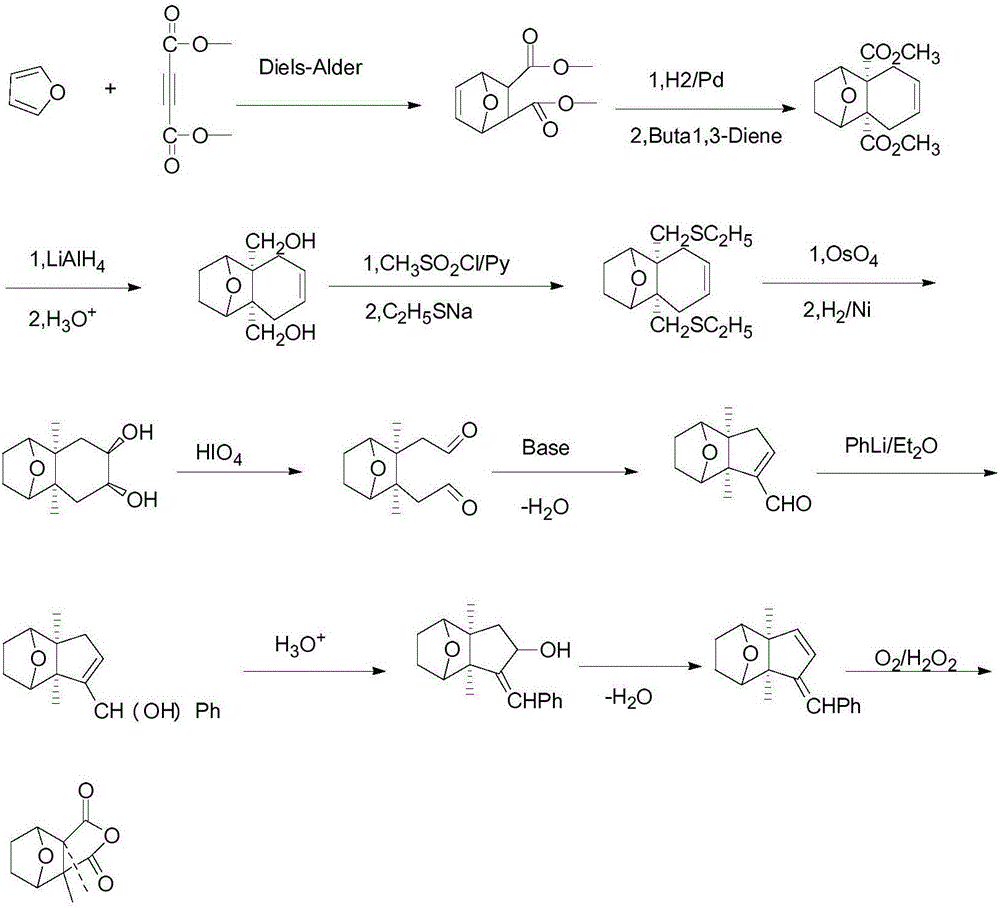
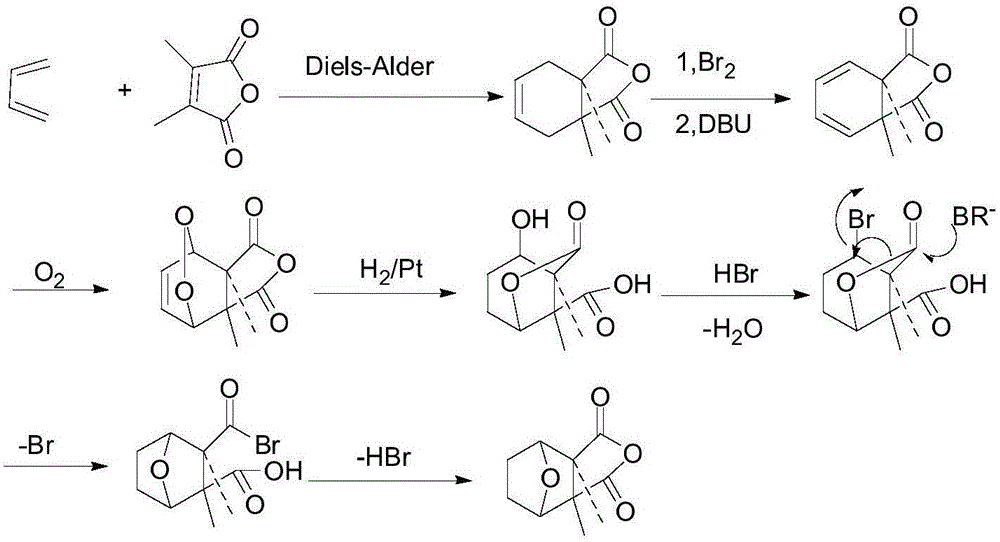
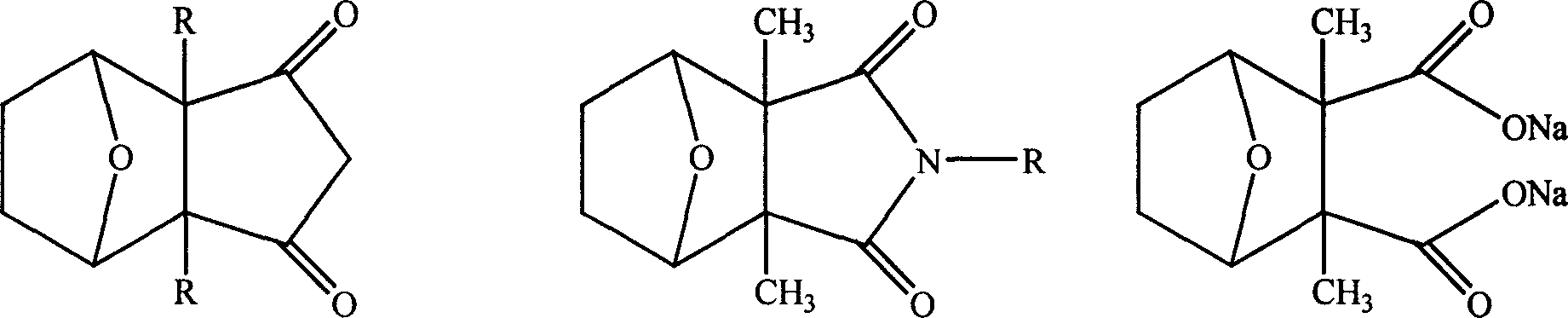
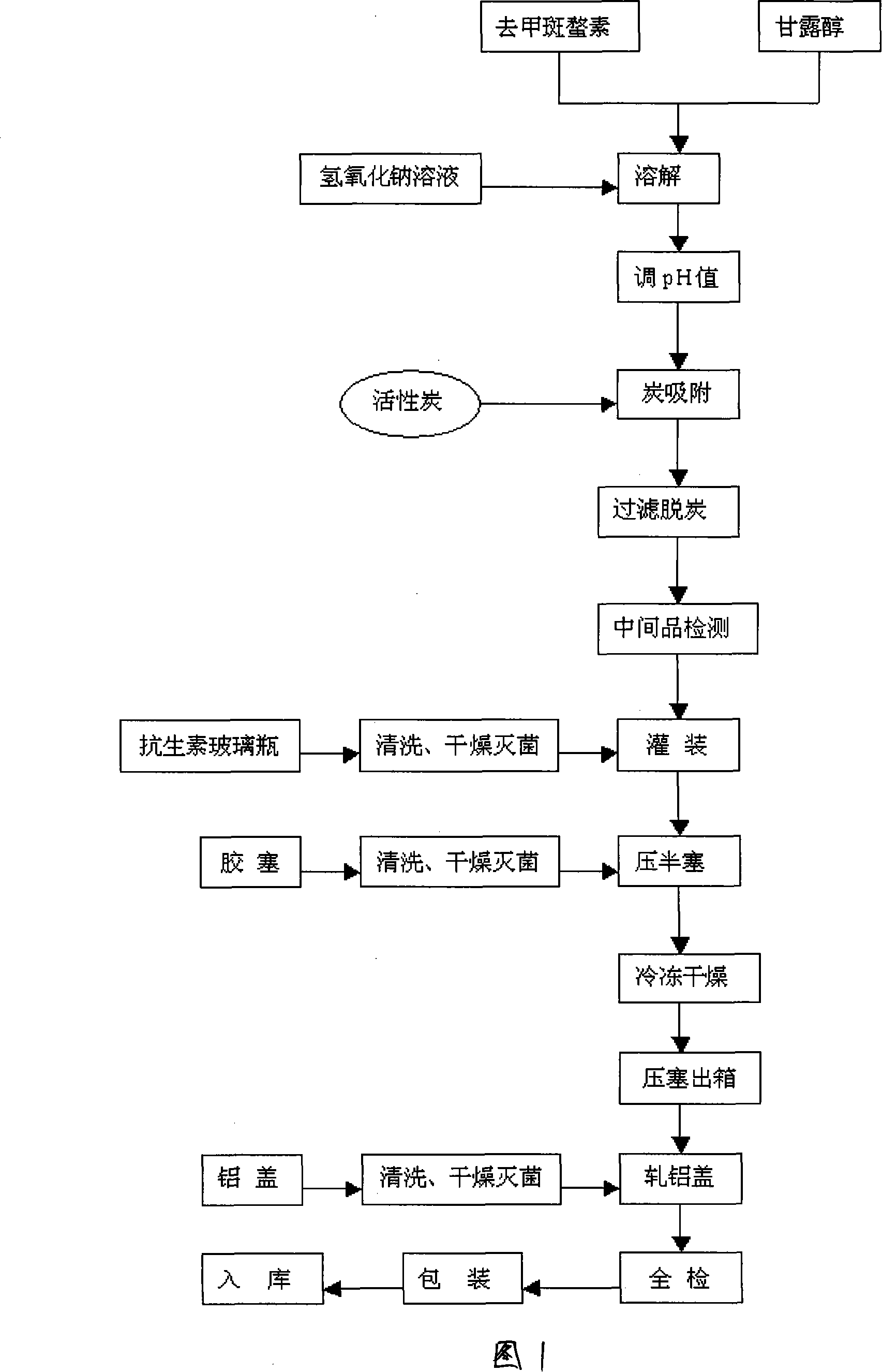
Patents
Literature
172 results about "Cantharidin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
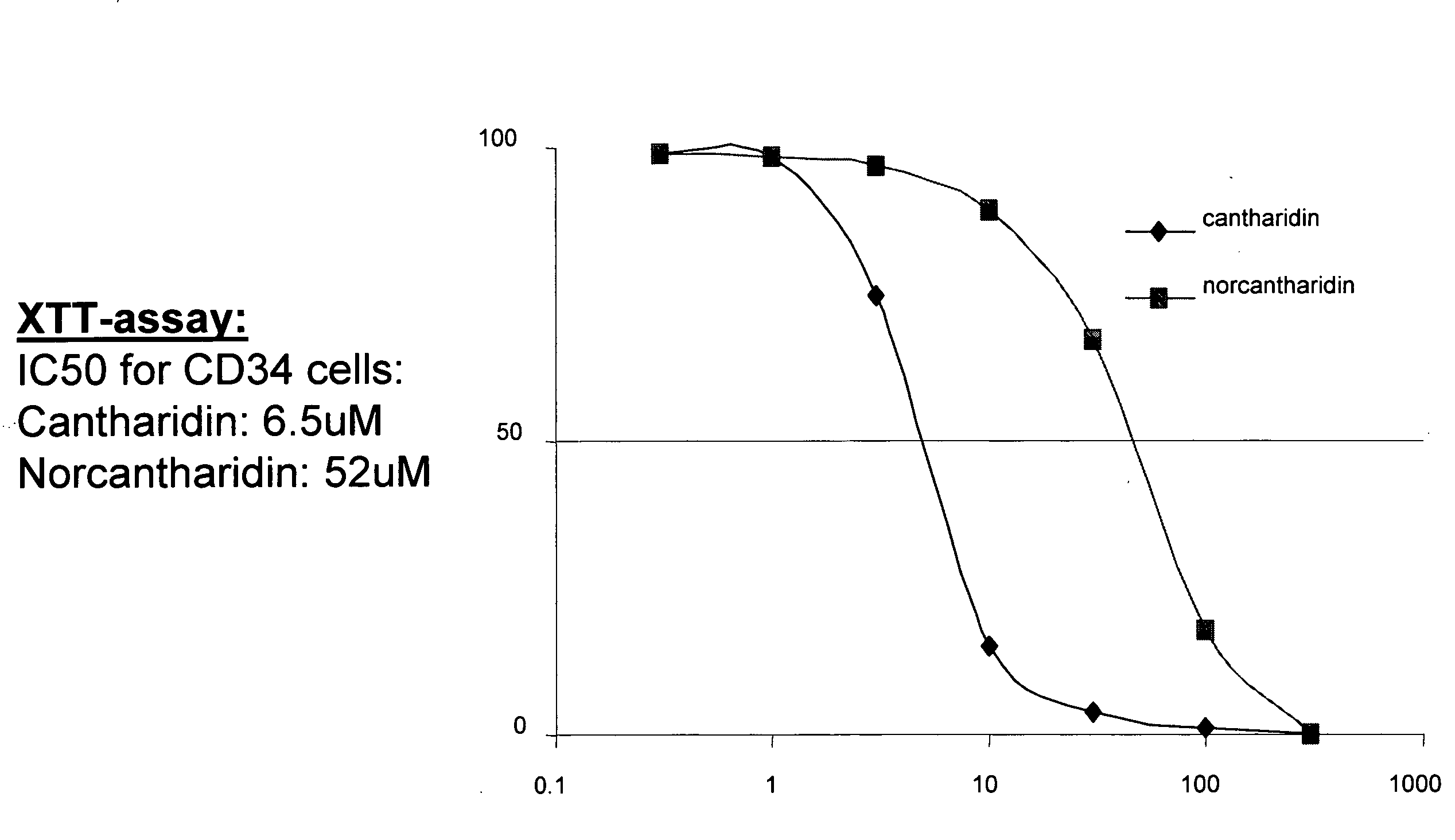
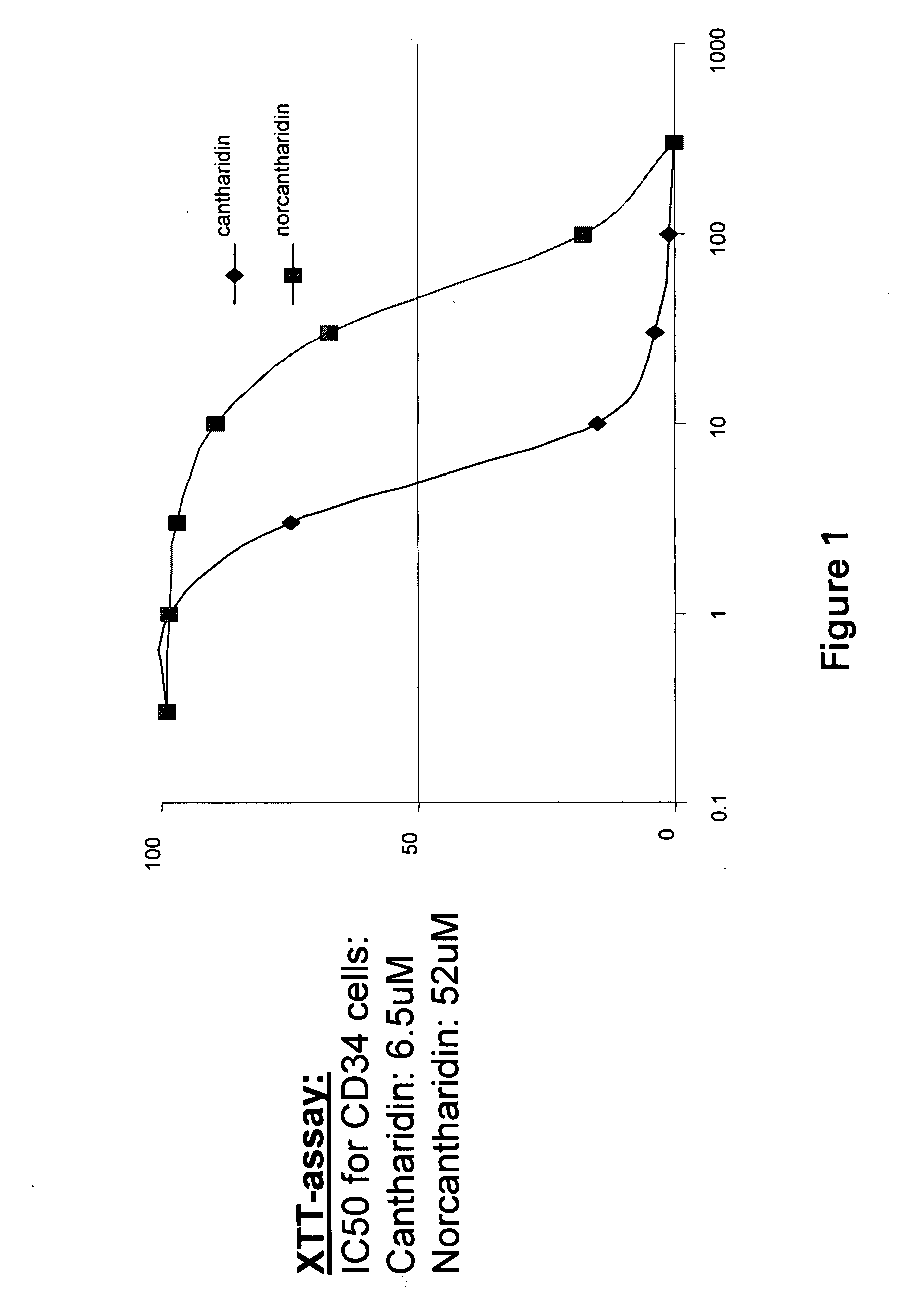
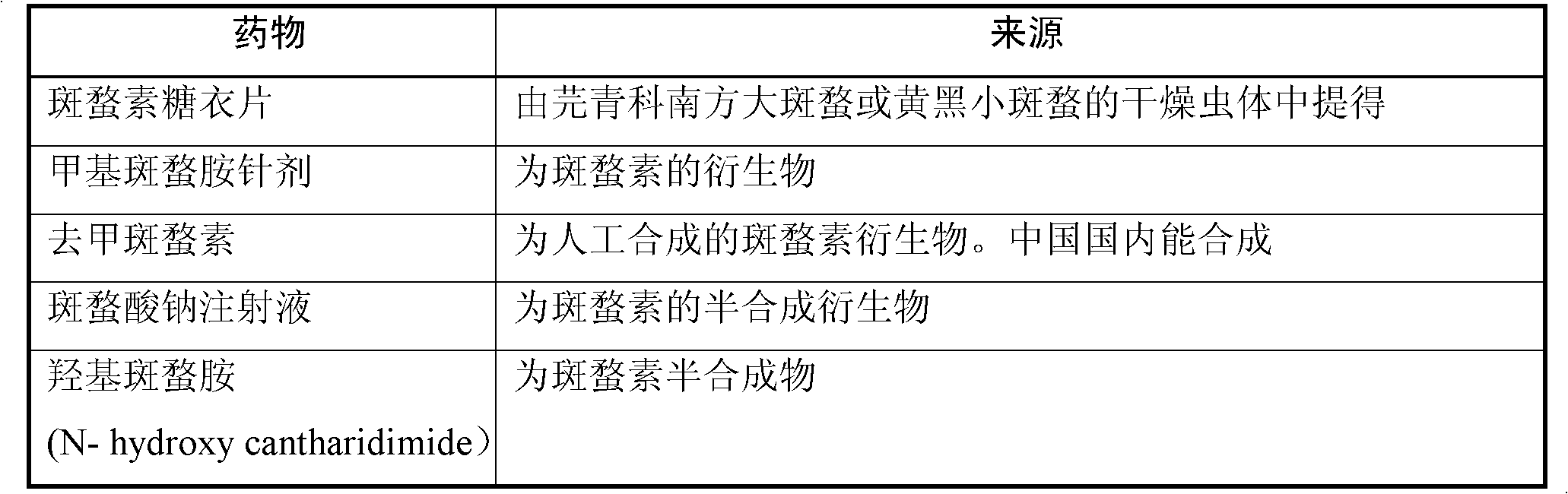
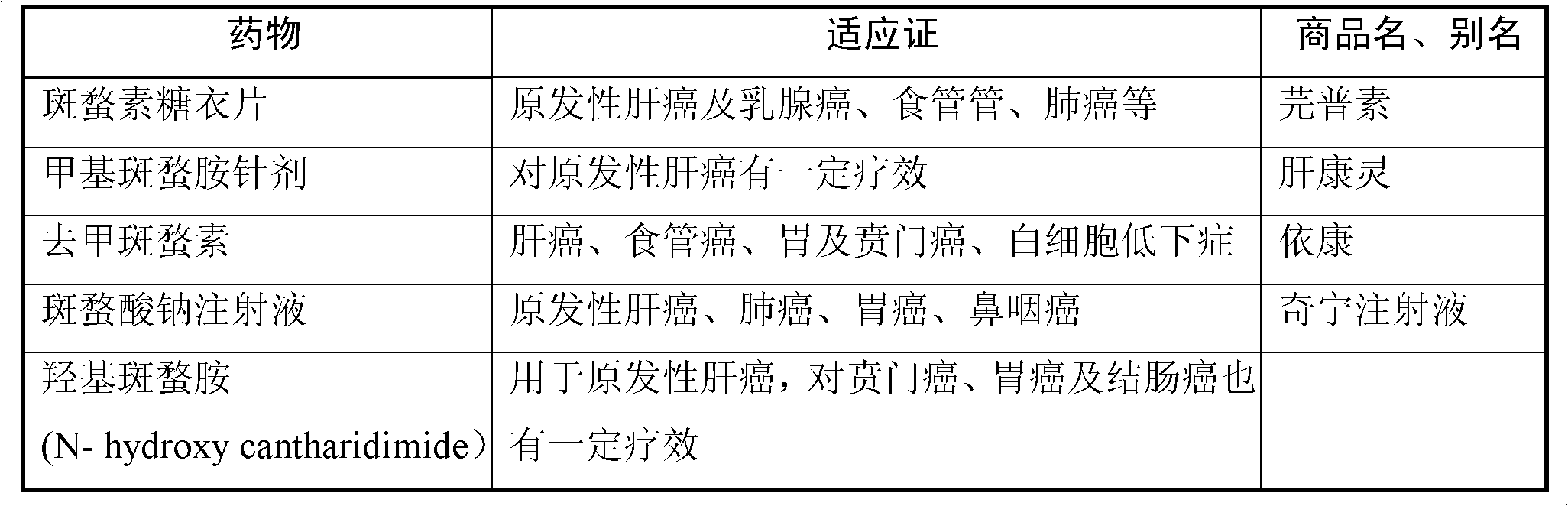
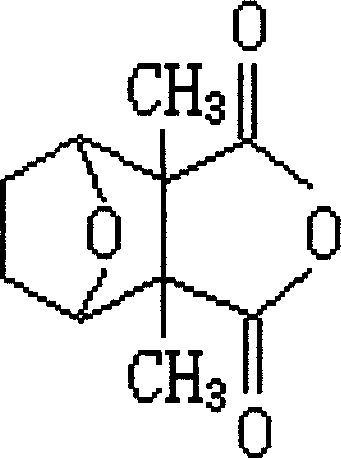
Cantharidin is an odorless, colorless fatty substance of the terpenoid class, which is secreted by many species of blister beetles. It is a burn agent or a poison in large doses, but preparations containing it were historically used as aphrodisiacs. In its natural form, cantharidin is secreted by the male blister beetle and given to the female as a copulatory gift during mating. Afterwards, the female beetle covers her eggs with it as a defense against predators.
