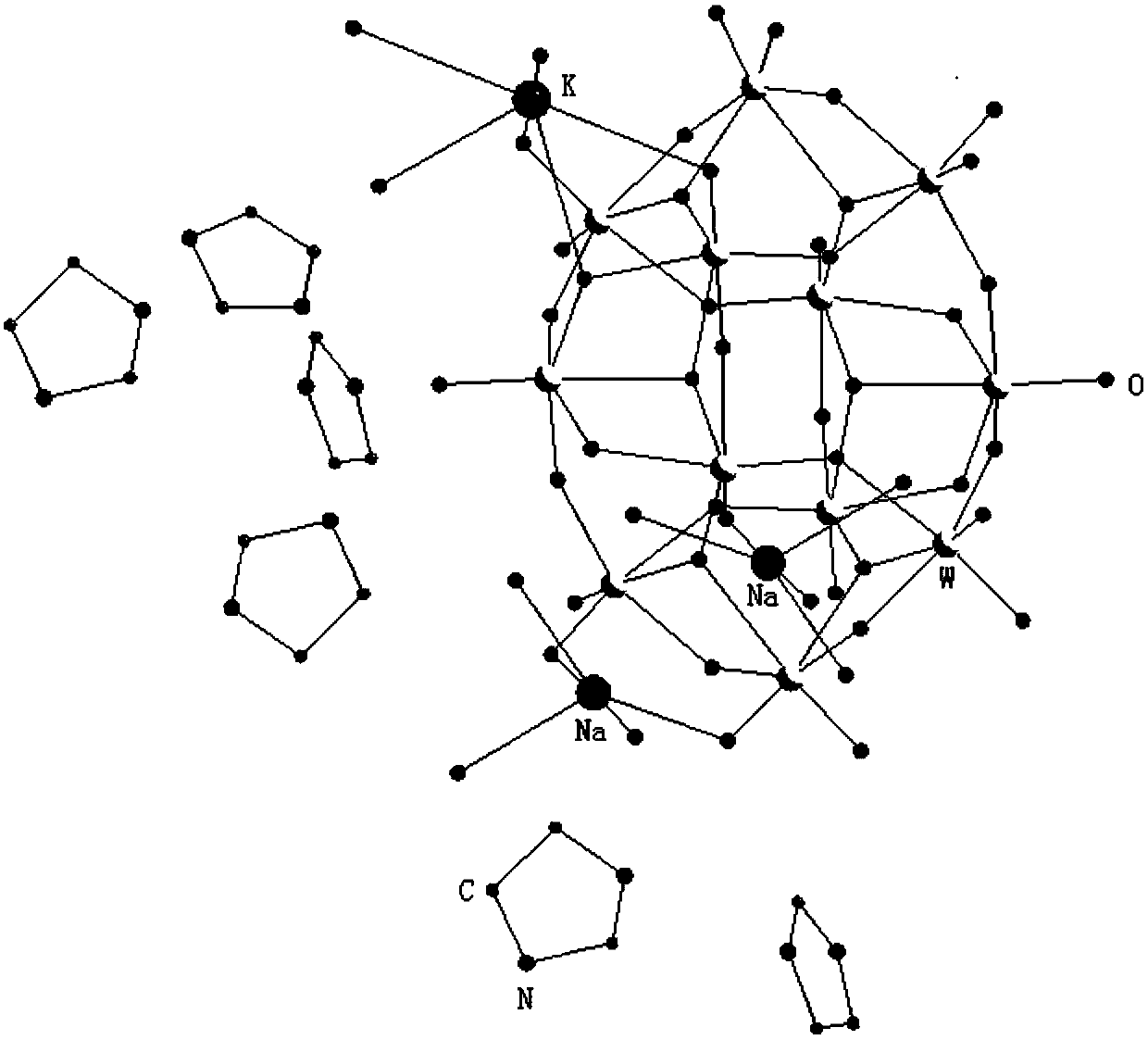
Dodeca-tungstate crystal material with two-dimensional nano pore cavity structure and preparation method of dodeca-tungstate crystal material
ActiveCN109518274AThe synthesis method is simpleEasy to operatePolycrystalline material growthFrom normal temperature solutionsCyclopenteneSpace group
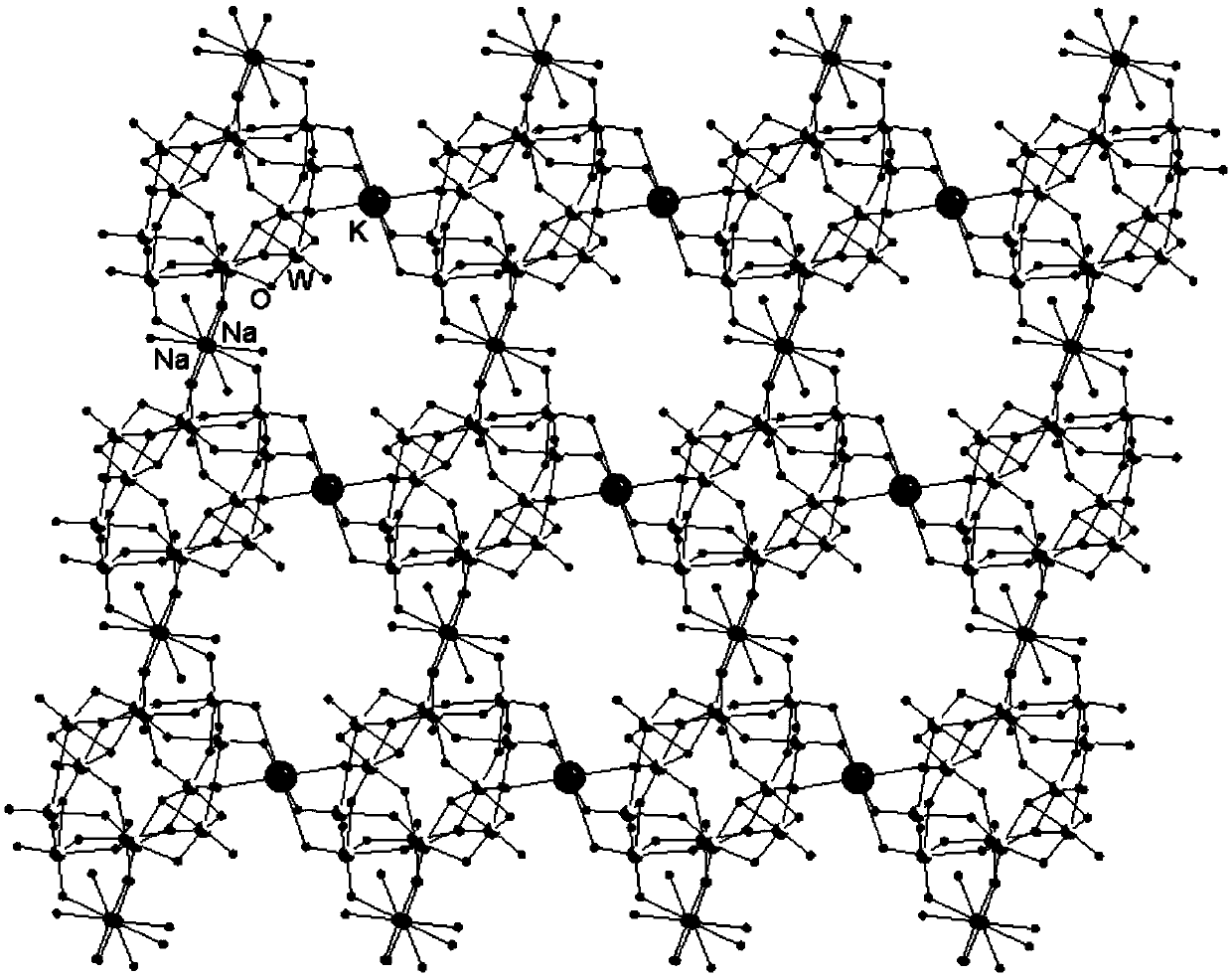
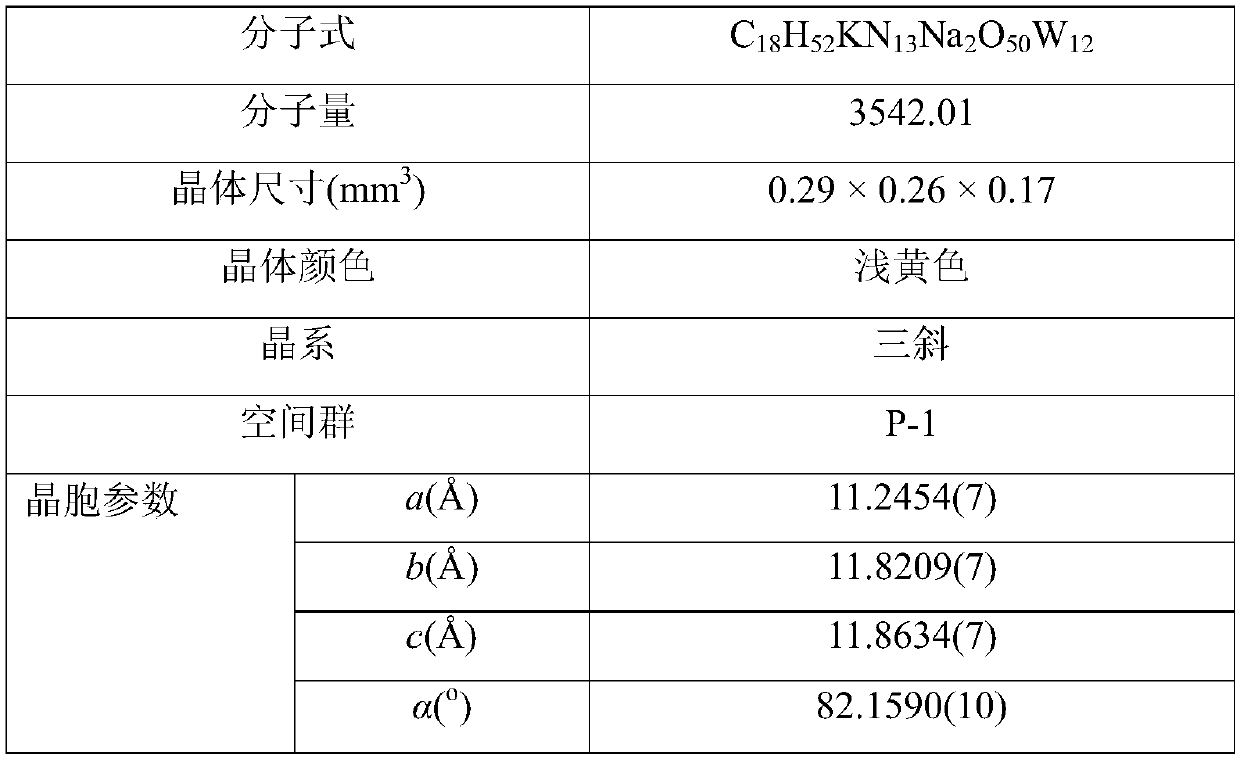
The invention discloses a dodeca-tungstate crystal material with a two-dimensional nano pore cavity structure and a preparation method of the dodeca-tungstate crystal material, and belongs to the technical field of metal oxygen cluster compound materials and preparation thereof. The chemical formula of the dodeca-tungstate crystal material is (NH4)Na2K(C3H5N2)6[H2W12O42].8H2O, the dodeca-tungstatecrystal material is a triclinic system, the space group is P-1, and the cell parameters are that a=11.2454(7) Angstrom, b=11.8209(7) Angstrom, and c=11.8634(7) Angstrom, wherein alpha is equal to 82.1590(10) degrees, and beta is equal to 87.7150(10) degrees. Raw materials such as sodium tungstate, tungsten acid, imidazole and ammonium chloride are subjected to hydrothermal synthesis, then the dodeca-tungstate crystal material provided with the two-dimensional nano pore cavity structure and capable of being widely applied to the field of catalytic materials can be prepared, the dodeca-tungstate crystal material can play a catalytic role when hydrogen peroxide oxidizes cyclopentene to prepare glutaraldehyde, the productive rate of a product can reach 60% or above, and the good catalytic activity is shown.
Owner:HEFEI UNIV
Mononuclear nickel coordination compound, and preparation method and application thereof
InactiveCN110818744ACheap and easy to useEasy to makeHydrocarbon from carbon oxidesOrganic-compounds/hydrides/coordination-complexes catalystsCrystal systemMethane gas
