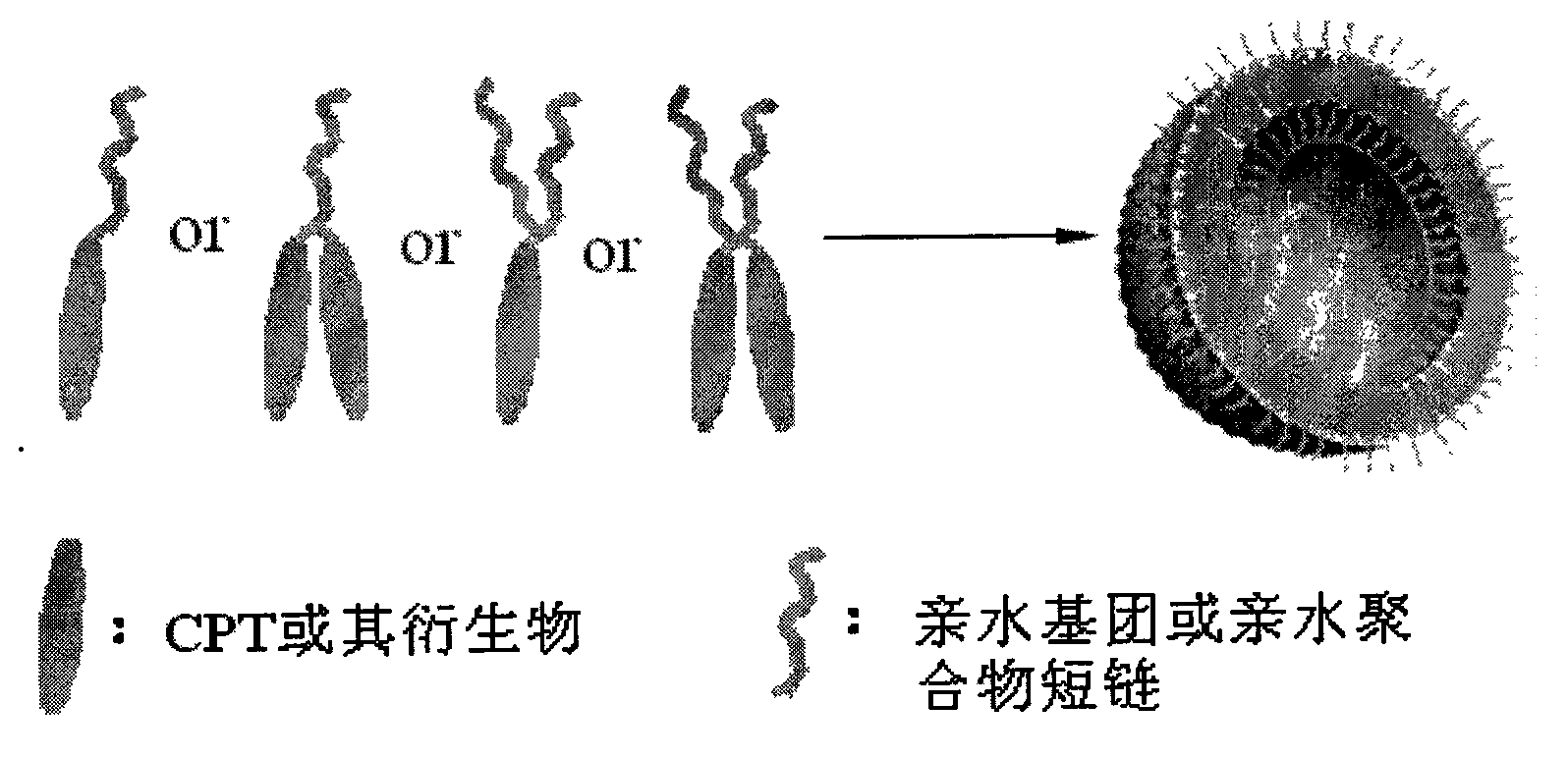
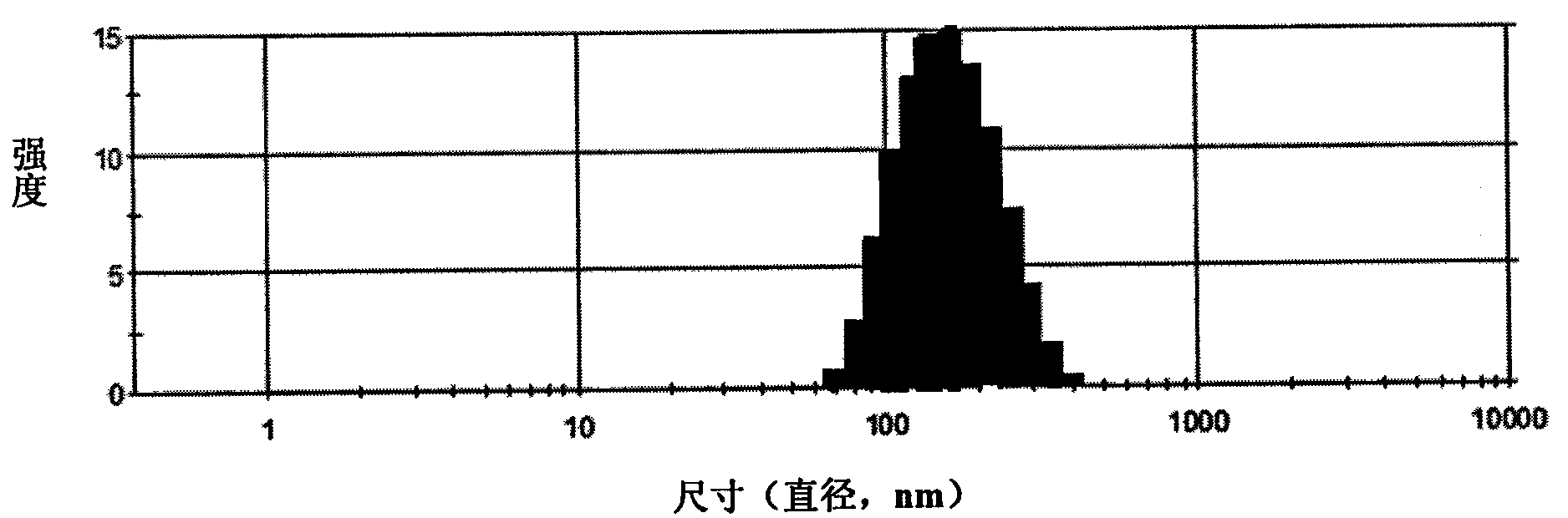
