Structure, preparation method and use of diethylenetriamine-based cantharidimide dimer derivative
A technology of cantharidin derivatives and crystals, which is applied in the field of unsaturated demethylcantharimid dimer structure, and can solve problems such as difficult crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 0.33g of unsaturated demethylcantharidin in 30ml of acetonitrile, add 0.10g of diethylenetriamine at a material ratio of 2:1, stir at room temperature for 20h, and obtain a white powdery precipitate. After the solvent evaporates to about 10ml remaining, After filtration, the resulting precipitate was recrystallized with methanol to obtain colorless rod-shaped crystals, which was the target product.
Embodiment 2
[0029] Dissolve 0.33g of unsaturated demethylcantharimid in 30ml of acetonitrile, add 0.23g of bis(2-bromoethyl)amine at a ratio of 2:1, stir at room temperature for 20h, and obtain a white powdery precipitate. The solvent was evaporated to about 10ml remaining, filtered, and the obtained precipitate was recrystallized with methanol to obtain colorless rod-shaped crystals, which was the target product.
[0030] Description of drawings.
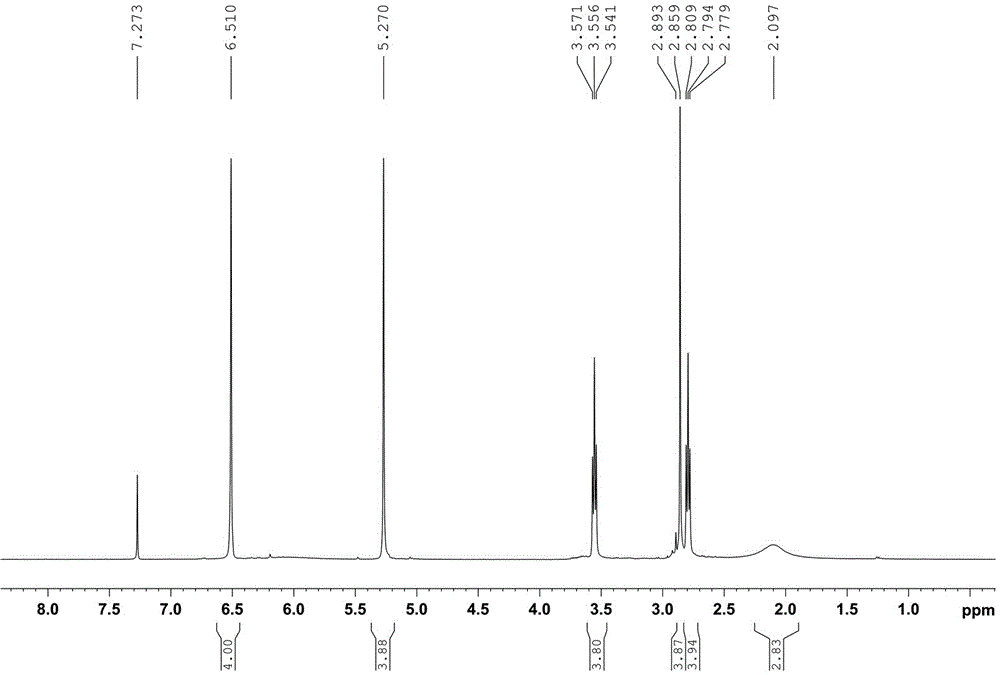
[0031] attached figure 1 is 2,2'-(3-azopentane-1,5-diyl)bis(3a,4,7,7a-tetrahydro-4,7-epoxy-1,3-dihydroisoindole -1,3-diketone) of 1 HNMR spectrum.