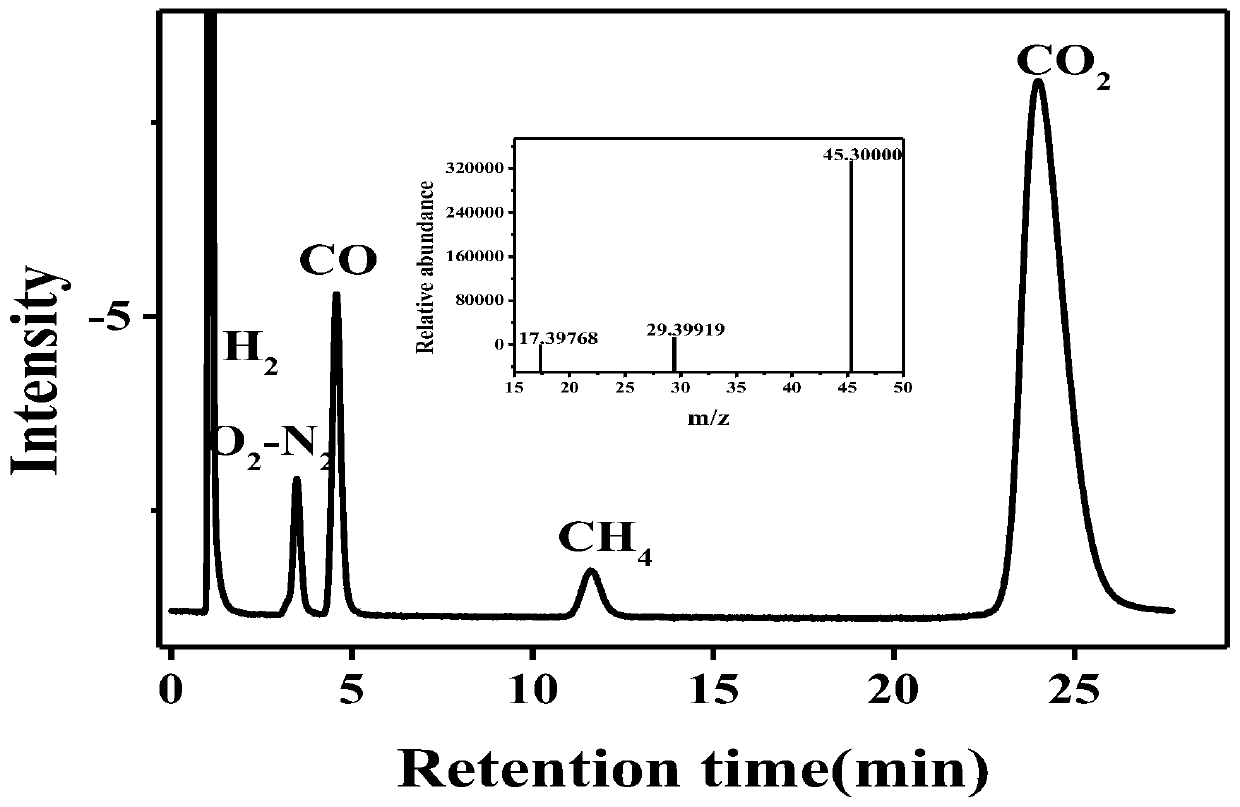
The invention relates to the technical field of coordination compound materials, and discloses a mononuclear nickel coordination compound, and a preparation method and an application thereof. An organic ligand 1,2-bis(dicyclohexylphosphino)ethane and nickel ions form the mononuclear nickel coordination compound having a chemical formula of C26H48Cl2NiP2 through a chlorine bridge bond. The coordination compound is a solid crystal, and the crystal belongs to a triclinic system and a P1 space group. The preparation method comprises the following steps: reacting a bis(triphenylphosphine)nickel chloride solid with 1,2-bis(dicyclohexylphosphino)ethane in an acetonitrile solution to obtain a yellowish-brown precipitate, then dissolving the precipitate in a mixed solution of acetonitrile and ethanol, and slowly volatilizing the solvents to obtain the yellow-green crystal. The compound can be used as a photocatalyst to convert greenhouse gas carbon dioxide into methane gas with high added values and CO. The preparation method has the advantages of simplicity, easiness in implementation, and high purity and high yield of the product, and the product has a good application prospect in the field of photocatalytic conversion of carbon dioxide.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
High-nuclear cobalt cluster substituted silicotungstate nano-cluster compound as well as preparation method and application thereof
ActiveCN111606360AHigh core countHigh metal oxygen-clusterMaterial analysis by electric/magnetic meansCobalt compoundsElectrochemical biosensorDichlorvos