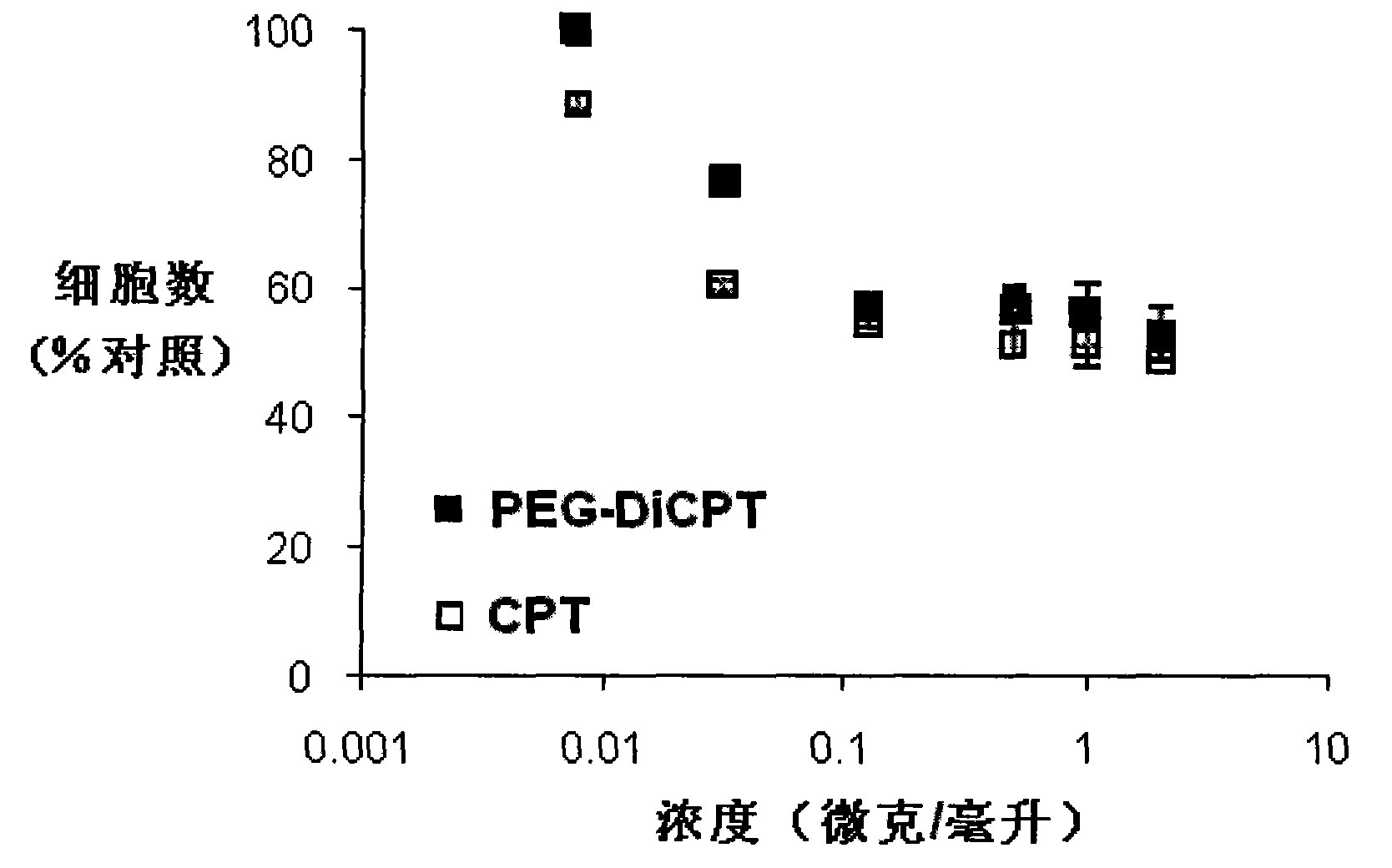
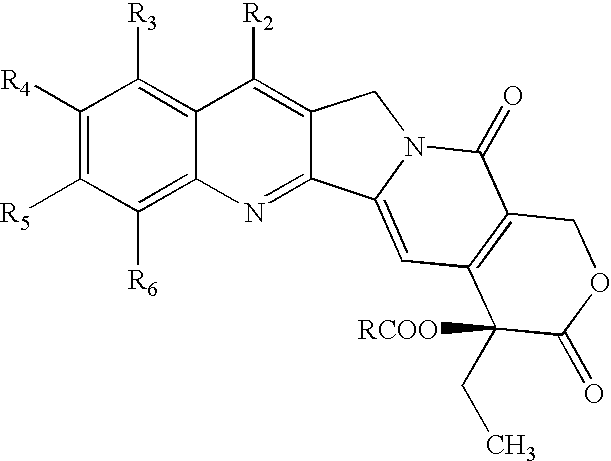
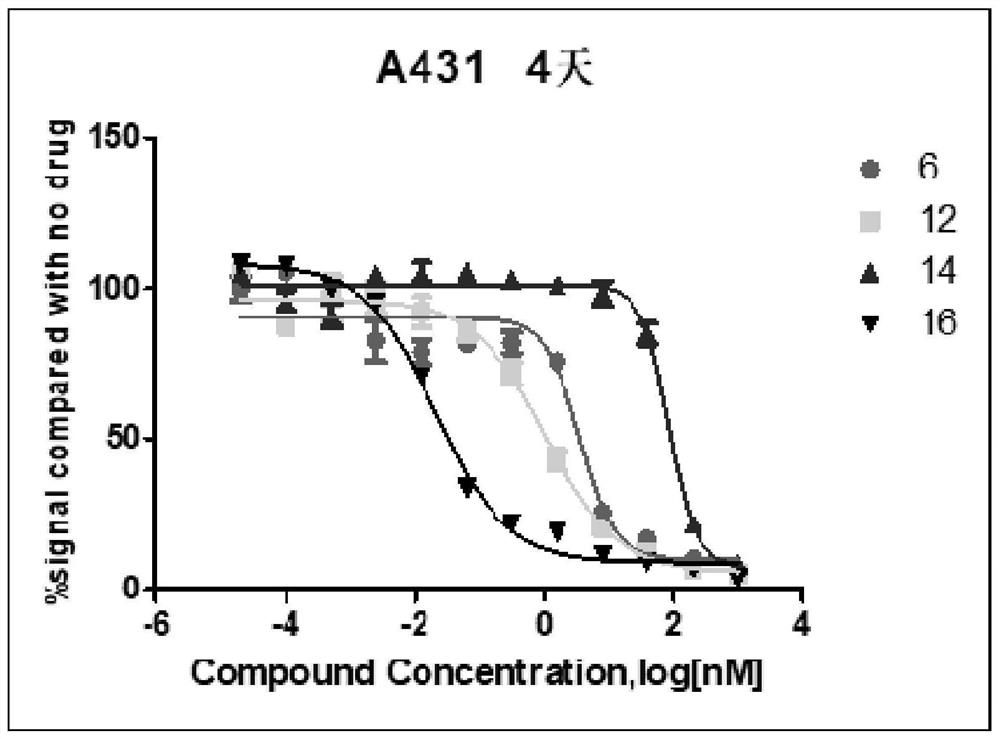