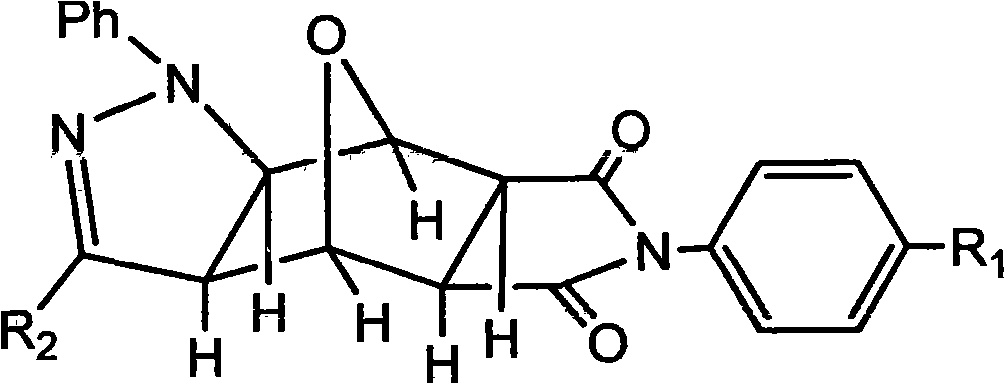
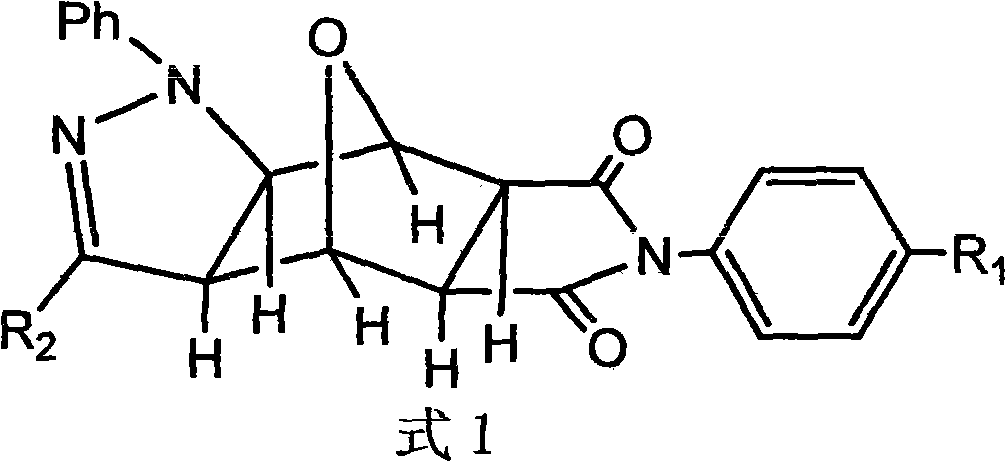
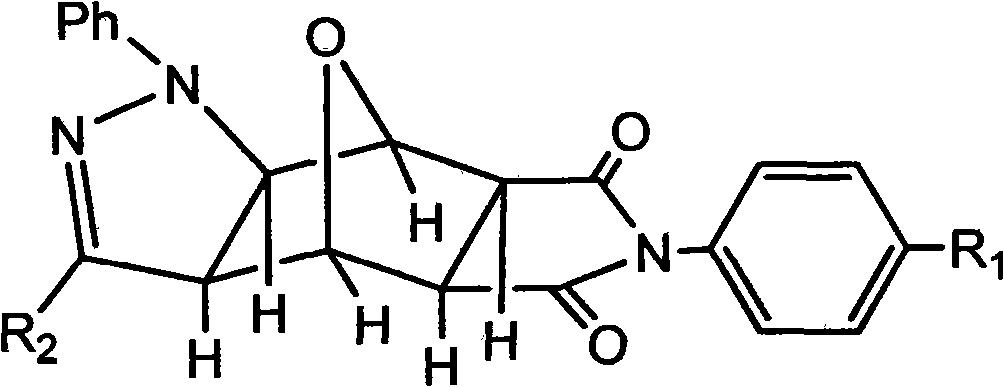
Pyrazolo N-substituted dehydronorcantharidin imide derivative as well as synthesis method, activity test method and application thereof
InactiveCN101812066AGood antitumor activityOrganic active ingredientsOrganic chemistryImideQuinoxaline
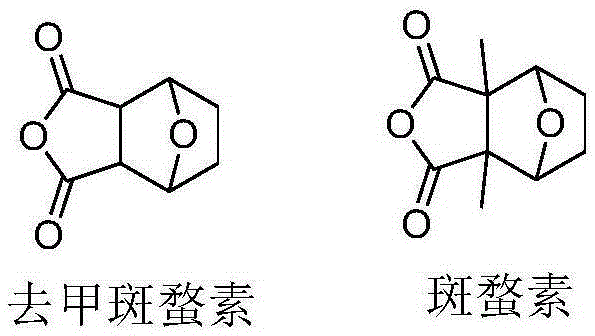
The invention discloses a pyrazolo N-substituted dehydronorcantharidin imide derivative as well as a synthesis method, an activity test method and application thereof, belonging to the field of cantharidin derivatives. The pyrazolo N-substituted dehydronorcantharidin imide derivative has a structural general formula shown as a formula 1: in the formula 1, R1 is H, C1, F, CH3, OCH3, OH or NO2; and R2 is 2-phenyl-2H-1,2,3-triazole-4-substituent or quinoxaline-2-substituent. The novel N-substituted dehydronorcantharidin imide derivative introduces five-membered heterocyclic pyrazole rings into norcantharidin substituted arylamine and has favorable anti-tumor activity.
Owner:SHAOXING UNIVERSITY
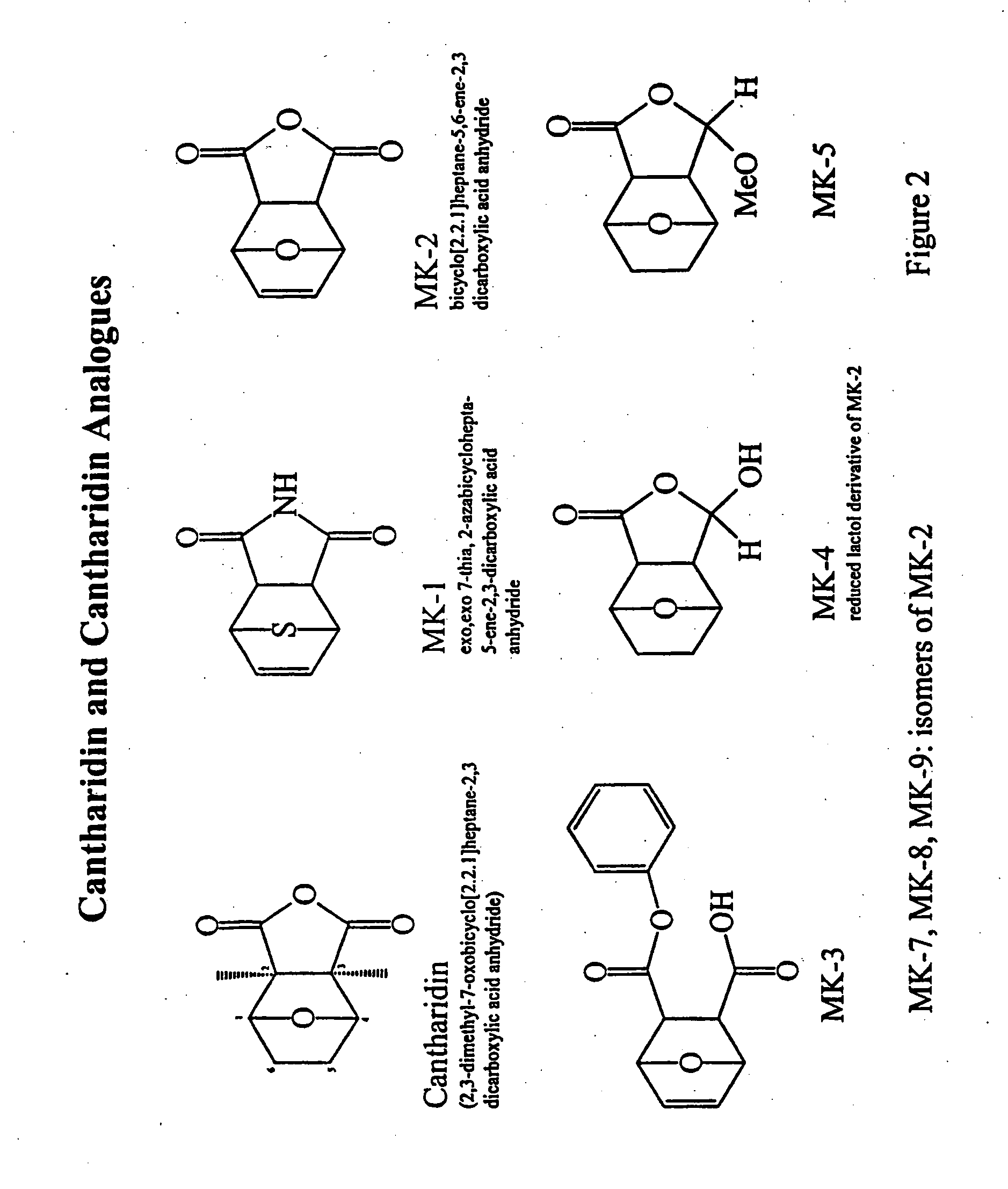
Anhydride modified cantharidin analogues useful in the treatment of cancer
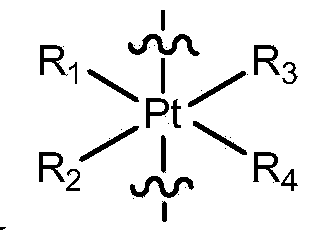
Anhydride modified cantharidin analogues useful in the treatment of certain forms of cancer also methods for the screening for anti-cancer activity of these analogues and / or their ability to sensitise cancer cells to cancer treatment. The modified cantharidin analogues have structure (I) or (II), wherein R1, R2, R3 and R4 are H, aryl or alkyl; X is O, N or S; Y is O, S, NH, NR; R is alkyl or aryl; A and B are H or CH3; W and Z are CHOH or C═O. These compounds inhibit protein phosphatase.
Owner:MCCLUSKEY ADAM +3
Method for extracting cantharidin
InactiveCN1966508AShorten the production cycleLess impuritiesOrganic chemistryRoom temperatureCantharidin
The invention discloses a method for extracting cantharidin, including extraction and recrystallization process. The said extraction process includes: (1) cold maceration process: drying and meshing mylabris worms into 40-60 meshes, adding maceration solvents and stirring for 5-10 h, and vacuum-filtering at a mylabris worms / hydrochloric acid / acetone weight ratio of 1:0.01-0.05:3-9; (2) concentration and standing process: concentrating and distilling the filtrate at 40-60degreeC until no liquid flows out, cooling to room temperature, and placing for 5-9 hours; and (3) washing process: vacuum-filtering the concentrated liquid, and washing with the mixed solution of ethanol and petroleum ether for several times until the liquid clears up. Compared with the existing technology, this invention has the advantages of simplified process, short production cycles, simple operation, and high yield (increase by 50% compared with the existing technology).
Owner:芜湖天远生物科技有限公司
Apparatus and method of wart treatment
InactiveUS20070111954A1Prevents the wart-removing composition from drying outCross-contamination between patients is preventedBiocideSalicyclic acid active ingredientsMollusca contagiosumMedicine
A single-use, delivery vehicle for the treatment of warts and molluscum contagiosum, consisting of a solution of cantharidin, salicylic acid, and podophyllin packaged in a crushable ampoule with a soft, absorbent, applicator tip. A method of delivering wart-removing and molluscum removing compositions to the skin, consisting of the steps of crushing an ampoule containing the wart-removing composition and pressing the absorbent tip against the wart or molluscum.