The invention discloses a high-nuclear cobalt cluster substituted silicotungstate nano-cluster compound as well as a preparation method and application thereof. The high-nuclear cobalt cluster substituted silicotungstate nano-cluster compound has the following chemical formula: Na18[Co(H2O)6]2{[Na(H2O)4]2[Na(H2O)2]2[Co(H2O)3]2[Co21(H2O)4(OH)12] (SiW10O37)6} 24H2O. The high-core cobalt cluster substituted silicotungstate nano-cluster compound is crystallized in a triclinic system, and the space group is P-1. An electrochemical biosensor based on the high-nuclear cobalt cluster substituted silicotungstate nano-cluster compound is simple and convenient when being used for detecting dichlorvos, and has the advantages of high selectivity, good repeatability, good stability, low detection limitand the like.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Minoxidil-salicylate crystal form for treating alopecia and preparation method of minoxidil-salicylate crystal form
InactiveCN113501791AGood treatment effectOrganic chemistry methodsCarboxylic compound separation/purificationCrystal systemSalicylic acid
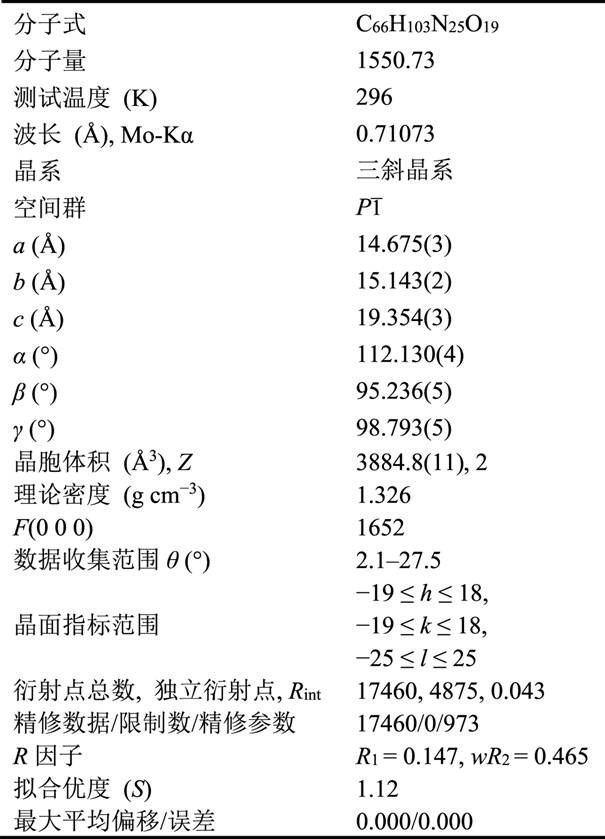
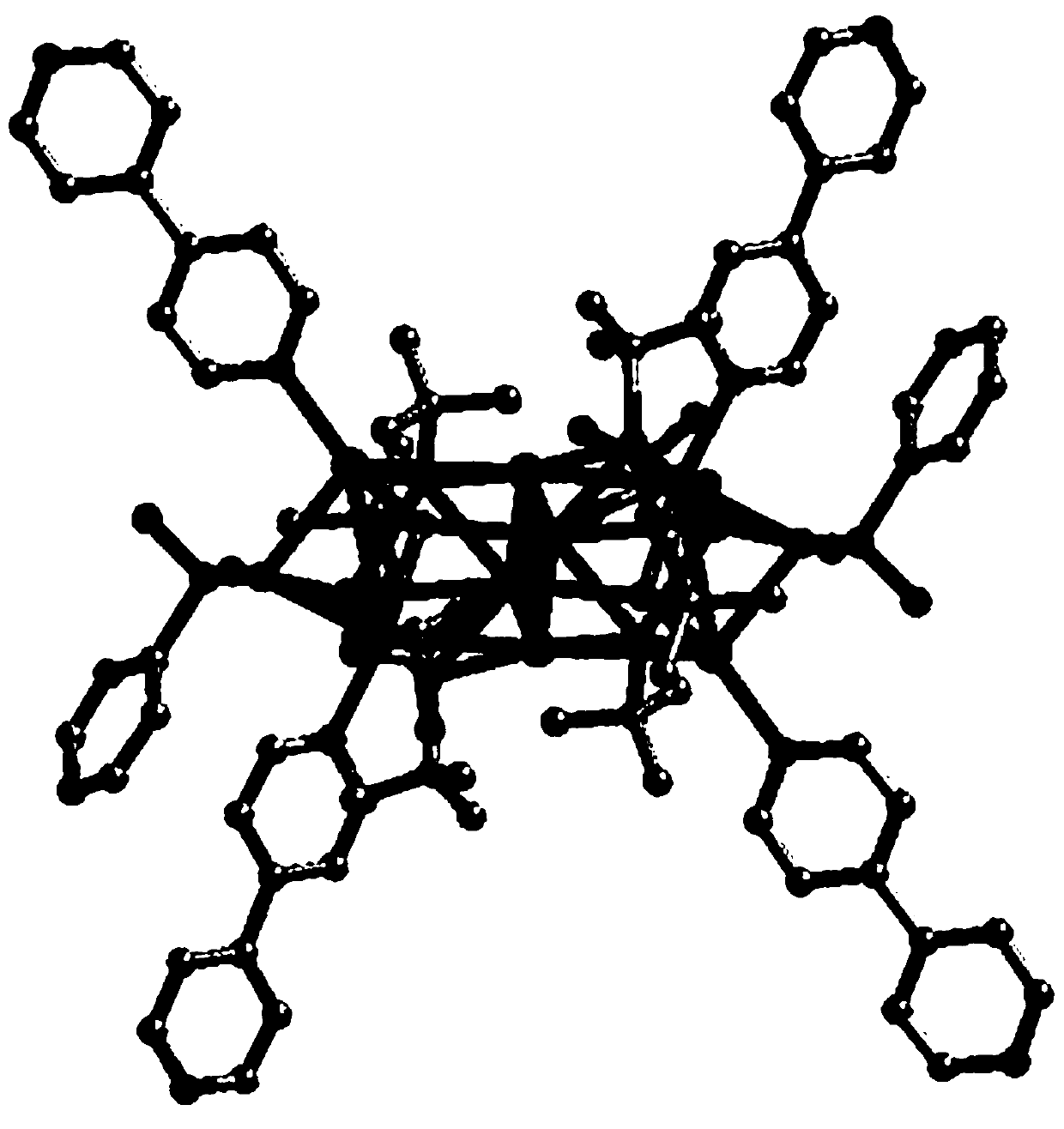
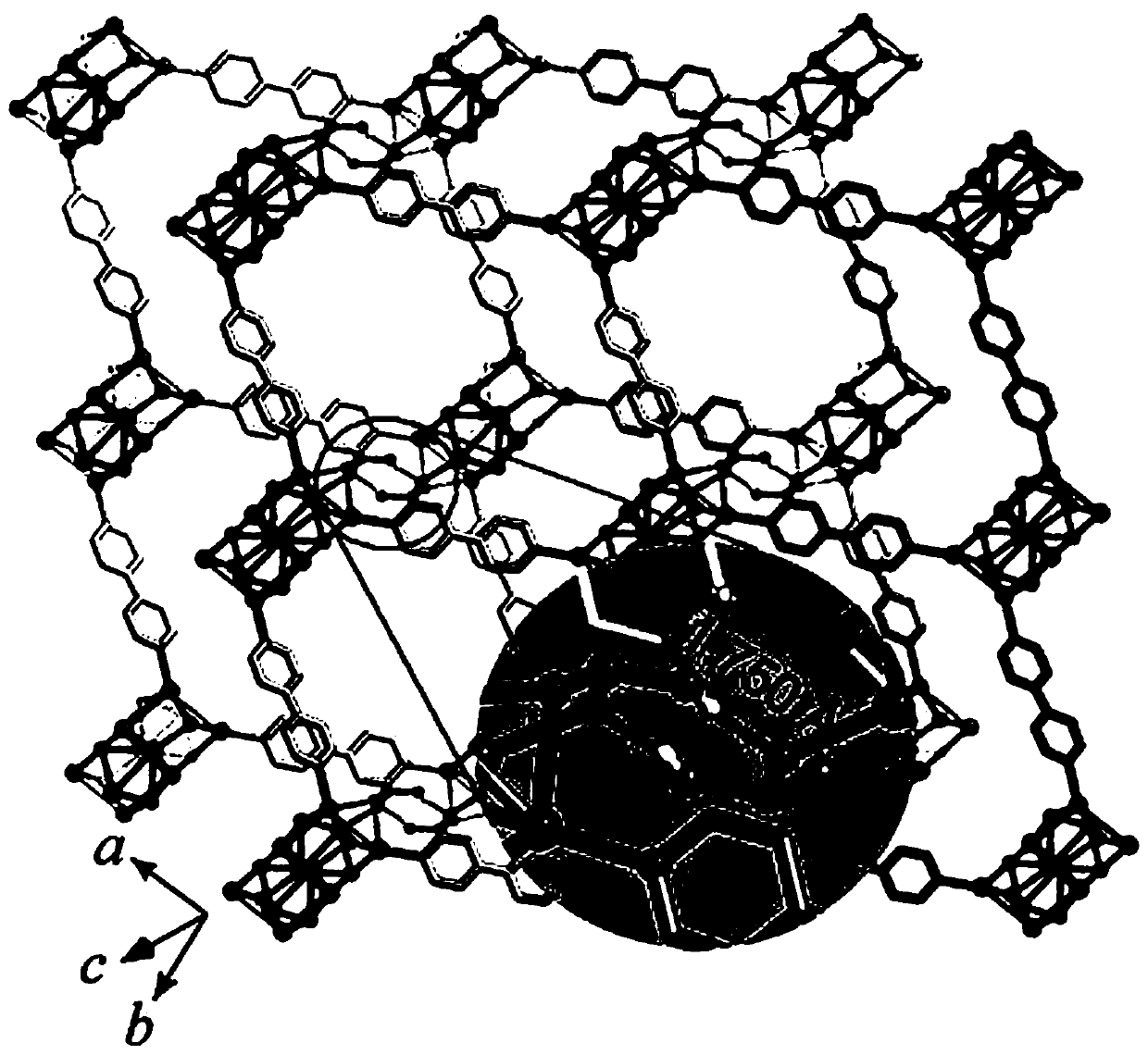
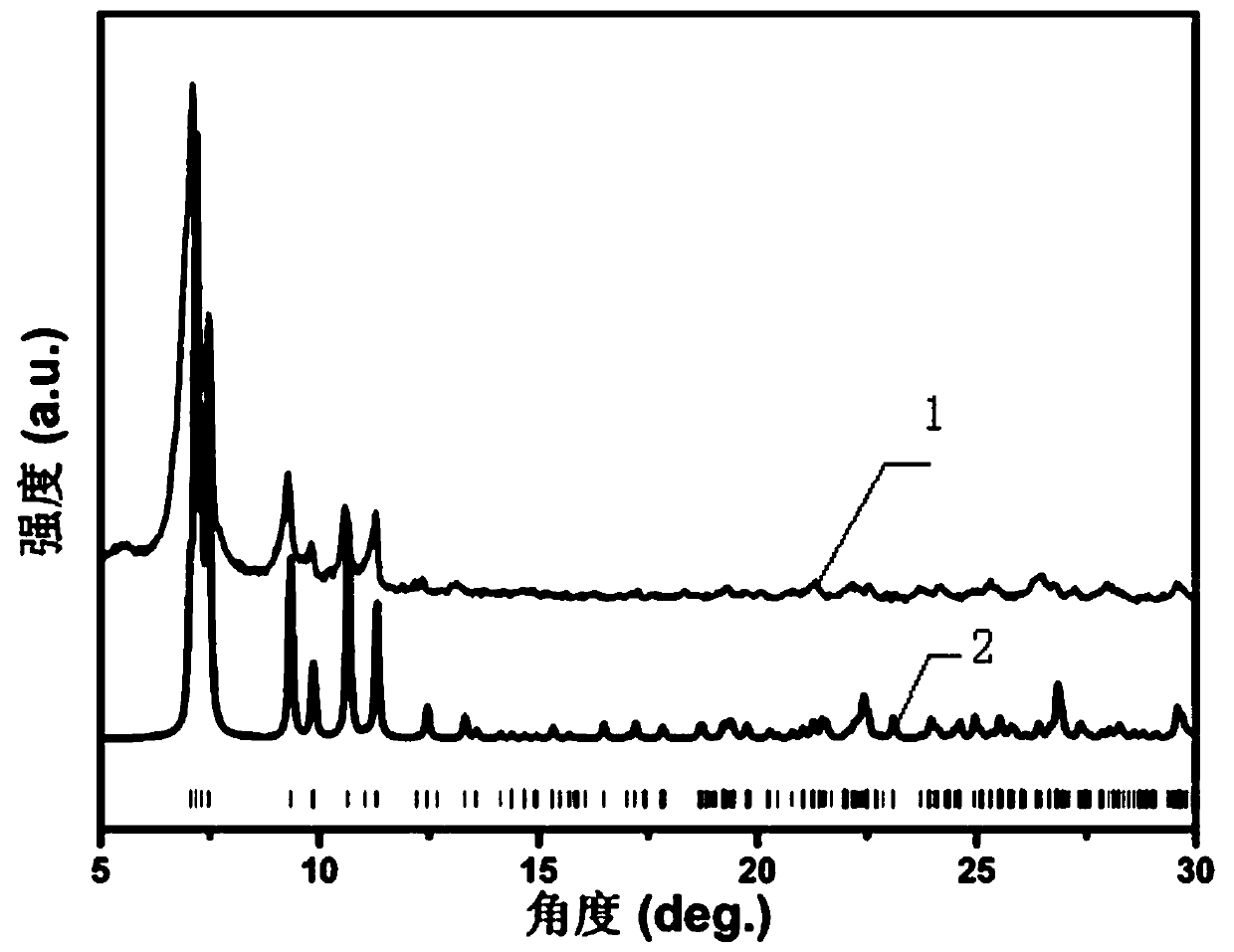
The invention relates to a minoxidil-salicylate crystal form for treating alopecia and a preparation method of the minoxidil-salicylate crystal form. The minoxidil-salicylate crystal form is formed by combining three minoxidil cations, two minoxidil molecules, two salicylic acid anions, one salicylic acid molecule and five water molecules through interaction of ionic bonds and hydrogen bonds. The crystal structure belongs to a triclinic system, the space group is P-1, the axial length a is equal to 14.175 to 15.175 angstroms, the axial length b is equal to 14.643 to 15.643 angstroms, the axial length c is equal to 18.854 to 19.854 angstroms, the axial angle alpha is equal to 111.630 to 112.630 degrees, the axial angle beta is equal to 94.736 to 95.736 degrees, and the axial angle gamma is equal to 98.293 to 99.293 degrees. An animal model proves that the treatment effect of the minoxidil crystal form prepared by the method is superior to that of an original medicine.