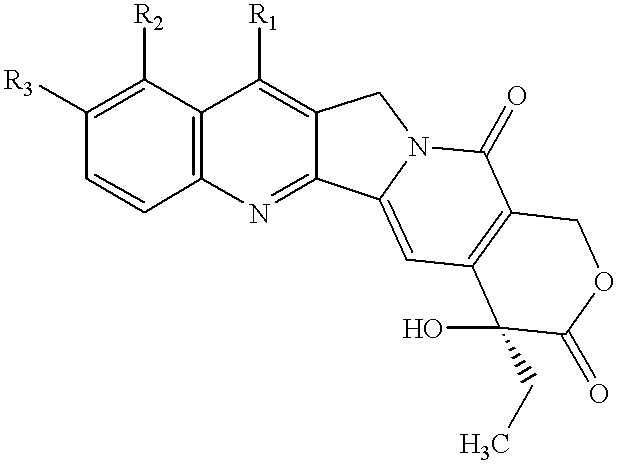
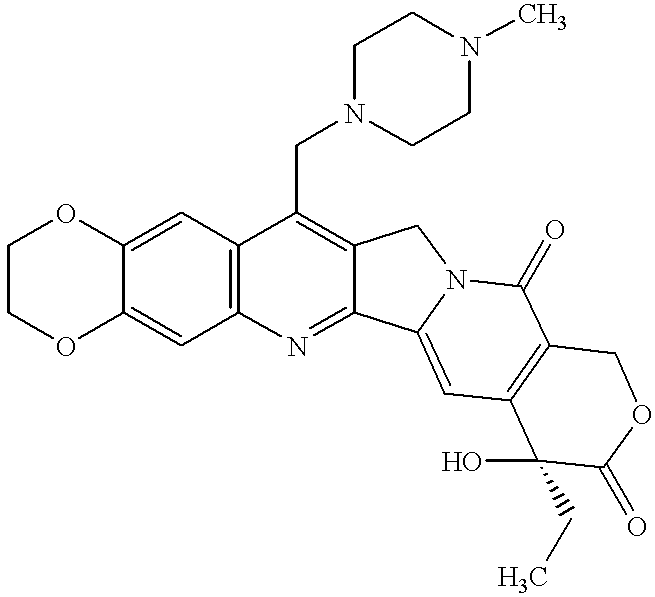