Owner:CUTICEUTICALS
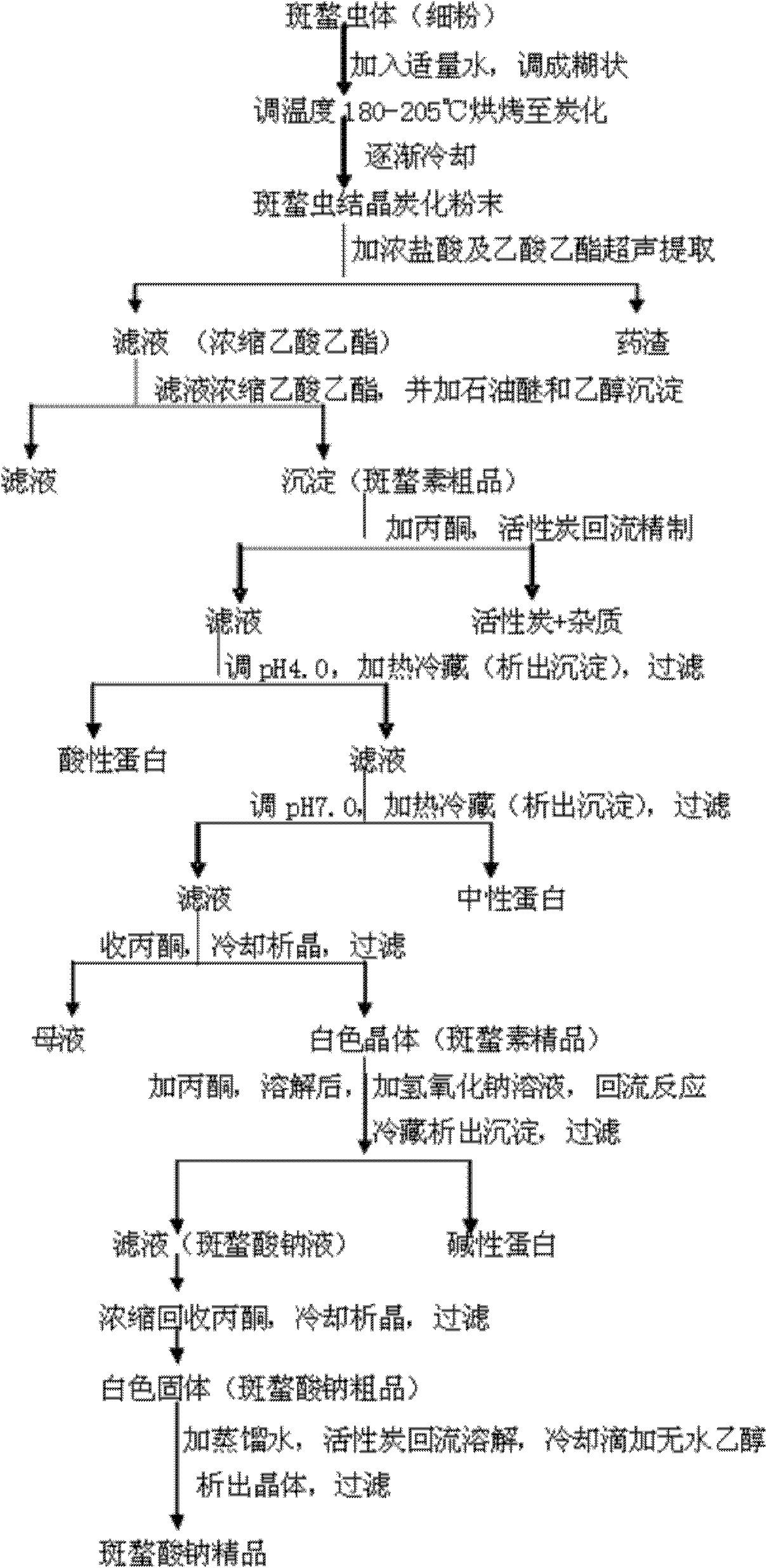
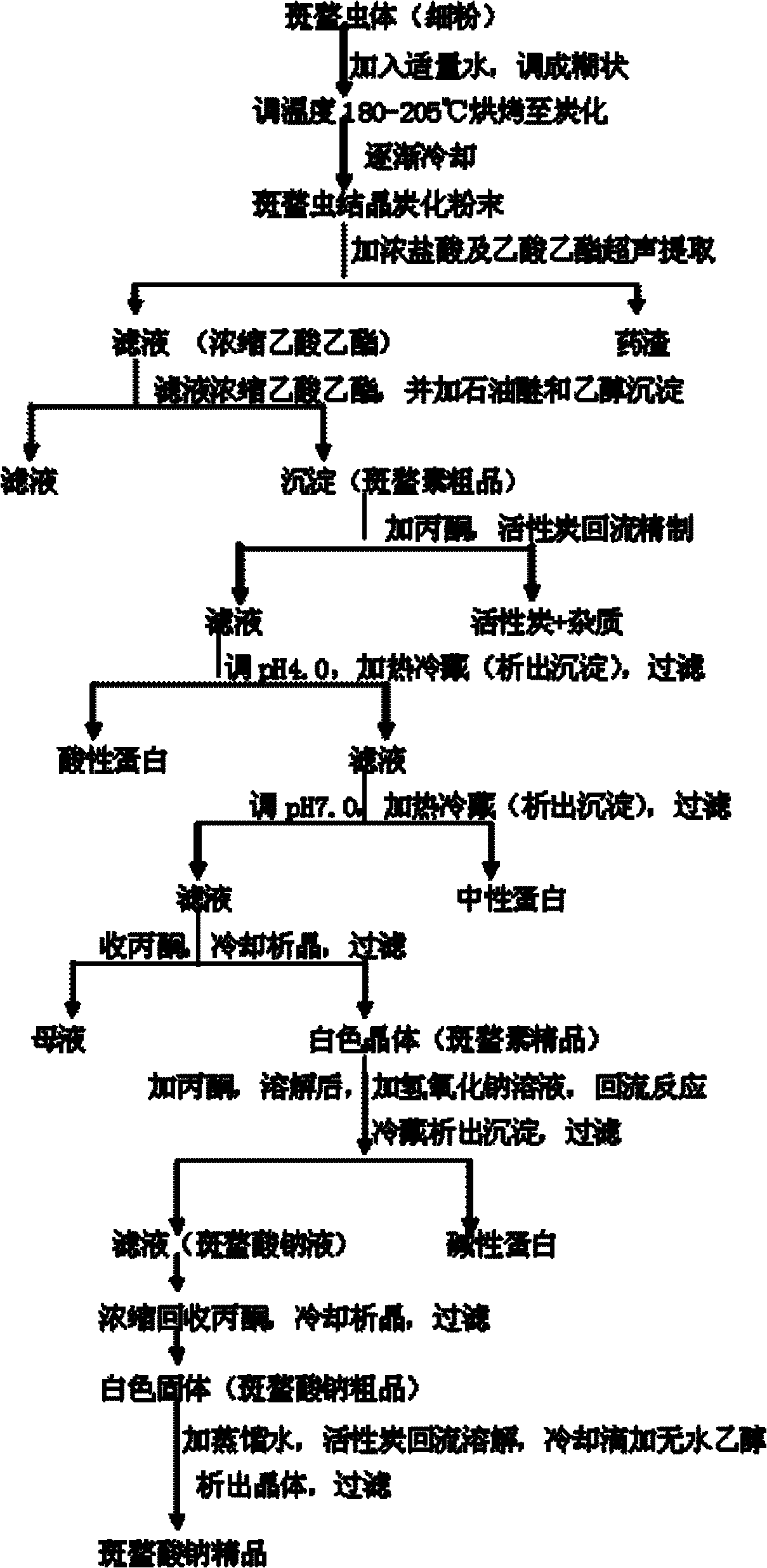
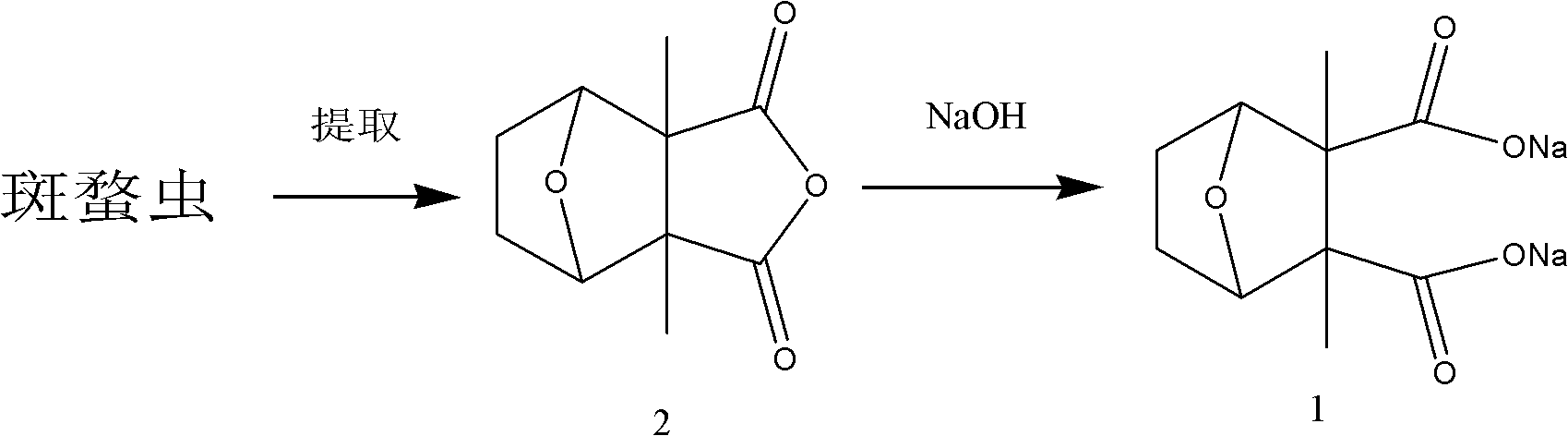
Preparing process of sodium cantharidinate
InactiveCN101012230AHigh purityStable in natureOrganic chemistryAnthropod material medical ingredientsCantharidinDosage form
The invention discloses a making technique of sodium cantharidate, which comprises the following steps: processing canthariasis; extracting cantharidin; purifying; refining; synthesizing sodium cantharidate; purifying sodium cantharidate.