Owner:SHANDONG UNIV OF TECH
Fluorescence color-change material capable of directionally identifying dichloromethane, trichloromethane and tetrachloromethane and preparation method of fluorescence color-change material
ActiveCN108165256AImprove adsorption capacityObvious breathing effectMaterial analysis using wave/particle radiationFluorescence/phosphorescencePorositySpace group
Owner:HENAN POLYTECHNIC UNIV
Photoluminescent crystal material praseodymium potassium borate, and preparation method and application thereof
InactiveCN106480500ARaw materials are cheap and easy to getThe synthesis method is simplePolycrystalline material growthFrom melt solutionsSpace groupPhotoluminescence
The invention discloses a photoluminescent crystal material praseodymium potassium borate, and a preparation method and an application thereof, and belongs to the technical field of rare earth luminescent materials. A technical scheme comprises the key point that the photoluminescent crystal material praseodymium potassium borate has the chemical formula of K3Pr3(BO3)4, belongs to a triclinic crystal system, and has the space group of P-1; the unit cell parameters comprise a=9.0814 angstroms, b=10.8052 angstroms, c=14.0885 angstroms, alpha=69.800 DEG, beta=89.922 DEG, gamma=89.892 DEG, Z=4, and V=1297.4 angstrom<3>. The method comprises the steps: synthesizing praseodymium potassium metaborate monocrystals by a high temperature solution method; placing a fully and evenly ground mixed raw material in a platinum crucible, placing the crucible in a box-type oven, and carrying out heating synthesis; and keeping a constant temperature at the maximum temperature, and then slowly cooling down to room temperature to obtain monocrystals of the compound. The crystal material can be excited by 445 nm light to emit red fluorescence, can be used in fluorescent luminescent materials and devices, and meets the demand of electronic industry, public place display, home appliance display and the like.