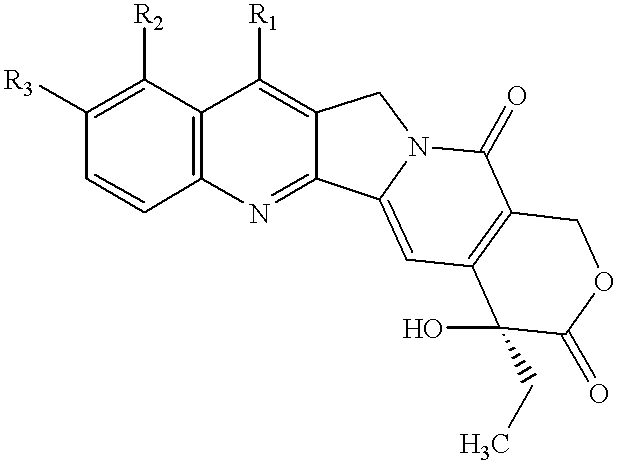
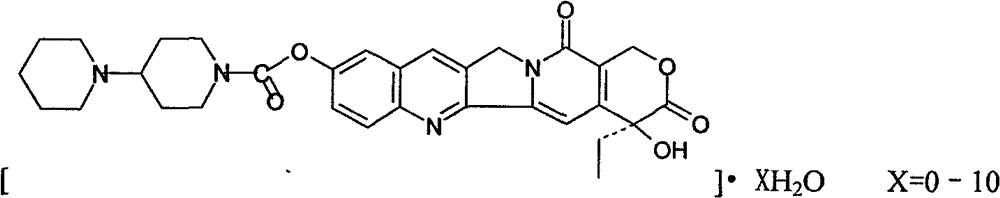
