Amphipathic polymer with main chain containing double anticancer drugs, as well as preparation method and nano-micelle of amphipathic polymer
A technology of amphiphilic polymers and anticancer drugs, which is applied in the field of preparation of amphiphilic polymers and nanomicelles containing double anticancer drugs in the main chain, which can solve the problems of high toxicity and side effects, low bioavailability, and easy metabolism And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing amphiphilic polymers containing double anticancer drugs in the main chain, including the following steps:
[0049] Step 1: reacting a platinum (II) compound with hydrogen peroxide to obtain a platinum (IV) compound;
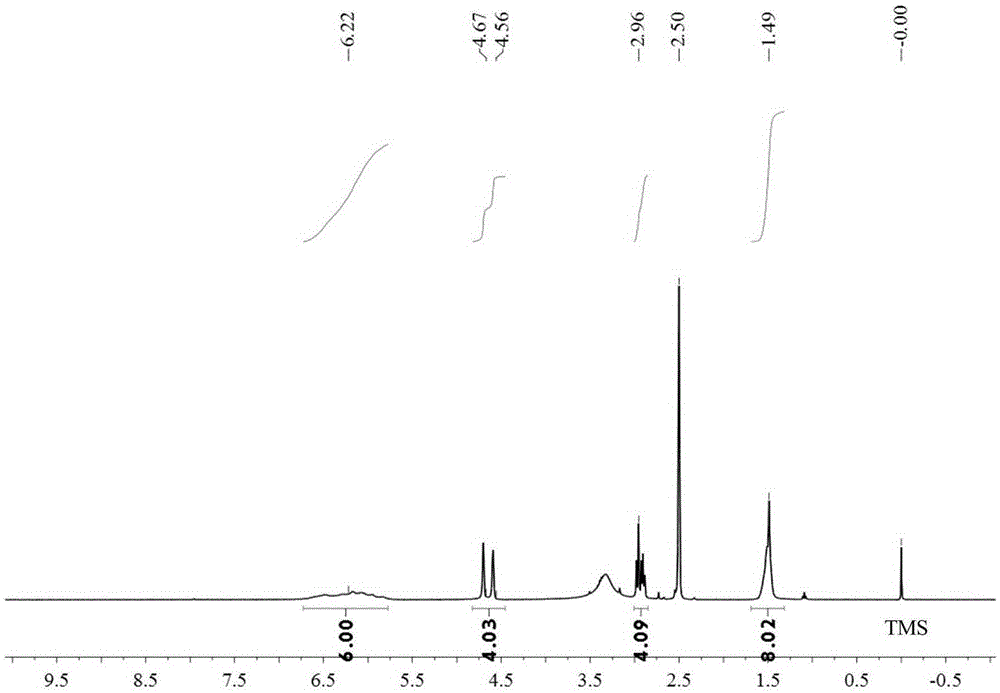
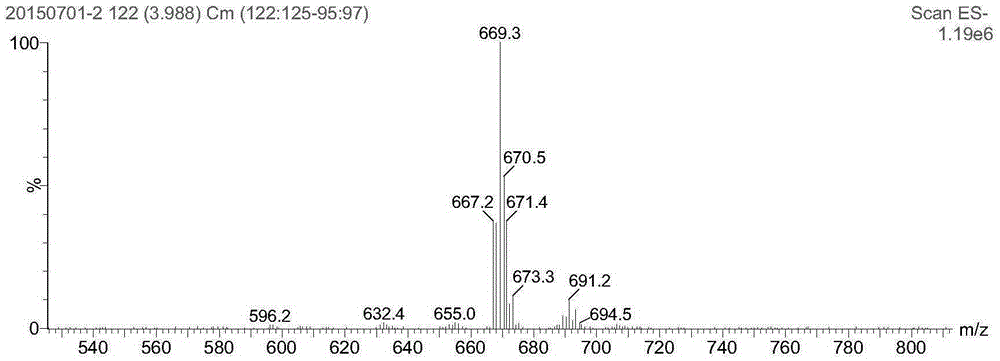
[0050] Step 2: reacting the platinum (IV) compound obtained in step 1 with cantharidin or demethylcantharidin to obtain a platinum (IV) cantharidin complex or a platinum (IV) norcantharidin complex;
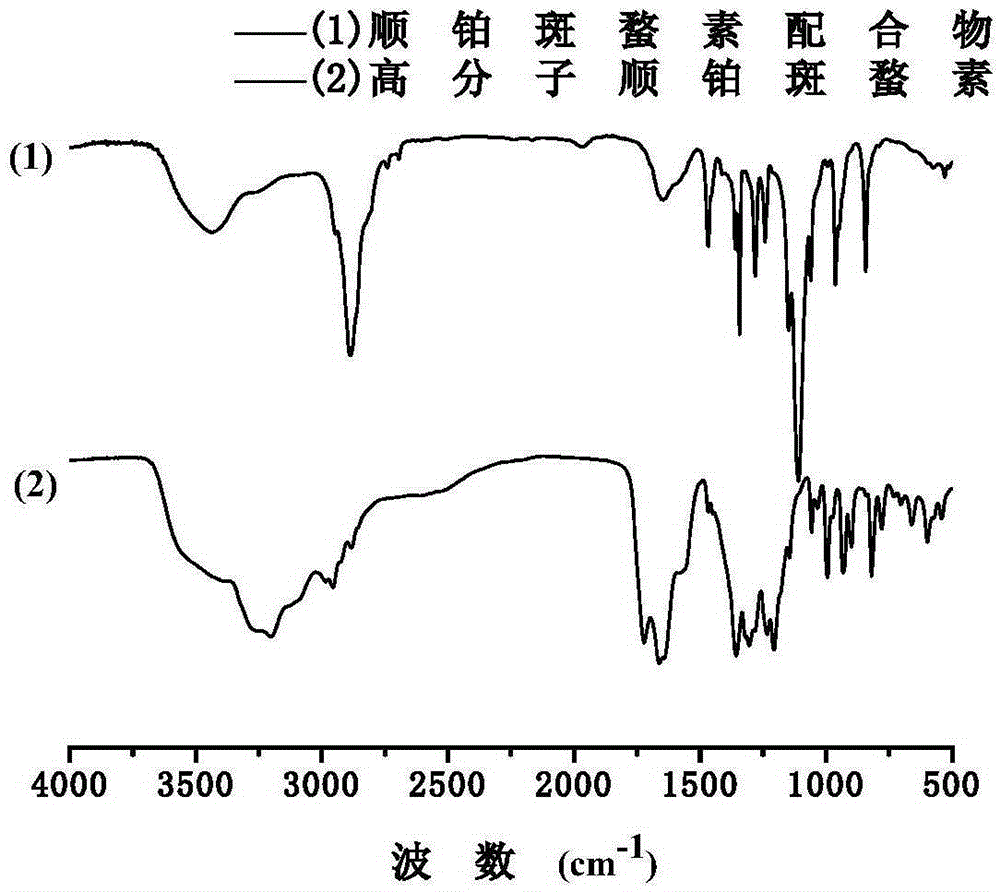
[0051] Step 3: react the platinum (IV) cantharidin complex or platinum (IV) demethylcantharidin complex obtained in step 2 with diamine and polyethylene glycol to obtain a main chain containing double anticancer drug with high amphipathic molecular.
[0052] According to the present invention, the platinum (II) compound is first reacted with hydrogen peroxide to obtain the platinum (IV) compound, the reaction temperature is preferably room temperature, the reaction time is preferably 12h, the platinu...
Embodiment 1c