Owner:HENAN POLYTECHNIC UNIV
Birefringent crystal for UV-visible band, powder and preparation methods thereof
ActiveCN110184642ASynthetic method is fastPolycrystalline material growthFrom solid stateSpace groupBirefringent crystal
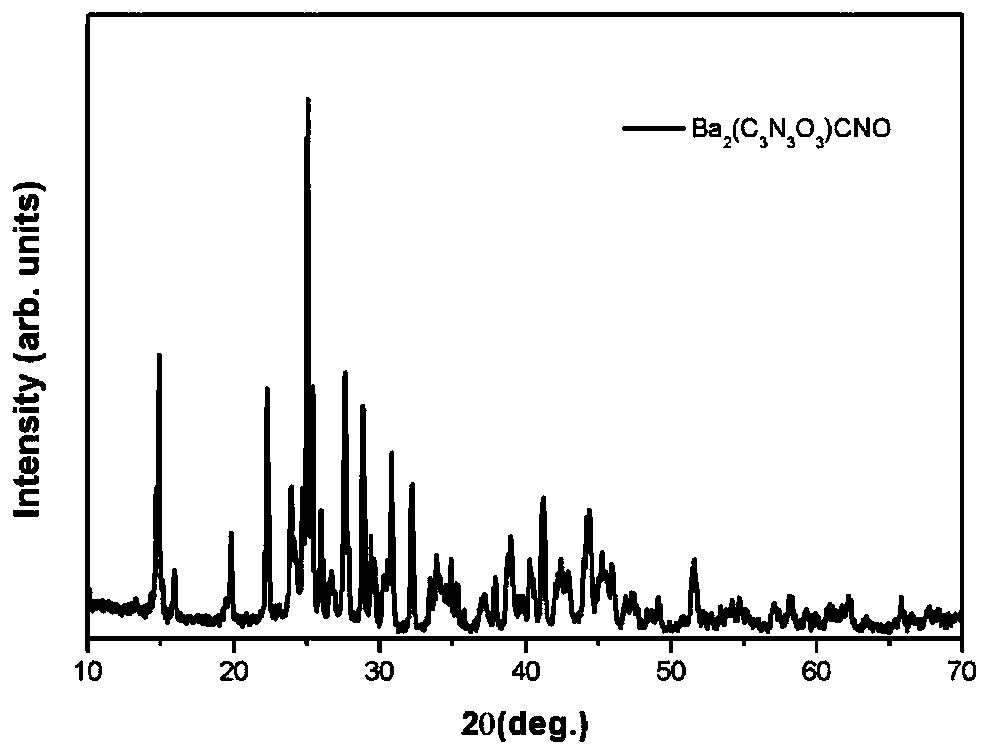
The invention discloses a birefringent crystal for a UV-visible band; the chemical formula of the crystal is Ba2(C3N3O3)CNO. The crystal belongs to a triclinic system, and the space group has the cellparameters that alpha is equal to 82.146 degrees, beta is equal to 75.303 degrees, gamma is equal to 60.689 degrees, and Z is equal to 2; the transmission range of the crystal is 240-2000 nm; the calculated value delta n of birefringence is equal to 0.36 at lambda being equal to 800 nm, and the value is twice that of a calcite CaCO3 crystal; the birefringent crystal can be used for fabricating photoelectric elements such as polarization polarizing prisms and polarization beam splitter prisms in the UV-visible band. The invention also provides preparation methods of the crystal and a pure powder phase.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS +1
Capacitor
InactiveCN104143442ALarge capacity per unit massLarge capacityElectrolytic capacitorsSpace groupPolymer chemistry
The invention provides a capacitor. The capacitor comprises a compound crystal shown in the formula I. A crystal compound belongs to the triclinic crystal system, according to a P-1 space group, a is equal to 1.09091, b is equal to 1.16257, c is equal to 1.94343, alpha is equal to 81.38 degrees, beta is equal to 84.47 degrees, gamma is equal to 65.29 degrees and V is equal to 2.2122.