Owner:南通集智知识产权服务有限公司
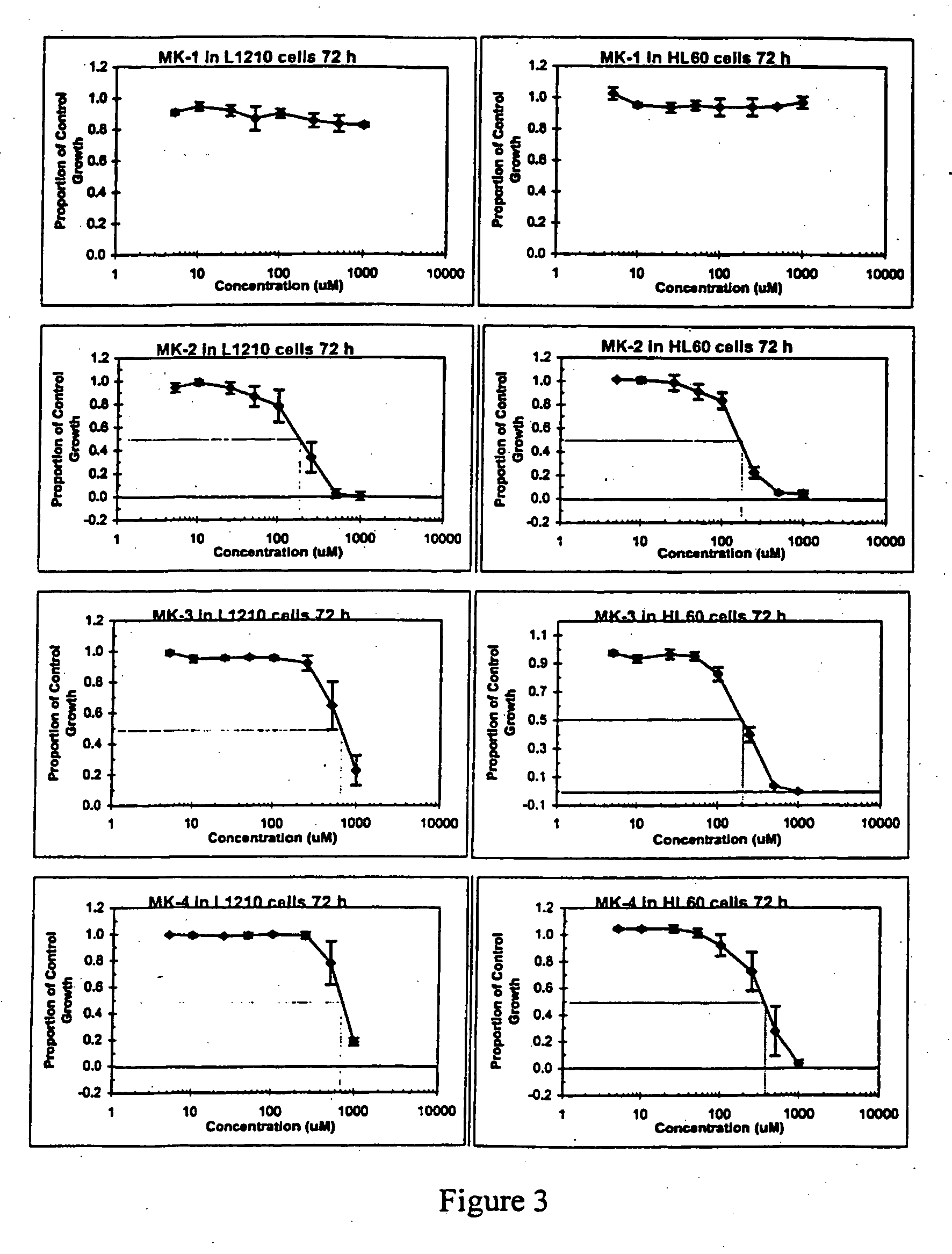
Cancer therapy with cantharidin and cantharidin analogs
InactiveUS20080267947A1Low toxicityGood curative effectBiocideAntibody ingredientsCancer cellRegimen
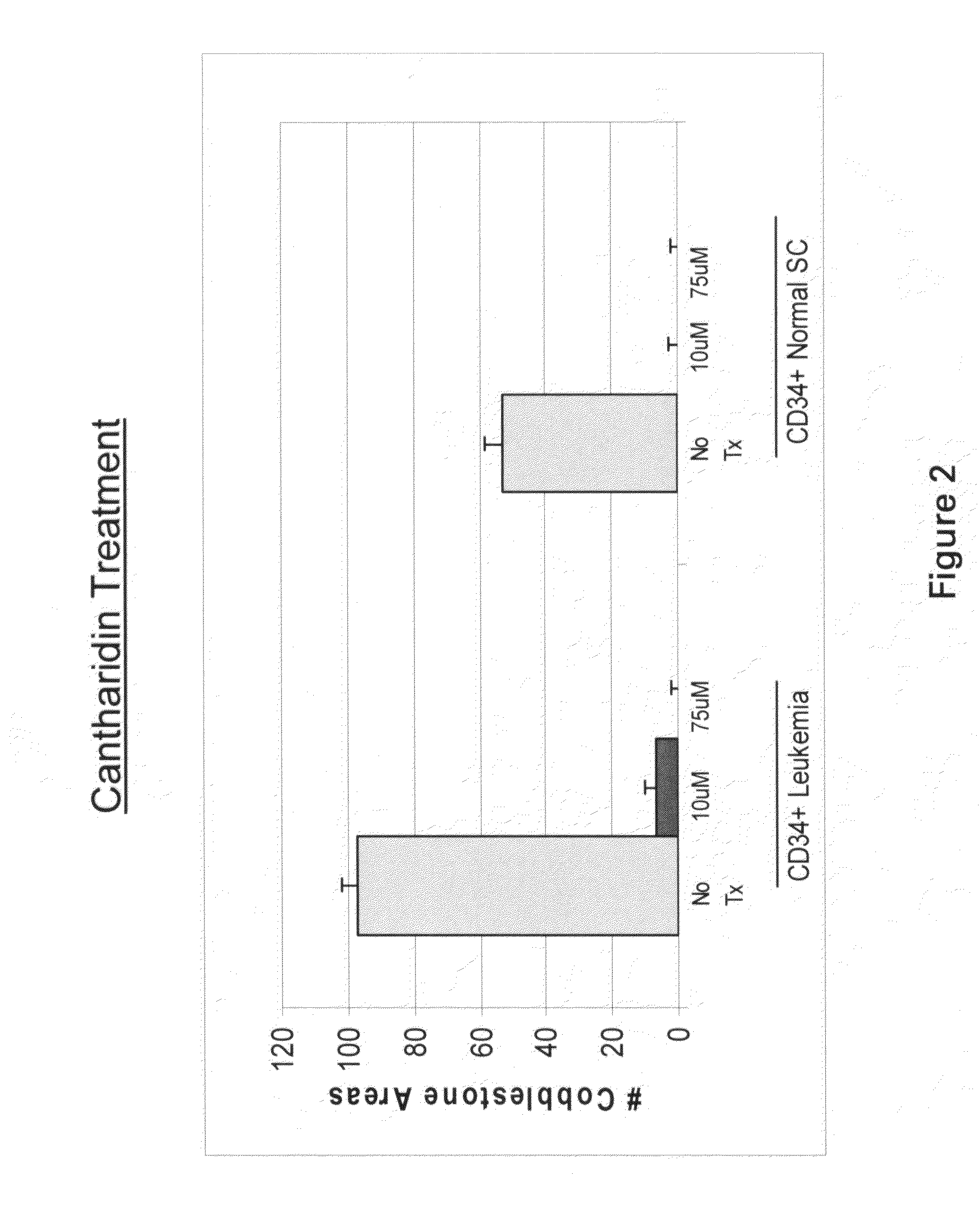
Provided are methods for treating cancer in a patient, comprising administration of a therapeutically effective regimen of cantharidin or cantharidin analog of formula of formula I, II or IIIwherein R1, R2, R3, R4, R5, R6, R7, R8, R11, R12, A, Y and Z are as set forth herein, or a pharmaceutically acceptable salt thereof, to a patient in need thereof. In some embodiments of the methods, the therapeutically effective regimen stabilizes, reduces or eliminates cancer stem cells. In some embodiments of the methods, the therapeutically effective regimen reduces or eliminates cancer cells.
Owner:STEMLINE THERAPEUTICS
Compound having anticancer activity and preparation method
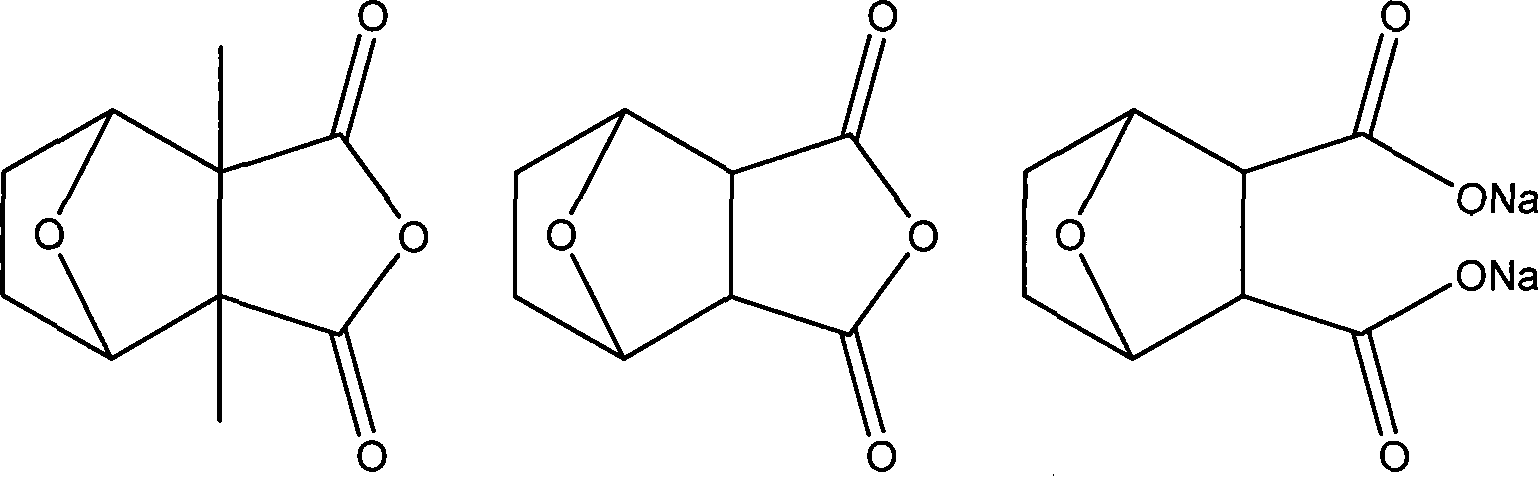
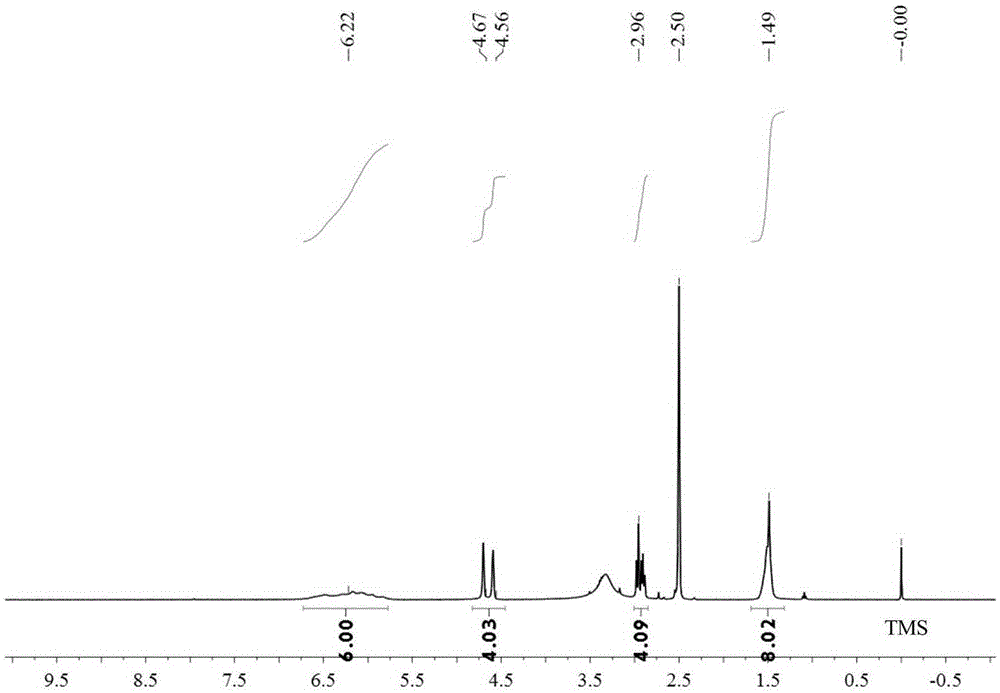
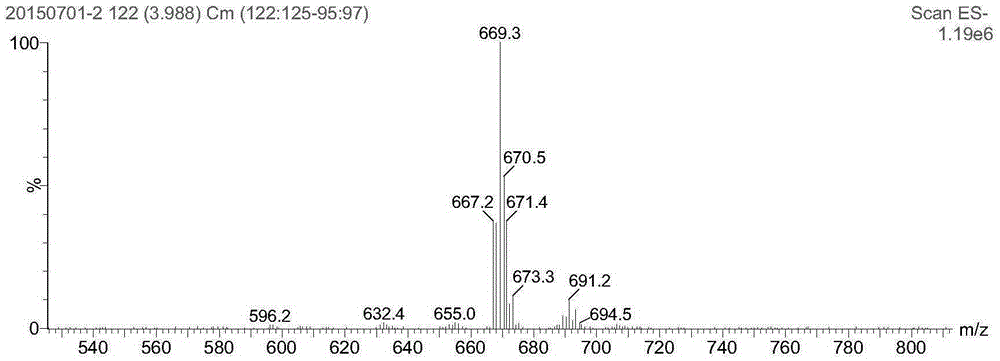
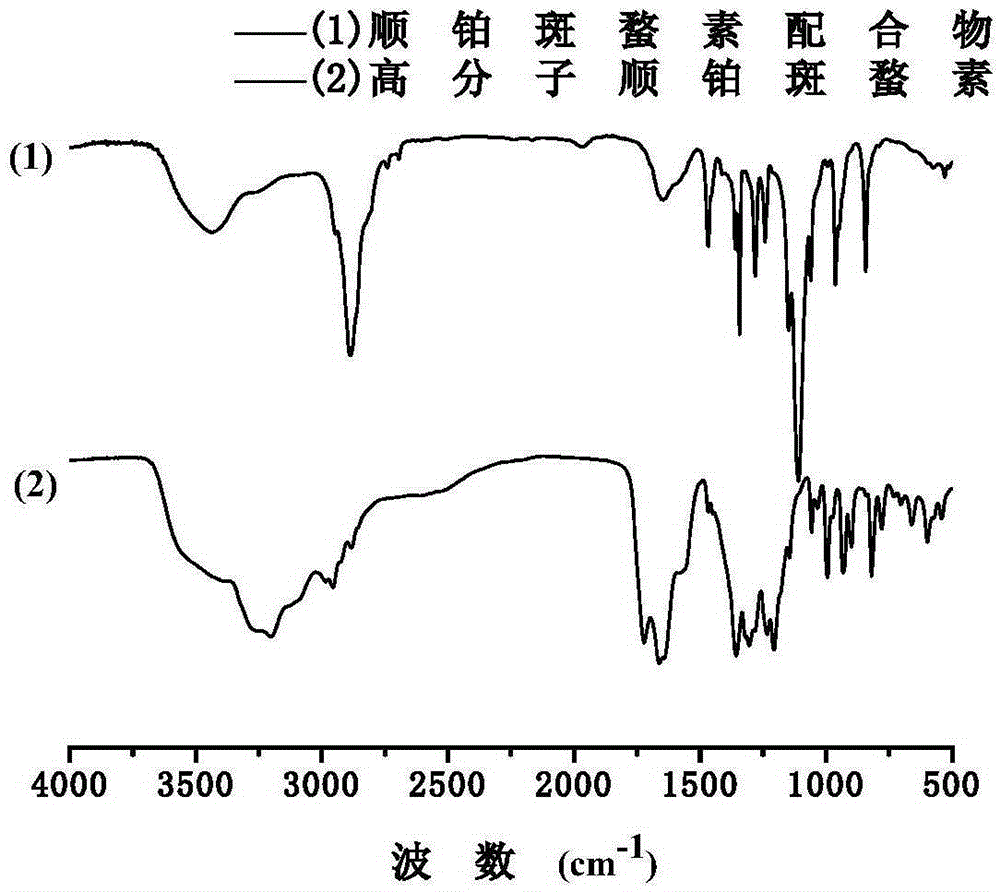
ActiveCN103588818ALow cytotoxicityImprove anti-cancer effectGroup 8/9/10/18 element organic compoundsAntineoplastic agentsL929 cellCantharidin
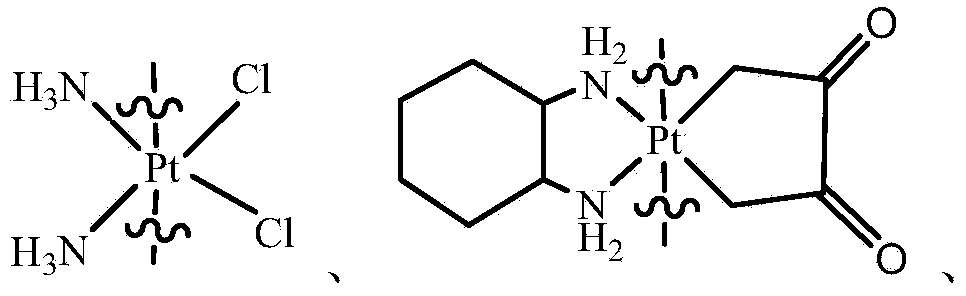
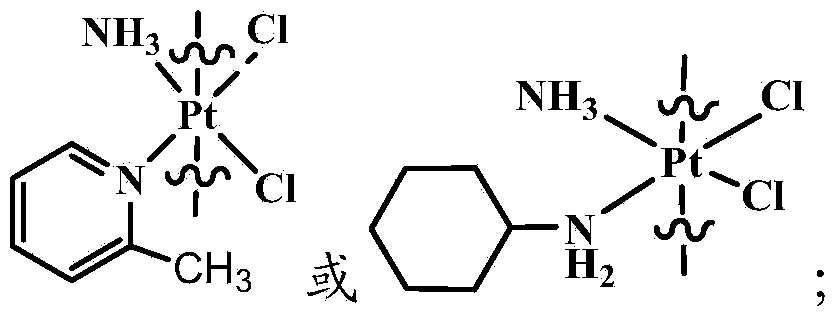
The invention provides a compound having anticancer activity, which is obtained by cooperation of Norabieta cantharidin and platinum (IV) and modification of a piperazine derivative. The compound is characterized in that a Norabieta cantharidin piperazine derivative ligand is introduced, thereby cytotoxicity of a platinum (IV) compound is reduced, and high antineoplastic activity is provided. The result of the cytotoxicity experiments shows that the provided platinum (IV) compound has less destruction to normal mice fibroblast L929, but has good killing effect to cancer cells such as human breast cancer MCF-7 cell, human cervical carcinoma HeLa cell and human lung adenocarcinoma A549 cell, so that the compound having anticancer activity is capable of reducing the system toxicity and having equal anticancer activity with cisplatin. The compound having anticancer activity has good killing effect by aiming at cisplatin resistance human lung adenocarcinoma A549 / DDP cell. Therefore, the toxicity is reduced, the cisplatin resistance can be reversed, and anticancer effect of the platinum medicines can be increased.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
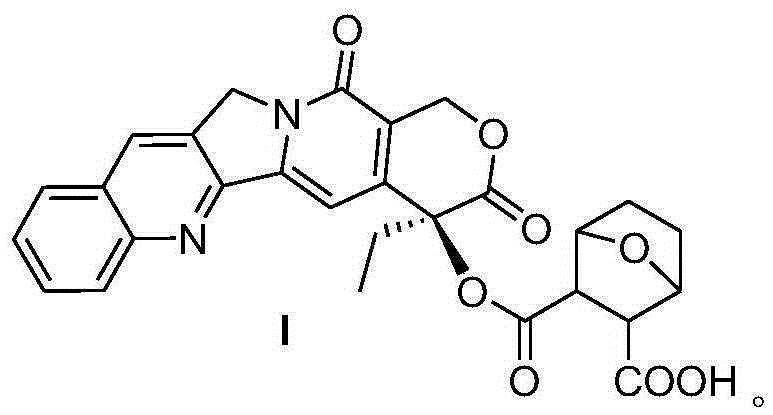
Acid-sensitive camptothecin-site 20 norcantharidate derivative and antineoplastic application thereof
InactiveCN105399757AImprove anti-tumor effectHigh inhibition rate in vitroOrganic chemistryAntineoplastic agentsSolubilityCantharidin
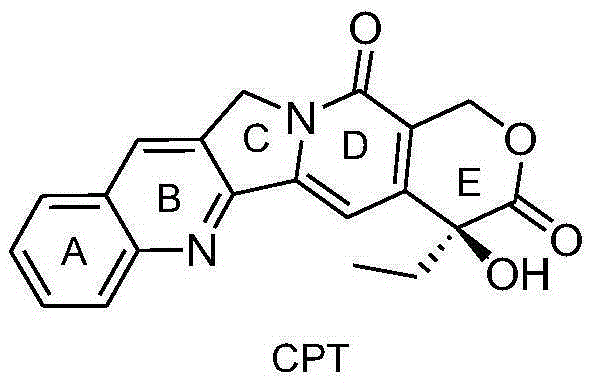