[0067] Example 1c, c-[Pt(II)(CDA)(N 3 ) 2 ] preparation
[0068] (1) 1.57g (5mmol) of c,c-[Pt(II)(CDA)Cl 2 ] and 1.7g (10mmol) of silver nitrate were dissolved in 100ml of distilled water, stirred and reacted in the dark at room temperature for 12h, and the precipitate AgCl was removed by filtration to obtain divalent platinum hydrate nitrate c,c-[Pt 2+ (II)(CDA)(H 2 0) 2 ](NO 3 - ) 2 ;
[0069] (2) The above c,c-[Pt 2+ (II)(CDA)(H 2 0) 2 ](NO 3 - ) 2 The aqueous solution was reacted with 0.65g (10mmol) of sodium azide at room temperature for 4h to obtain the divalent platinum diazide complex c,c-[Pt(II)(CDA)(N 3 ) 2 ] Yellow precipitate.
Embodiment 2c
[0070] Example 2c, c-[Pt(II)(CHDA)(N 3 ) 2 ] preparation
[0071] (1) Dissolve 1.14g (10mmol) trans-1,2-cyclohexanediamine and 4.15g (10mmmol) potassium chloroplatinite in 100ml double-distilled water, and stir for 12 hours in the dark to obtain a complex of divalent platinum c,c-[Pt(II)(CHDA)Cl 2 ];
[0072] (2) 1.9 g (5 mmol) of c,c-[Pt(II)(CHDA)Cl 2 ] and 1.7g (10mmol) of silver nitrate were dissolved in 100ml of distilled water, stirred and reacted in the dark at room temperature for 12h, and the precipitate AgCl was removed by filtration to obtain divalent platinum hydrate nitrate c,c-[Pt 2+ (II)(CHDA)(H 2 0) 2 ](NO 3 - ) 2 ;
[0073] (3) The above c,c-[Pt 2+ (II)(CHDA)(H 2 0) 2 ](NO 3 - ) 2 The aqueous solution was reacted with 0.65g (10mmol) of sodium azide at room temperature for 4h to obtain the divalent platinum bis-azide complex c,c-[Pt(II)(CHDA)(N 3 ) 2 ] Yellow precipitate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com