Owner:CHENGDU JINGRONG ELECTRONICS
Mononuclear copper complex with fluorescence property and preparation method thereof
ActiveCN110330527AStrong fluorescenceImprove thermal stabilityGroup 5/15 element organic compoundsOrganic chemistry methodsFluorescenceStructural formula
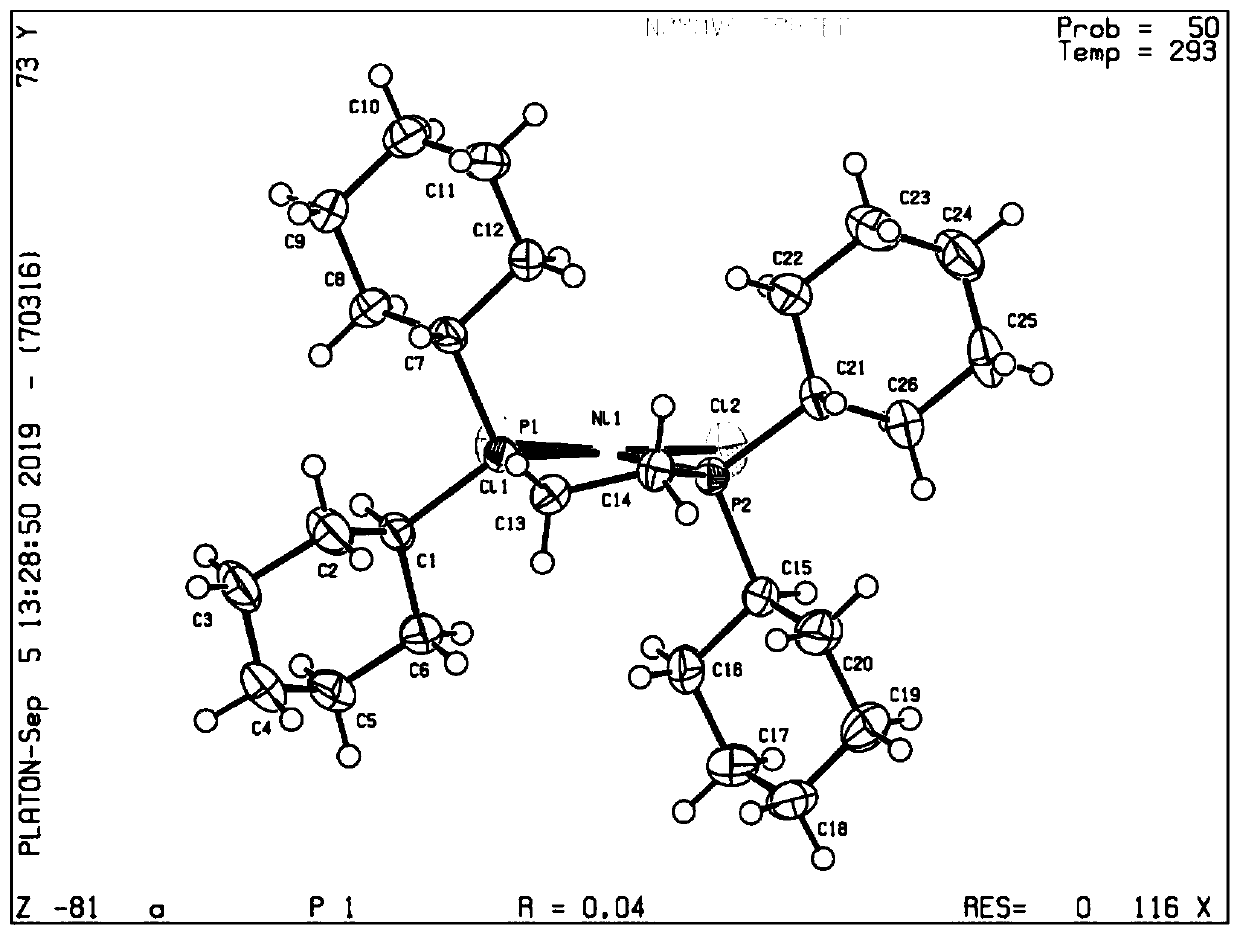
The invention discloses a mononuclear copper complex with fluorescence property and a preparation method thereof. The molecular formula of the complex is C63H51CuClN3O2P2, and the structural formula of the complex is shown in the specification. The crystal of the complex belongs to a triclinic system, and the cell parameters are as follows: alpha = 79.21 degrees, beta = 87.78 degrees and gamma = 86.12 degrees. Each complex molecule comprises a copper ion, a benzoimidazolylphosphine ligand and a triphenylphosphine auxiliary ligand. In the crystal, Cu ions in the center of the complex are respectively coordinated with Cl ions, N and P atoms in benzimidazolylphosphine ligands and P atoms in the auxiliary ligand triphenylphosphine to form a tetra-coordinated twisted tetrahedral configuration.The invention also provides a preparation method of the complex. The method is mild in reaction condition, simple in preparation process and low in cost. The copper complex has good thermal stabilitywhile having fluorescence property.