The invention provides a camptothecin-site 20 norcantharidate derivative with a structural formula as shown in I which is described in the specification, and a preparation method and antineoplastic application thereof. Results of activity test prove that the camptothecin-site 20 norcantharidate derivative as shown in I is an appropriate candidate antineoplastic drug, especially a candidate drug for resisting liver cancers, stomach cancers and colorectal cancers. Compared with positive contrast drugs, i.e., camptothecin and cantharidin, the camptothecin-site 20 norcantharidate derivative has improved water solubility and stability; and the camptothecin-site 20 norcantharidate derivative is sensitive to acid and can be easily hydrolyzed. Moreover, the preparation method for the camptothecin-site 20 norcantharidate derivative employs easily available raw materials, has high yield and is easy to operate and implement.
Owner:ZUNYI MEDICAL UNIVERSITY
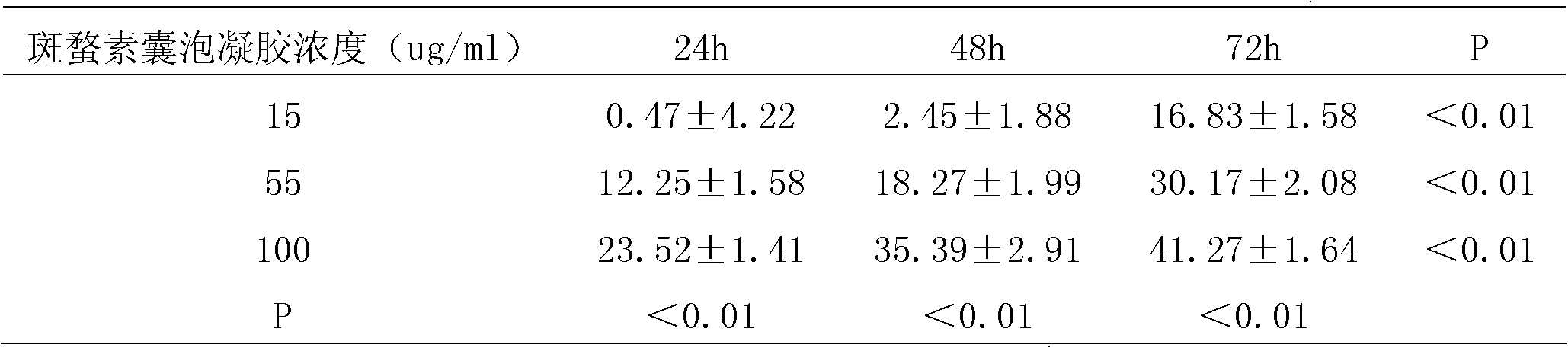
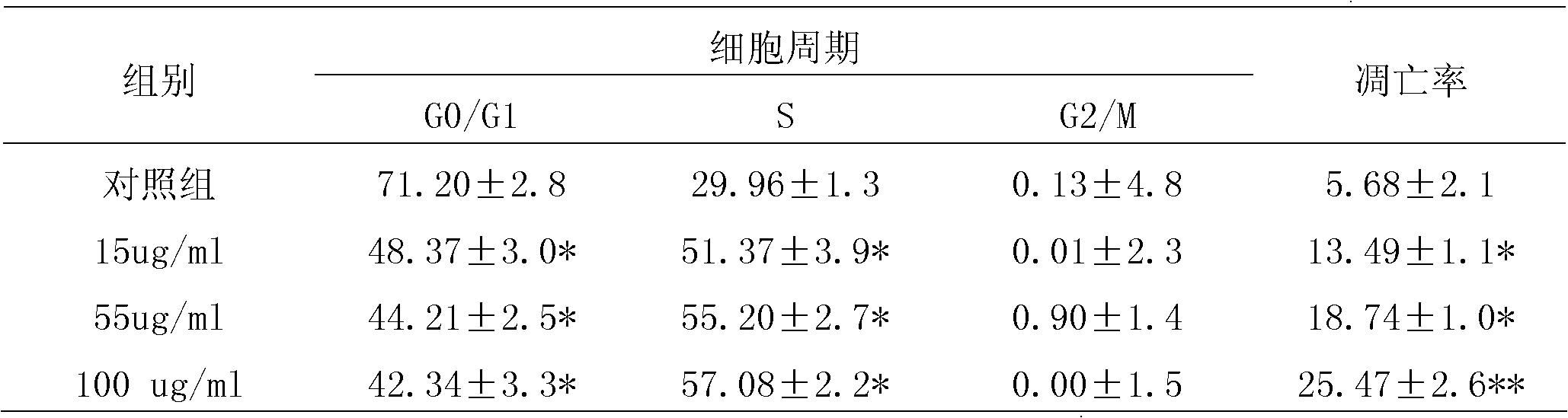
Cantharidin vesicles and preparation thereof and preparation methods of cantharidin vesicles and preparation thereof
InactiveCN102579317APromote growthSignificant effectPharmaceutical delivery mechanismDermatological disorderClinical efficacyMedicine
The invention discloses cantharidin vesicles and a preparation method thereof, and a preparation containing the cantharidin vesicles and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The cantharidin vesicles are prepared from cantharidin, a nonionic surfactant and an additive and are preferably prepared into an external gel preparation. A cantharidin percutaneous delivery external preparation has unique advantage over the medicament and is applied to a novel vesicle administration system, so that the percutaneous absorption of the medicament is increased, the clinical curative effect is enhanced, and the cantharidin percutaneous delivery external preparation can be used for treating cancer, specifically liver cancer, and has a liver targeting effect.
Owner:BEIJING INCREASEPHARM CORP LTD
Method of treating acquired perforating dermatosis with cantharidin
A method of treating acquired perforating dermatosis. The method has the step of applying an amount of cantharidin to an area of skin in need of treatment thereof.
Owner:LEVITT JACOB
Preparation method of sodium cantharidate
ActiveCN102146086ASimple extraction processSimple manufacturing processOrganic chemistryCarbonizationCantharidin
The invention discloses a preparation method of sodium cantharidate. The method comprises the following processing steps: performing high temperature carbonization of cantharis, extracting cantharidin, degreasing cantharidin and precipitating, refining cantharidin, synthetizing sodium cantharidate, refining sodium cantharidate, etc. By adopting the preparation method, the extracting time of cantharidin can be greatly shortened, lipidic and peptdic proteins and impurities in crude cantharidin and refined cantharidin can be completely removed, and the yield and purity of cantharidin can be increased. Therefore, sodium cantharidate with higher purity and yield can be obtained, the quality and safety of sodium cantharidate can be further increased and the quality reliability of sodium cantharidate can be increased when used in an injection.