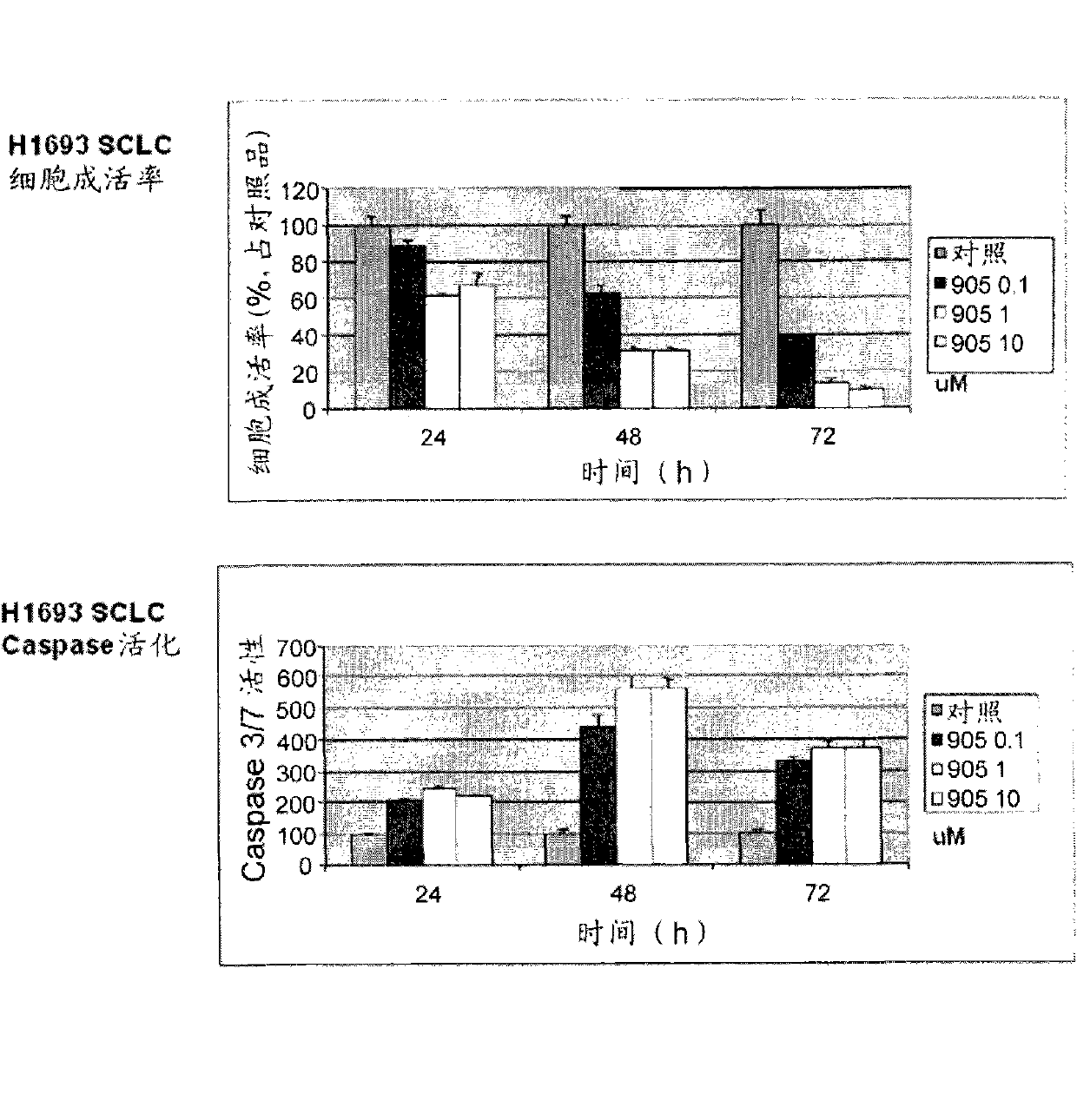
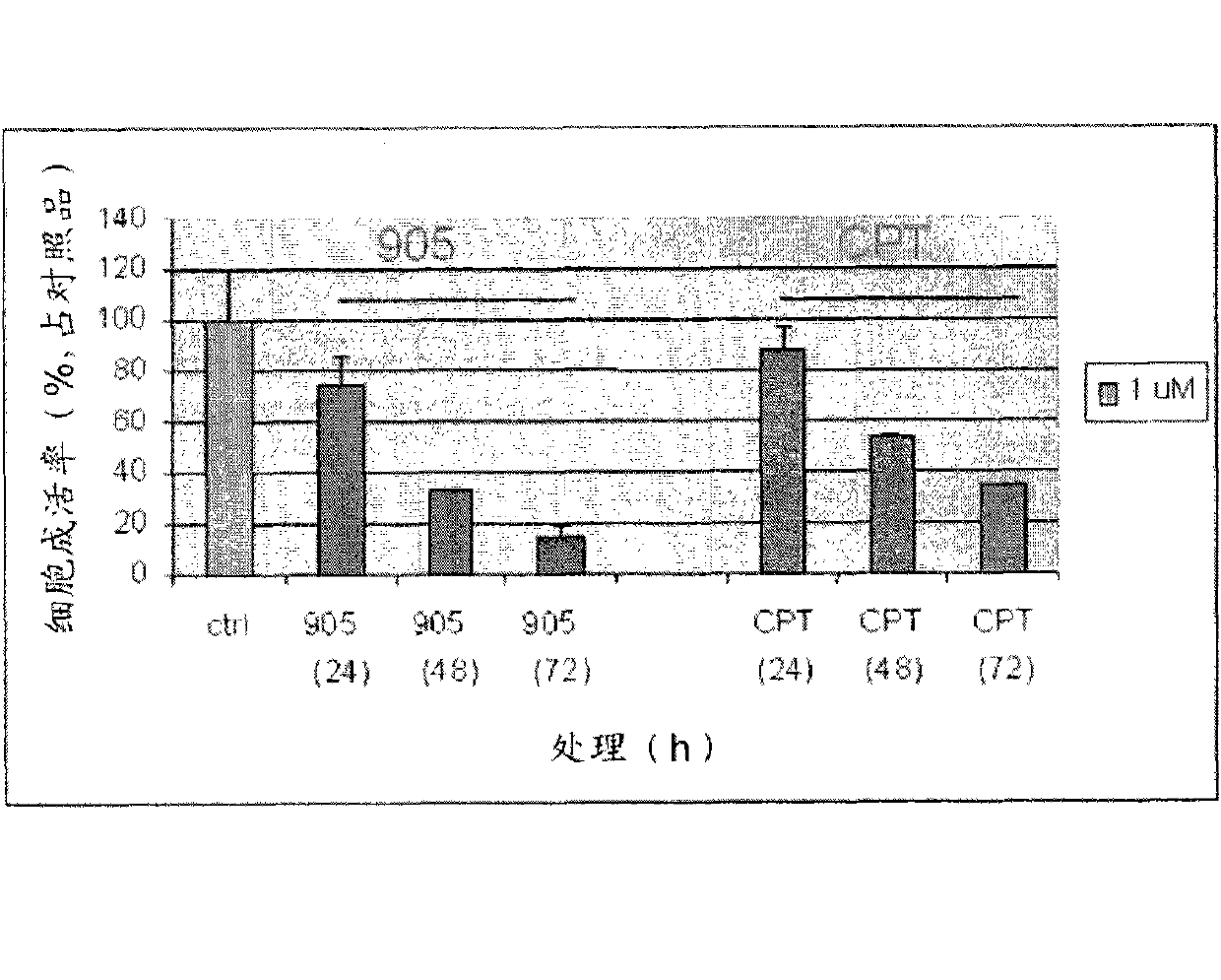
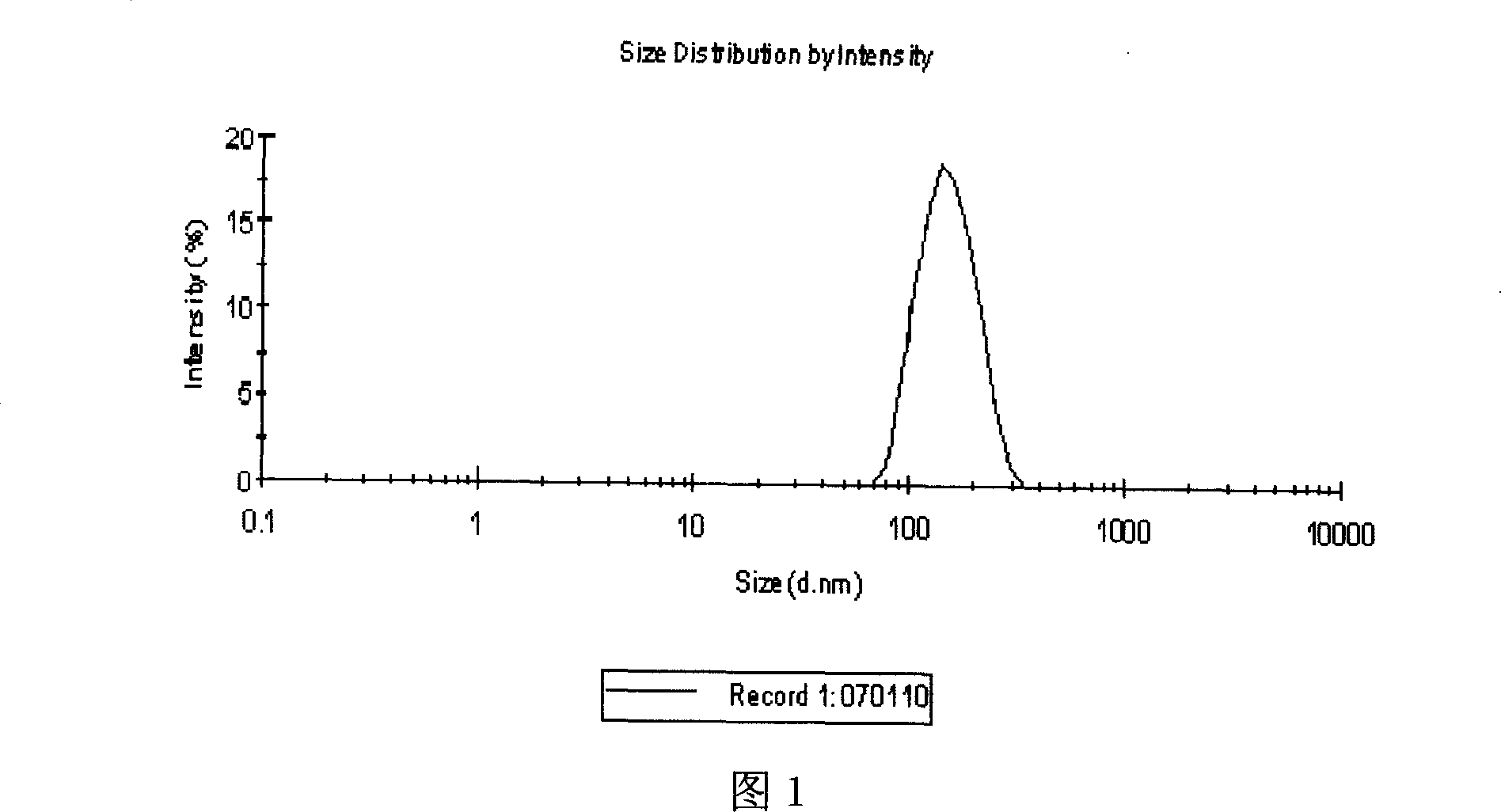