Owner:LUOYANG INST OF SCI & TECH
Ruthenium complex of semi-sandwich structure and preparation method thereof
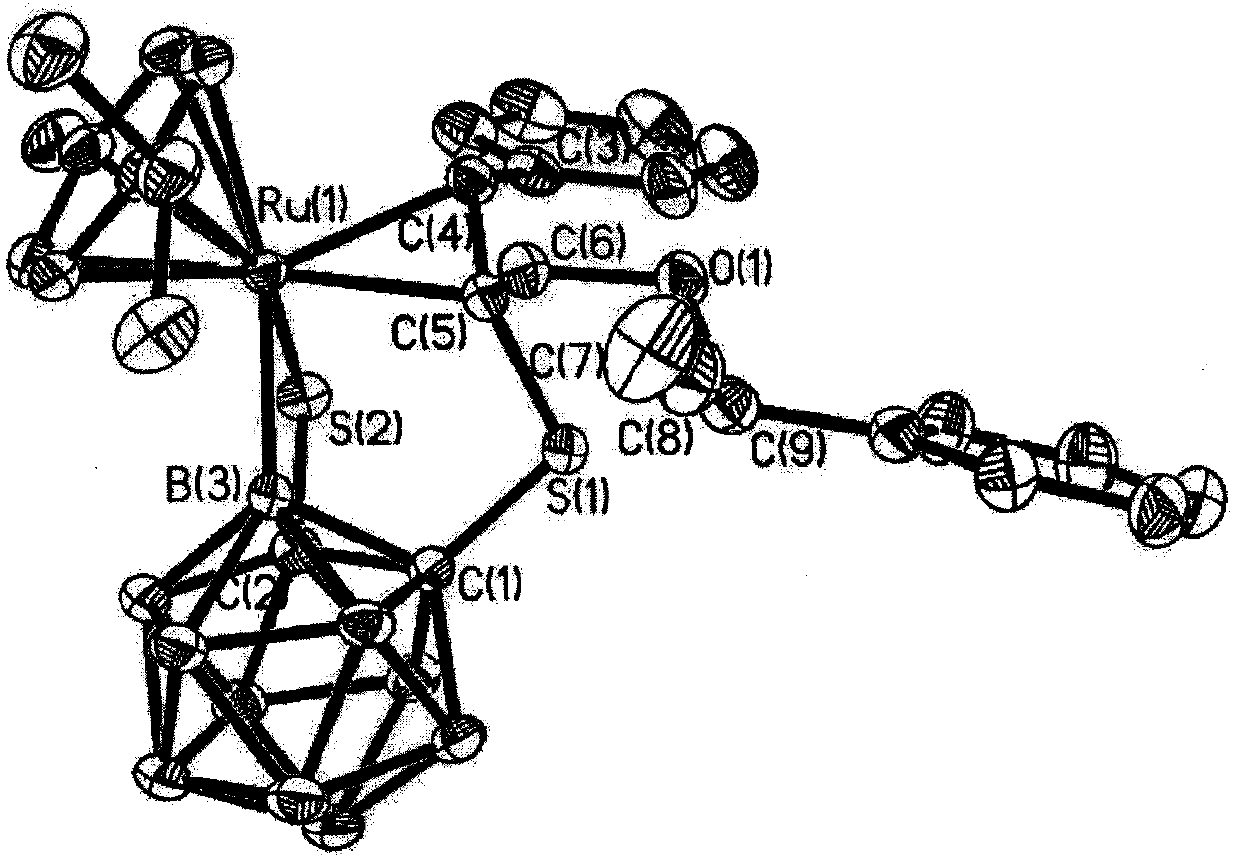
InactiveCN107814822ALow costMild reaction conditionsRuthenium organic compoundsOrganic chemistry methodsChromatographic separationSolubility
The invention relates to a ruthenium complex crystal of a semi-sandwich structure and a preparation method thereof and belongs to the technical field of organometallic chemistry. The structure of thecomplex is represented and confirmed by virtue of methods such as single crystal X-ray diffraction and nuclear magnetic resonance, the crystal belongs to a triclinic crystal system, a space group is P-1, a molecular formula is C3OH38B10ORuS2, the molecular weight is 687.89, and cell parameters are described in the specification. 1,2-dicarbon-based-closed-dodecocarborane, n-butyl lithium, sulfur, dichloro(p-methyl isopropyl phenyl) ruthenium (II) dimer and 1-phenyl-2-propyne-1-alcohol are used as raw materials, and column chromatographic separation is adopted, so that the product is obtained. The complex crystal has relatively good chemical stability and solubility, and the preparation method has the advantages of simplicity and high yield, so that the ruthenium complex crystal provided bythe invention has potential drug application value.
Owner:SHANGRAO NORMAL UNIV
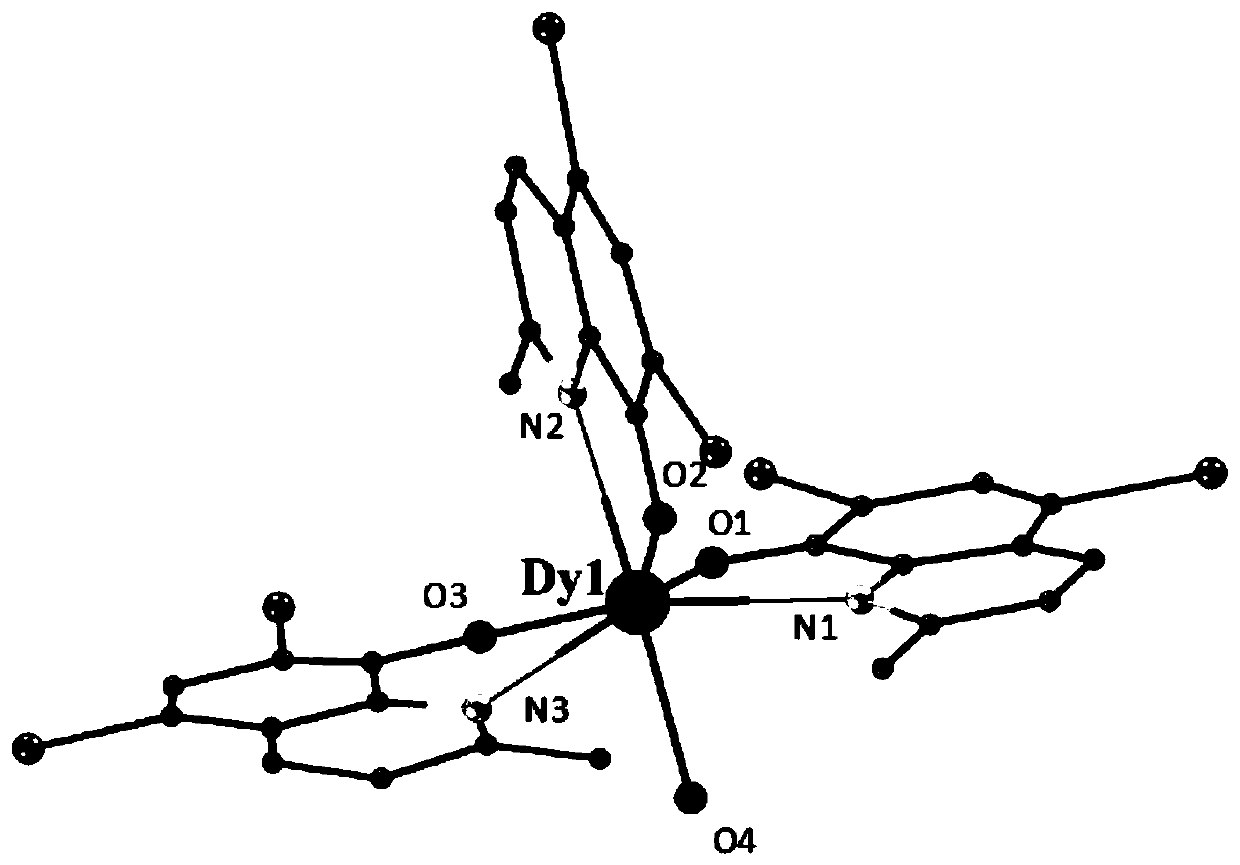
Mononuclear dysprosium complex based on 2-methyl-5,7-dichloro-8-hydroxyquinoline as a ligand and its preparation method and application
ActiveCN107089944BEasy to prepareLow costOrganic chemistry methodsOrganic/organic-metallic materials magnetismSpace groupHydrogen atom
The invention discloses a mononuclear dysprosium complex based on 2-methyl-5,7-dichloro-8-hydroxyquinoline as a ligand and a preparation method and application thereof. The chemical formula of the complex is [Dy (L) 3 (H2O)], wherein L refers to that a hydroxyl hydrogen atom of 2-methyl-5,7-dichloro-8-hydroxyquinoline is removed and 2-methyl-5,7-dichloro-8-hydroxyquinoline carries a negative charge; the complex belongs to a triclinic crystal system and a P-1 space group. The preparation method of the complex comprises the steps that Dy(NO3) 3.6H2O and 2-methyl-5,7-dichloro-8-hydroxyquinoline are obtained and dissolved with water, an obtained solution is adjusted to make the pH equal to 6.5-7.8, mixture liquid reacts under the heating condition, and the complex is obtained. The complex is simple in preparation method, low in cost and good in repeatability, a field-induced slow-relaxation magnetic behavior is presented at low temperature, and the complex can be used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
Tunable laser crystal chrome-doped sodium magnesium molybdate and preparation method thereof
InactiveCN103898609AModerate mechanical propertiesWide tunable laser bandPolycrystalline material growthFrom melt solutionsSpace groupMolybdate