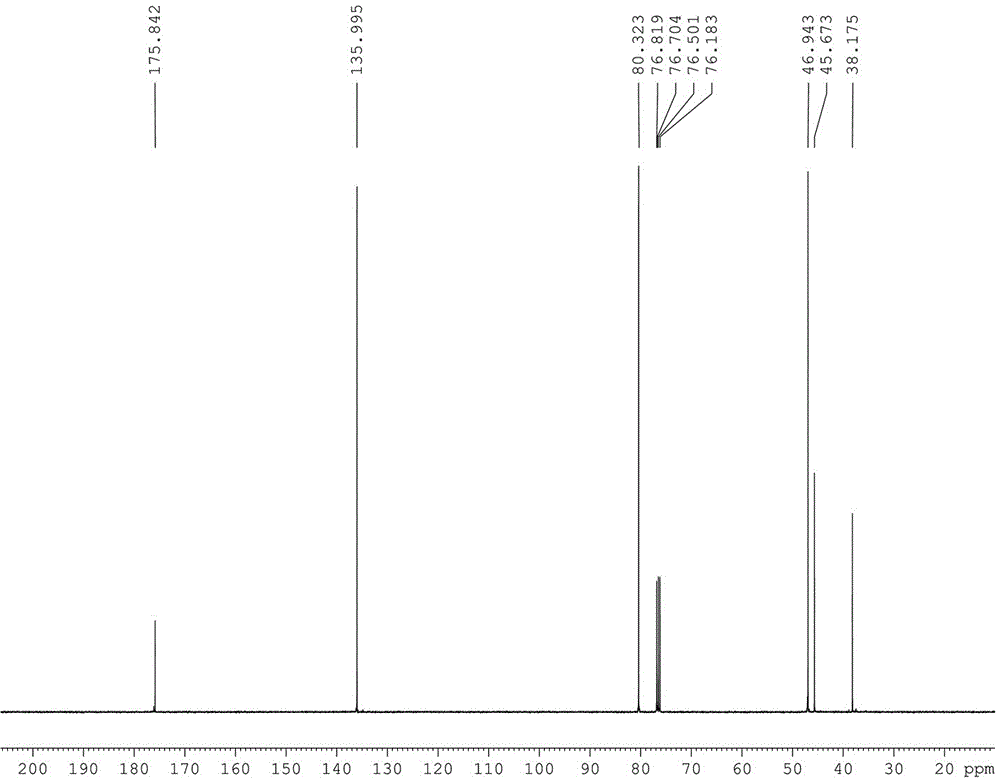
[0032] attached figure 2 is 2,2'-(3-azopentane-1,5-diyl)bis(3a,4,7,7a-tetrahydro-4,7-epoxy-1,3-dihydroisoindole -1,3-diketone) of 13 CNMR spectrum.
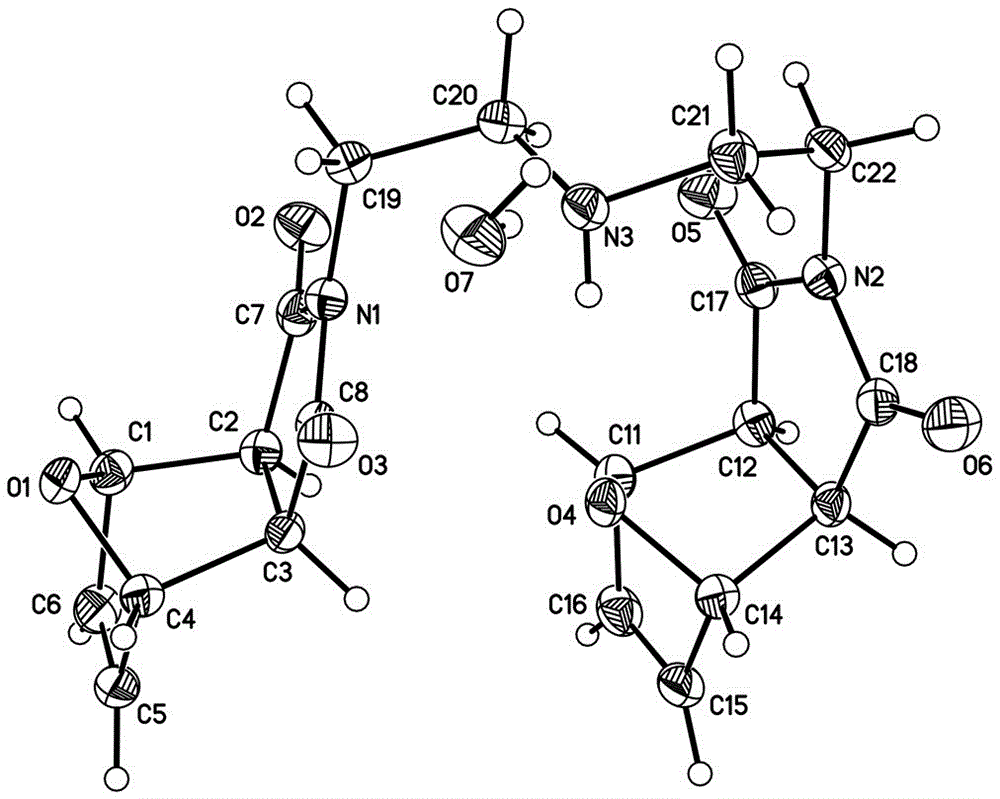
[0033] attached image 3 is 2,2'-(3-azopentane-1,5-diyl)bis(3a,4,7,7a-tetrahydro-4,7-epoxy-1,3-dihydroisoindole -1,3-dione) crystal structure, ellipsoidal probability 30%.
[0034] attached Figure 4 is 2,2'-(3-azopentane-1,5-diyl)bis(3a,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com