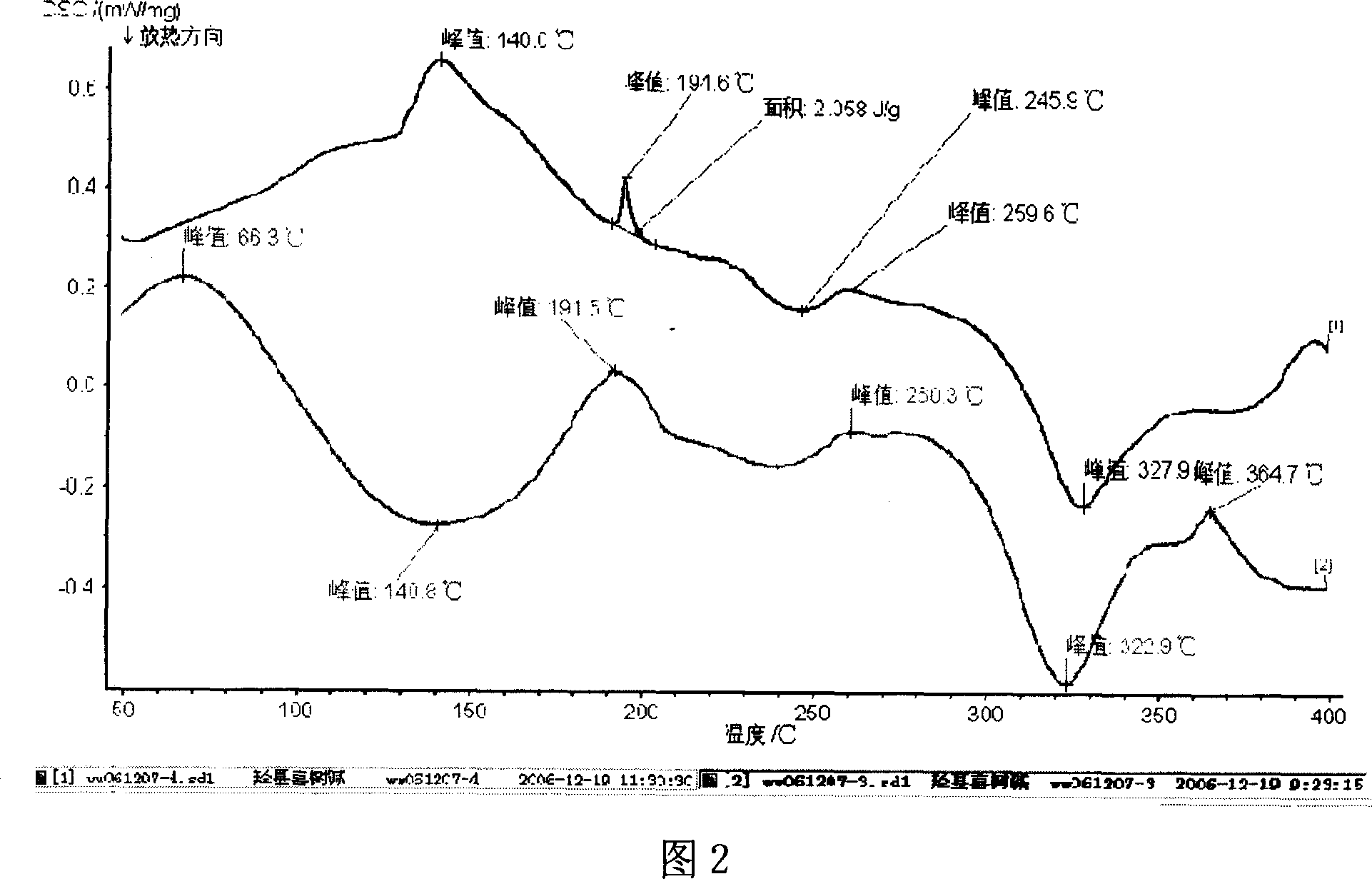
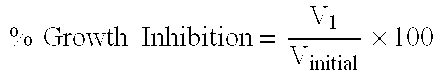Patents