The invention provides tunable laser crystal chrome-doped sodium magnesium molybdate and a preparation method thereof. A molten salt top seed crystal method is adopted, 55-80at.% of Na2Mo2O7 is used as a melting assistant, and the growth parameters are as follows: the cooling velocity is 0.5-3 DEG C / day, the growth temperature is 800-880 DEG C, the rotation speed is 5-30rpm / minute, and a Cr<3+>:Na2Mg5(MoO4)6 crystal which is relatively good in quality and relatively large in size is grown, wherein the doping concentration of Cr<3+> is 0.2-5at.%. The crystal belongs to a triclinic system, and has a P-1 space group structure as shown in the specification. The crystal has the characteristics that the mechanical property is moderate, the tunable laser wave band is wide, the emission cross section is large, and the like, and is a relatively good tunable laser crystal material.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Dihydropyridine calcium antagonist co-crystal and preparation method and application thereof
InactiveCN110372575AImprove solubilityImprove stabilityOrganic chemistry methodsHydroxy compound separation/purificationSolubilitySpace group
The invention relates to a dihydropyridine calcium antagonist co-crystal and a preparation method and application thereof. The molecular formula of the co-crystal is (C20H25ClN2O5).(C6H5COOH).(Y)n, wherein Y is any one of water molecule, ethanol molecule or isopropanol molecule, n is greater than or equal to 0 and less than or equal to 3, the co-crystal is crystallized in a triclinic crystal system and a P1 chiral space group, the unit cell dimensions are that alpha=97.1-97.5 degrees, beta=92.2-92.6 degrees, and gamma=111.5-112.1 degrees. According to the dihydropyridine calcium antagonist co-crystal and the preparation method and application thereof, the solubility and stability of an existing levamlodipine co-crystal crystal form are improved, and potentially, improvement of tablet stability of antihypertensive drugs and improvement of bioavailability in large-scale tablet production are facilitated.
Owner:FUDAN UNIV +1
14-core gold phosphine sulfur cluster compound as well as preparation method and application thereof
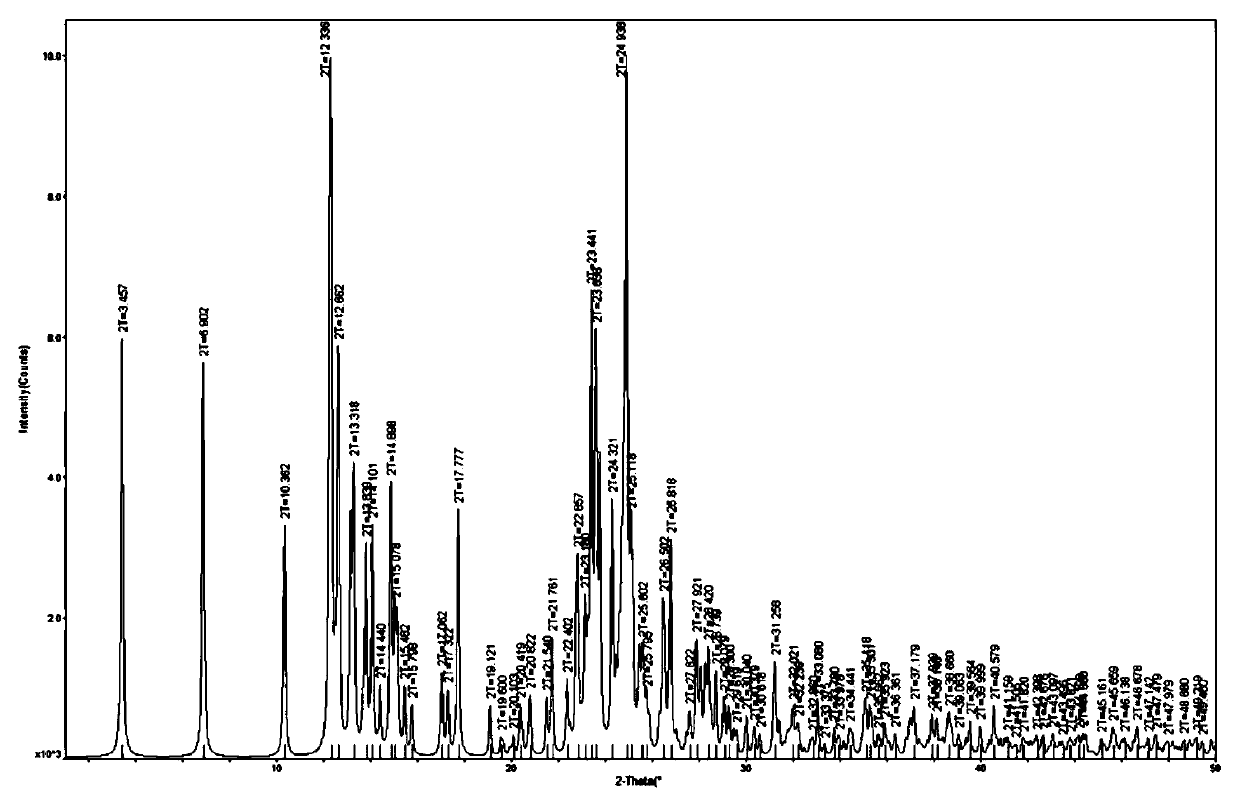
ActiveCN109134517AGood fluorescence propertiesLow cytotoxicityGroup 1/11 organic compounds without C-metal linkagesOrganic chemistry methodsQuantum yieldSpace group
The invention discloses a double-core gold phosphine sulfur cluster compound and a 14-core gold phosphine sulfur cluster compound. The cluster compound is a bulky crystal, the molecular formula of which is C155H136Au14Cl2N10P10S6 and the chemical formula of which is [Au14S6(bdppmapy)5]Cl2, wherein bdppmapy represents N,N-di(diphenylphosphine methyl)-2-aminopyridine; the 14-core gold phosphine sulfur cluster compound is a triclinic system, the space group of which is Pi and the cell parameters of which are shown in a formula, wherein alpha is equal to 87.453(3) degrees, beta is equal to 76.042(2) degrees and gamma is equal to 75.797(2) degrees. The invention further discloses an application of the 14-core gold phosphine sulfur cluster compound as a lysosome targeted probe. The 14-core goldphosphine sulfur cluster compound has a relatively good fluorescent property, a relatively high quantum yield and relatively low cytotoxicity.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com