Owner:贵州君之堂制药有限公司
A method for extracting cantharidin with compound enzyme
InactiveCN102268006AStrong anti virusImprove antibacterialAntibacterial agentsAntimycoticsPectinaseFiltration
The invention relates to a method for extracting cantharidin from compound enzyme. The method comprises the following steps of: performing enzymolysis on a mylabris medicinal material with cellulase and pectic enzyme; leaching a hydrolysate with a mixed liquid with the volume ratio of chloroform to absolute ethanol to concentrated hydrochloric acid of (1-2):(1-2):(1-2); performing suction filtration on a leached product in hot state and adding chloroform-absolute ethanol-concentrated hydrochloric acid in the volume ratio of (1-2):(1-2):(1-2) for leaching; combining filtrates obtained in two times and performing rotary steaming; dissolving the obtained solid by using a mixed liquid in the volume ratio of water to chloroform of (1-2):(1-2); extracting a lower layer organic phase and performing rotary steaming on the lower layer organic phase; adding a mixed liquor in the volume ratio of absolute ethanol to petroleum ether of (1-2):(1-2) into the obtained solid for dissolving; and recrystallizing with acetone to obtain cantharidin. Compared with the conventional extraction method, the method has the advantages of high extracting speed, low cost, time saving, labor saving, high extraction rate, lower impurity content, easiness for purification and the like, and is suitable for large-scale industrial production.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Preparation method of cantharidin-loaded tumor cell membrane-encapsulated tellurium elementary substance nanoparticles
ActiveCN110623939AAvoid clearingInhibit expressionOrganic active ingredientsPhotodynamic therapySide effectCell membrane
The present invention provides a preparation method of cantharidin-loaded tumor cell membrane-encapsulated tellurium elementary substance nanoparticles and belongs to the field of nano biomedicine. The nanoparticles prepared from tellurium elementary substance as a core, cantharidin as a model drug and tumor cell membranes as an outer layer coating. The tellurium nano-material and the cantharidinhaving toxic and side effects on organisms are encapsulated by the tumor cell membranes in the nanoparticles, so that biocompatibility of the nanoparticles is improved, immune cells are effectively prevented from being cleared, and the nanoparticles have homologous targeting capability; after intravenous injection, the nanoparticles are massively gathered at tumor sites through an EPR effect and ahomologous targeting property, the tellurium elementary substance is rapidly heated under near-infrared stimulation to cause outer cell membrane rupture, and the leaked cantharidin inhibits a heat shock reaction of tumor cells, enhances a photo-thermal treatment effect of the tellurium elementary substance, and kills tumor cells. The method is reasonable in design, simple in preparation technology and wide in application prospect, and lays a foundation for design and development of a corresponding drug delivery system.
Owner:DALIAN UNIV OF TECH
Separation purification process of cantharidin
The invention discloses a cantharidin separation and purification method, which comprises the following steps: (1) the crude extract of the cantharidin is put in a vessel which is sealed, and is connected with air or CO2 and heated to 140 DEG C. to 150 DEG C., the crude extract of the cantharidin is melted and boiled and the large amount of white smog is taken to the cooling device by the air to gain the white power. (2) the ethyl acetate with 80 times volume is added to dissolve the white powder and is heated and refluxed under 80 DEG C. for 10min, and is filtered while heating and is cooled naturally to recrystallize, and conducts pumping filtration, is washed using the volume rate of 1 : 2 between the absolute ethyl alcohol and (30 DEG C. to 60 DEG C.) petroleum ether, and dried under room temperature to gain the cantharidin. The invention is characterized in that the invention has high product purity, high yield, low consumption of the organic solvent and simplified technology.
Owner:GUIZHOU UNIV
Cancer Therapy With Cantharidin And Cantharidin Analogs
InactiveUS20150224083A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationBiocideAnimal repellantsCancer cellRegimen
Provided are methods for treating cancer in a patient, comprising administration of a therapeutically effective regimen of cantharidin or cantharidin analog of formula of formula I, II or IIIwherein R1, R2, R3, R4, R5, R6, R7, R8, R11, R12, A, Y and Z are as set forth herein, or a pharmaceutically acceptable salt thereof, to a patient in need thereof. In some embodiments of the methods, the therapeutically effective regimen stabilizes, reduces or eliminates cancer stem cells. In some embodiments of the methods, the therapeutically effective regimen reduces or eliminates cancer cells.
Owner:STEMLINE THERAPEUTICS
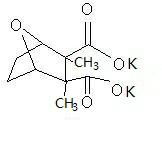
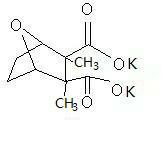
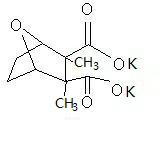
Potassium cantharidate and preparation method thereof
InactiveCN102093383AHigh purityStable in natureOrganic chemistryAntineoplastic agentsPotassium hydroxideDistilled water
The invention discloses potassium cantharidate of a structural formula below. The preparation method comprises: adding trichloromethane or isopropanol into pure cantharidin according to a weight ratio of (30-50):1, heating to dissolve the trichloromethane or isopropanol, and adding distilled water into potassium hydroxide according to a molar ratio of the potassium hydroxide to the cantharidin of 3:(2-3) to prepare solution at a weight concentration of 10 percent; adding the solution of potassium hydroxide into the trichloromethane or isopropanol solution containing cantharidin, heating the mixed solution to 60 to 75 DEG C, volatilizing trichloromethane or isopropanol till dryness, and filtering to obtain colorless transparent solution; and adding trichloromethane or isopropanol in a volume which is 2 to 5 times that the filtrate into filtrate, separating to obtain upper colorless transparent aqueous solution, placing the colorless transparent aqueous solution in an oven for concentration and drying to obtain white crystalline powder. The potassium cantharidate has high anticancer activity, the process is simple, easy to operate and very safe, and the finished product is stable in properties and high in purity.
Owner:ZUNYI MEDICAL UNIVERSITY
Externally used Chinese patent drug for treating ecthyma and its preparation
InactiveCN101011503ANo hospitalizationShort course of treatment for acneHeavy metal active ingredientsAnthropod material medical ingredientsCantharidinChinese patent
Disclosed is a Chinese medicament for treating ecthyma and its preparing process, which is prepared from the following medicinal materials (by weight portion): zanthoxylum piperitum 10-15, bunge corydalis herb 10-15, Chinese ephedra 10-15, banksia rose 15-20, native copper 15-20, Chinese gall 5-10, cantharidin 0.5-1, allume 5-10, orpiment 5-10, mercurous chloride 0.5-1 and red precipitate 0.5-1.
Owner:李笑银
Process for preparing sodium cantharidinate
The invention discloses a process for preparing sodium cantharidinate, which comprises the following steps: treating cantharis; extracting cantharidin; purifying; refining; synthesizing the sodium cantharidinate; purifying and refining the sodium cantharidinate, and the like. The invention has the advantages of simplicity, practicability, good safety, high purity of prepared finished products, stable quality, low production cost, and the like and can be applied to the production of various dosage forms of big injections, big transfusions, tablets, granules, capsules, oral liquid, ointments, and the like.
Owner:江苏睿玻生物科技有限公司
The preparation method of sodium cantharidinate
InactiveCN102276619AReduce pollutionEnhance pharmacological effectsOrganic chemistryAntineoplastic agentsCantharidinPollution
The invention relates to the technical fields of medicine, food and health products, and discloses a highly efficient preparation method of antitumor drug sodium cantharidinate. Add cantharidin essence into sodium hydroxide aqueous solution, heat to dissolve, add activated carbon to reflux for decolorization for 0.5-1 hour, heat filter, wash with water, concentrate under reduced pressure, add ethanol, cool to room temperature, precipitate solid and dry to obtain white powder. Wherein the equivalent ratio of cantharidin fines and sodium hydroxide is 1:2-3, and the weight ratio of cantharidin and ethanol is 1:3-10. The synthesis of sodium cantharidinate and the decolorization and refining of activated carbon are completed simultaneously, and the crystallization is carried out with ethanol-water system. The invention provides a synchronous method for synthesizing sodium cantharidate and decolorizing and refining activated carbon, the preparation process is simpler, the reaction cycle is shortened, the application of a large amount of acetone and isopropanol is omitted in the reaction process, the production cost is reduced, and The pollution to the environment is small, the quality control is simpler, the production efficiency is improved, and the industrial production is convenient.
Owner:GUIZHOU JINQIAO PHARMA
Pesticide composition containing cantharidin
The invention discloses a pesticide composition containing cantharidin, which comprises the following constituents (by mass percent): cantharidin 0.1-10.0%, insecticide 0.1-30.0%, and balancing auxiliary agent for pesticides.
Owner:杨凌农林科大昆虫资源研究发展中心
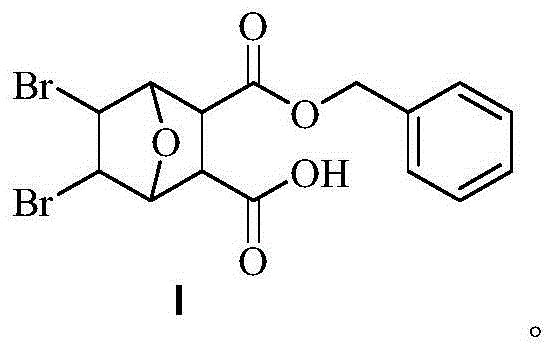
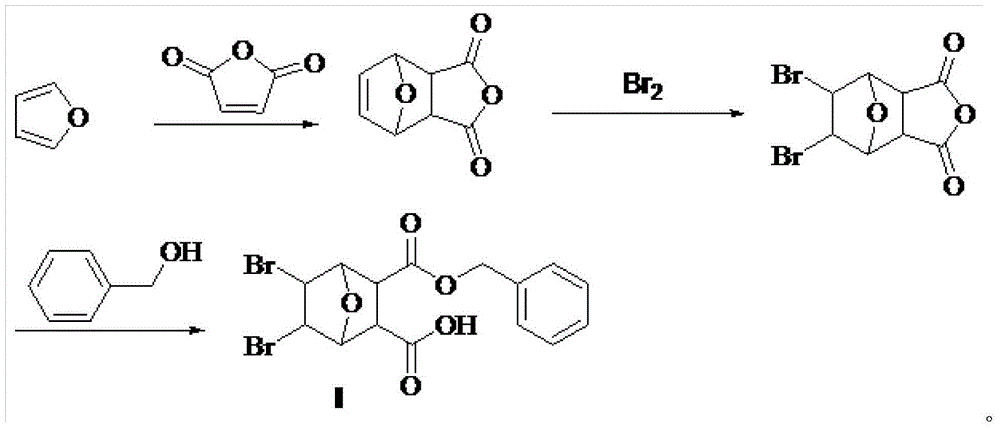
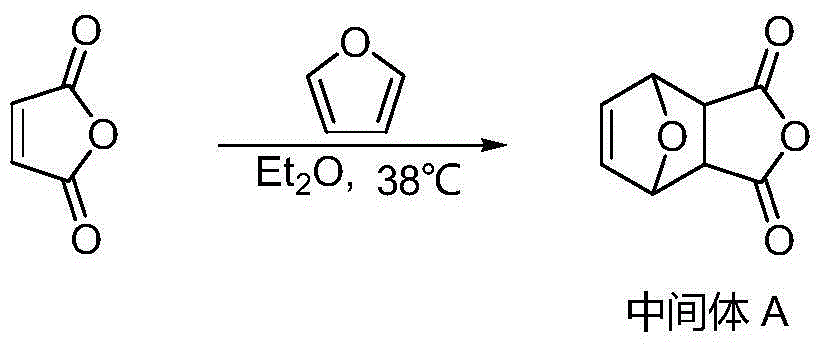
Bromo-norcantharidin acid-benzyl ester, and synthetic method and application thereof
The invention discloses a bromo-norcantharidin acid-benzyl ester shown as a structural formula I. The bromo-norcantharidin acid-benzyl ester is an open-loop 5,6-dibromo-norcantharidin acid benzyl ester, and an active test proves that the bromo-norcantharidin acid-benzyl ester has a good anti-liver-cancer effect and can be used as a high-efficiency and low-toxicity cantharidin anticancer medicine The synthesis process is excellent in selectivity, raw materials are readily available, the cost is low, the synthetic route is simple, operation is easy, a product obtained by the synthesis has low toxicity, high yield and high purity, and the process is high-efficiency, convenient and low in cost.
Owner:GUIZHOU BAIQIANG PHARMA
Method for extracting cantharidin from cantharides
The invention relates to a method for extracting cantharidin from cantharides. The method is characterized by comprising the following steps of: performing continuous countercurrent ultrasonic extraction on powder of cantharides by using acetone, removing impurities from an extracting solution, concentrating under reduced pressure, evaporating to remove partial solvent, and cooling and crystallizing a concentrated solution to obtain a crude product of cantharidin; heating to dissolve the crude product by using chloroform, filtering to remove insoluble substances, and cooling and crystallizing filtrate to obtain the cantharidin. The continuous countercurrent ultrasonic extraction is adopted, the impurities are removed from the extracting solution by an elutriation method, and recrystallization is carried out by using the chloroform, so that the cantharidin with the content of over 98 percent is obtained, steps of degreasing by using an organic solvent and the like are eliminated, the operation is simple, the production is safe, the extraction yield is high, and the method is suitable for mass production.
Owner:JIANGSU SIHUAN BIOENG
Injectio Natarii Norcantharidatis freeze-drying powder injection and its preparation method
InactiveCN1557298AGood effectInstant in waterOrganic active ingredientsPowder deliveryFreeze-dryingCantharidin
The present invention is freeze dried demethyl sodium cantharidinate powder for injection and its preparation process, and belongs to the field of medicine technology. The freeze dried demethyl sodium cantharidinate powder for injection contains demethyl cantharidin 0.25-70 wt%, sodium hydroxide 0.1-35 wt% and excipient 0-99 wt%. The preparation process includes compounding demethyl cantharidin and sodium hydroxide in liquid compounding tank, adding injection water and heating to dissolve, adding excipient, dissolving and regulating pH value; adding proper amount of carbon for injection, stirring for 30 min for adsorption, filtering to eliminate carbon and sterilize, packing in Schering bottle, and freeze drying to obtain the freeze dried demethyl sodium cantharidinate powder for injection. The medicine is clinically used in treating liver cancer, esophagus cancer, hepatitis, etc.
Owner:肖广常 +1
Method for preparing cantharidin from cantharides
InactiveCN101798309AReduce dosageImprove efficiencyOrganic chemistryBulk chemical productionCantharidinSolvent
The invention provides a novel process for preparing cantharidin from cantharides. The process comprises the following steps of: by using supercritical CO2 as a solvent and acetone as an entrainer, performing static immersion of the cantharides for 1 to 1.5 hours at an extraction pressure of 10 to 20MPa and an extraction temperature of between 30 and 40 DEG C, performing dynamic extraction for 2 to 3 hours at an analytic pressure of 4 to 8MPa and an analytic temperature of between 40 and 50 DEG C, removing impurities from the obtained extracts by using petroleum ether, and crystallizing the obtained extracts by using acetone. The process has the advantages of high extraction efficiency, short period, little pollution and low cost.
Owner:NANJING ZELANG MEDICAL TECH
Novel green and environment-friendly synthetic process for cantharidin
The invention relates to a novel green and environment-friendly synthetic process for preparing cantharidin under the condition of normal pressure and belongs to the field of synthesis and preparation of medicines. The process comprises the following steps: preparing 3-cyano-3-hydroxy-4-methyl formate-2,5-tetrahydrothiophene from a NaCN aqueous solution; adding benzene, pyridine and POCl3 into thiophene to prepare 3-cyano-4-methyl formate-2,5-dihydrothiophene; then adding acetic acid and concentrated hydrochloric acid, and carrying out reflux reaction to obtain 2,5-dihydrothiophene-3,4-dicarboxylic acid powder; carrying out stirring and refluxing on the prepared dicarboxylic acid and thionyl chloride to prepare 2,5-dihydrothiophene-3,4-dicarboxylic anhydride; carrying out heating reaction according to the solid-liquid ratio of dicarboxylic anhydride to furfuran to ionic liquid being 1mg:(3.5 to 6[mu]l):(2 to 4[mu]l), extracting and carrying out Raney-Ni reflux to obtain the cantharidin. According to the process, superhigh pressure condition and equipment are not needed, and overnight reaction at the temperature of 20 to 50DEG C is carried out; the process has the advantages of mild conditions, high conversion rate, and high yield of the cantharidin, and provides favorable conditions for industrial production of the cantharidin.
Owner:海南亦璞科技有限公司
Traditional Chinese medicine for treating facial paralysis and method for preparing same
InactiveCN103142963AImprove facial paralysis symptomsQuick resultsNervous disorderAnthropod material medical ingredientsPollenCantharidin
The invention discloses a traditional Chinese medicine for treating facial paralysis and a method for preparing the traditional Chinese medicine. The traditional Chinese medicine is prepared from the following raw materials in parts by weight: 8-10 parts of astragalus, 7-12 parts of saposhnikovia divaricata, 7-11 parts of ginger, 5-12 parts of rhizoma atractylodis macrocephalae, 8-10 parts of angelica, 7-12 parts of safflower, 8-12 parts of ligusticum wallichii, 9-10 parts of pollen typhae, 7-11 parts of rhizoma sparganii, 5-12 parts of curcuma zedoary, 6-15 parts of peach kernel, 9-13 parts of cantharidin, 8-15 parts of nux vomica, 7-12 parts of leech, 9-17 parts of earthworm, 8-11 parts of centipede, 5-13 parts of stiff silkworm, 8-14 parts of scorpion and 8-12 parts of eel blood. The powder of all the raw materials is made into a paste. The traditional Chinese medicine can be applied to the diseased part of a patient to improve the symptoms of the facial paralysis in a short time, can take effect quickly, is high in effective rate and low in treatment cost and has good social and economic benefits.
Owner:高思山
An anticancer medicinal composition, preparing method and use thereof
InactiveCN101007026AMeet urgent clinical needsGood curative effectOrganic active ingredientsDigestive systemDiseaseMalignant lymphoma
The invention belongs to medical domain; it has disclosed a compound medicine that contains cantharidin orits derivates and at least one kind of medicine that can improve the immunity, the preparing method and application is also mentioned in the invention. The medicine that can improve immunity is chosen from one kind or various kinds of lentinan, ganoderma and hoelen. The medicine can be used to treat liver cancer, esophageal cancer, carcinoma of gastric cardia, lung cancer, malignant lymphoma and low blood corpuscle; it can also be used to treat hepatitis, cirrhosis or HB virus; it can be used as preoperative drug for cancer operation or be used in combination chemotherapy with the function of coordinated synergistic action. The product in the invention has good stability and the effects have been greatly improved than the single application of Norcantharidin, lentinan, ganoderma and hoelen. The medicine can be made into any medically acceptable dosage with any medically acceptable accessories so it has wide prospects.
Owner:海安江理工技术转移中心有限公司
Injectio natarii norcantharidatis freeze-dried powder for injection and preparing method thereof
ActiveCN101229135AImprove stabilityGood shape appearancePowder deliveryOrganic active ingredientsActivated carbonMANNITOL/SORBITOL
The invention relates to a freeze-dried powder of sodium norcantharidin for injection and a preparation method for the freeze-dried powder: adding the norcantharidin and mannitol into well prepared sodium hydroxide solutions and adjusting the ph; adding activated carbon and removing colors, filtering and removing the carbon and finely filtering through a 0.22Mu m filtering film and making loading separately; cooling the separately loaded medical solution rapidly to minus 60 to minus 55 DEG C with the speed of 15 to 25 DEG C per minute; preserving heat and freezing for 3 hours and pumping the vacuum of 5 to 15 Pa; heating slowly and uniformly to minus 5 to 5 DEG C with the speed of 3 to 3.5 DEG C per hour and preserving the heat for 2 to 5 hours and then heating with a uniform speed to 20 to 30 DEG C with 3 to 6 heating hours; preserving heat and drying for 5 hours and detecting, pressing a whole cover and packaging and warehousing. The freeze-dried powder obtained by the method is high in purity, beautiful in appearance and low in water content and the proportion of the nor cantharidin, the sodium hydroxide and the mannitol is provided as 2 to 1 to 20.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Amphipathic polymer with main chain containing double anticancer drugs, as well as preparation method and nano-micelle of amphipathic polymer
ActiveCN105254867AImprove anti-tumor activityLow cytotoxicityHeavy metal active ingredientsEmulsion deliveryChemical synthesisPolymer science
The invention provides an amphipathic polymer with a main chain containing double anticancer drugs, as well as a preparation method and a nano-micelle of the amphipathic polymer, and belongs to the technical field of chemical synthetic drugs. The structural formula of the amphipathic polymer is shown in the formula I. The amphipathic polymer is obtained by leading a quadrivalent platinum cantharidin complex or a quadrivalent platinum norcantharidine complex into the main chain of the polymer, wherein the quadrivalent platinum cantharidin complex or the quadrivalent platinum norcantharidine complex is taken as a main body, and is connected with the main chain through an amide bond to serve as a hydrophobic section, and a polyethylene glycol chain is taken as a hydrophilic section; the obtained polymer is relatively high in antineoplastic activity but relatively low in cytotoxicity. The invention further provides the preparation method of the mphipathic polymer, and the nano-micelle made of the amphipathic polymer.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation of norcantharidin targeted liposome and application of freeze-drying preparation of norcantharidin targeted liposome
InactiveCN107753429ASmall and uniform particle sizeEasy to operatePharmaceutical non-active ingredientsAntineoplastic agentsFreeze-dryingCantharidin
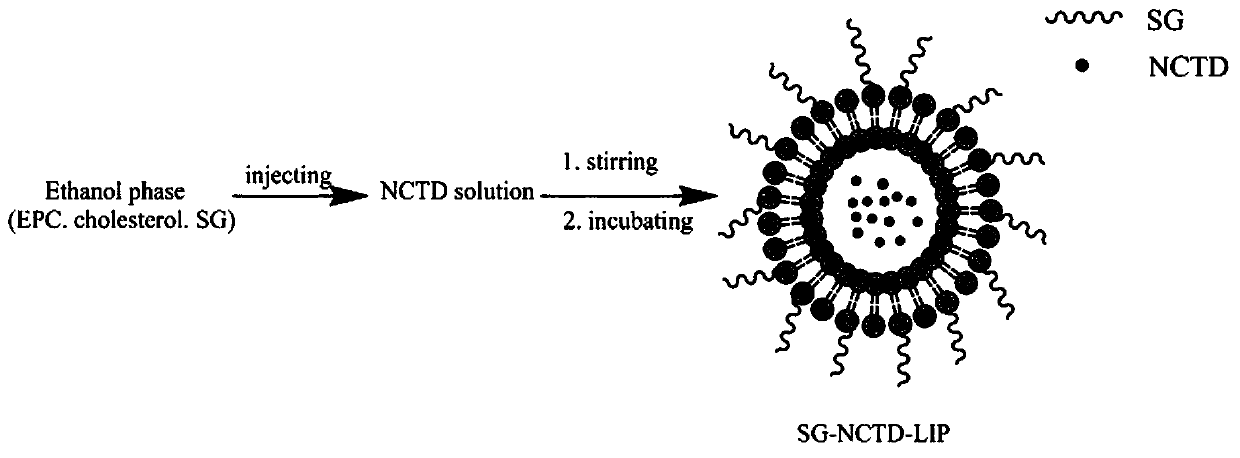
The invention belongs to the technical field of medicine. The invention discloses a norcantharidin liver-targeting liposome, a preparation method thereof and the application of a freeze-dried preparation. The norcantharidin liver-targeting liposome of the present invention is connected with a glycyrrhetic acid derivative on the surface of the liposome, which can be combined with the glycyrrhetic acid receptor on the surface of liver tumor cells and undergo endocytosis, thereby producing a tumor-suppressing effect. ; Norcantharidin is encapsulated in liposomes, which has better drug bioavailability and enhanced curative effect. The norcantharidin liposome freeze-dried preparation prepared by the invention has good stability, safety and reliability, is easy to store, and is beneficial to industrialized production.
Owner:GUILIN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com