Literature
265 results about "Camptothecin derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
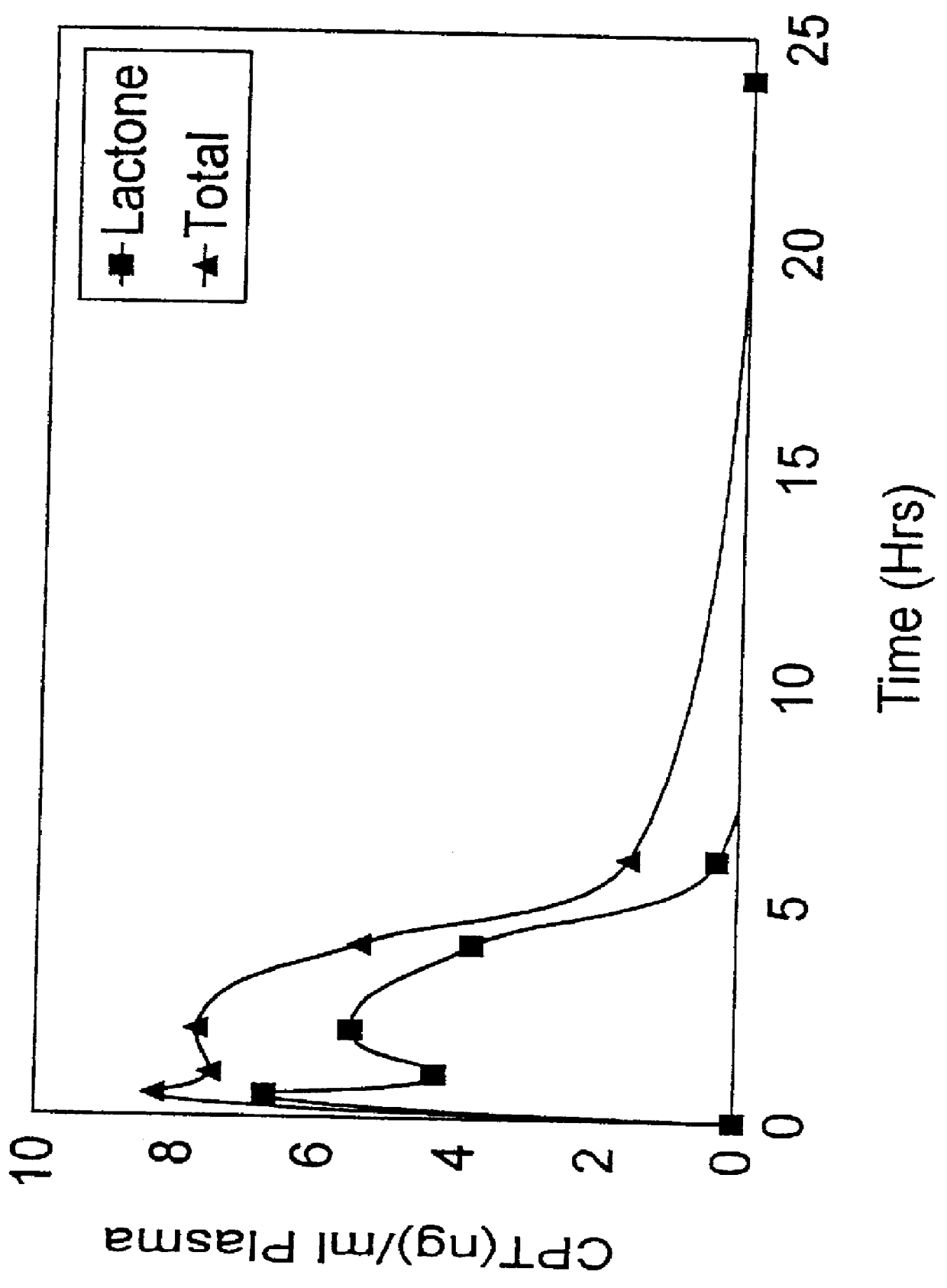
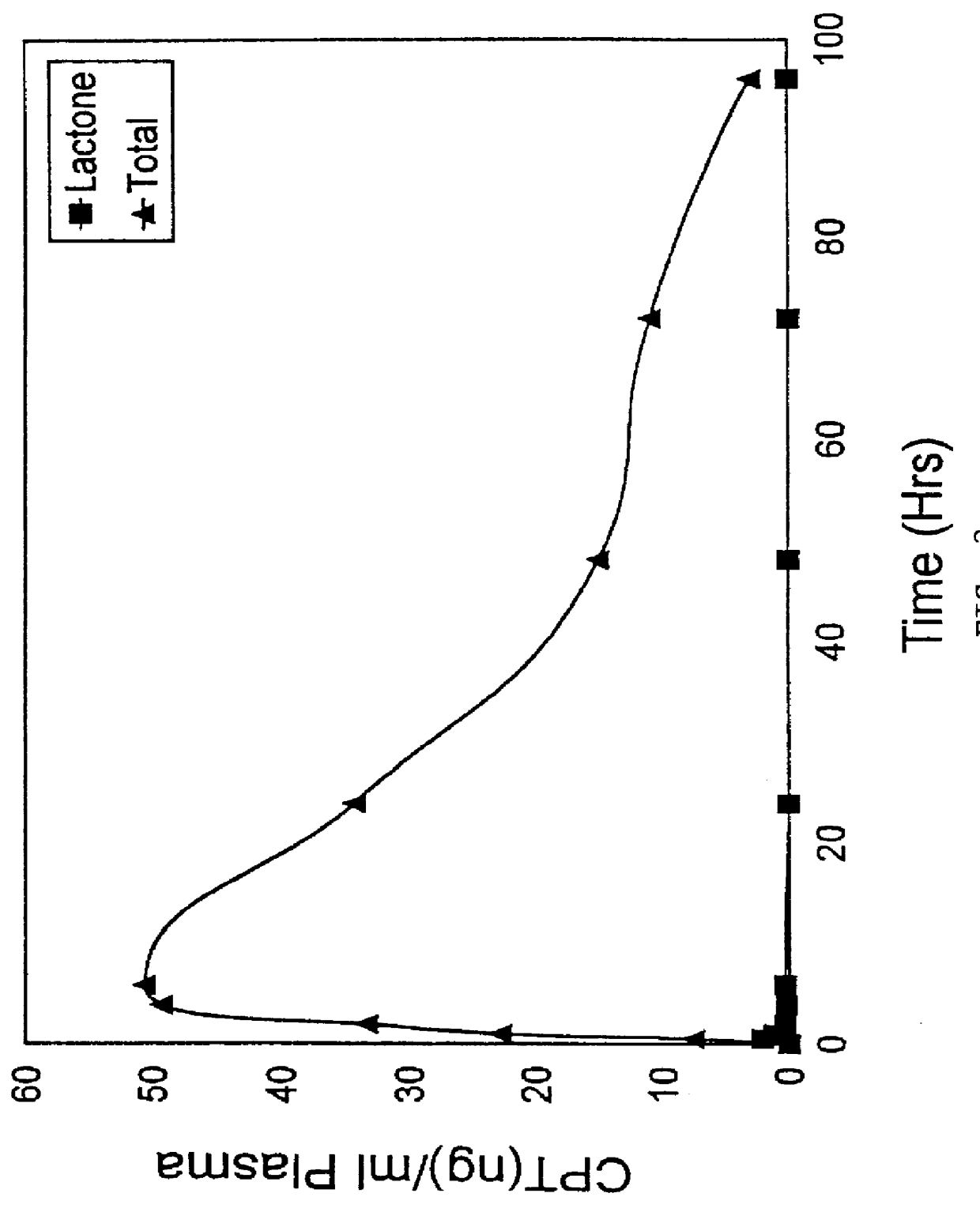
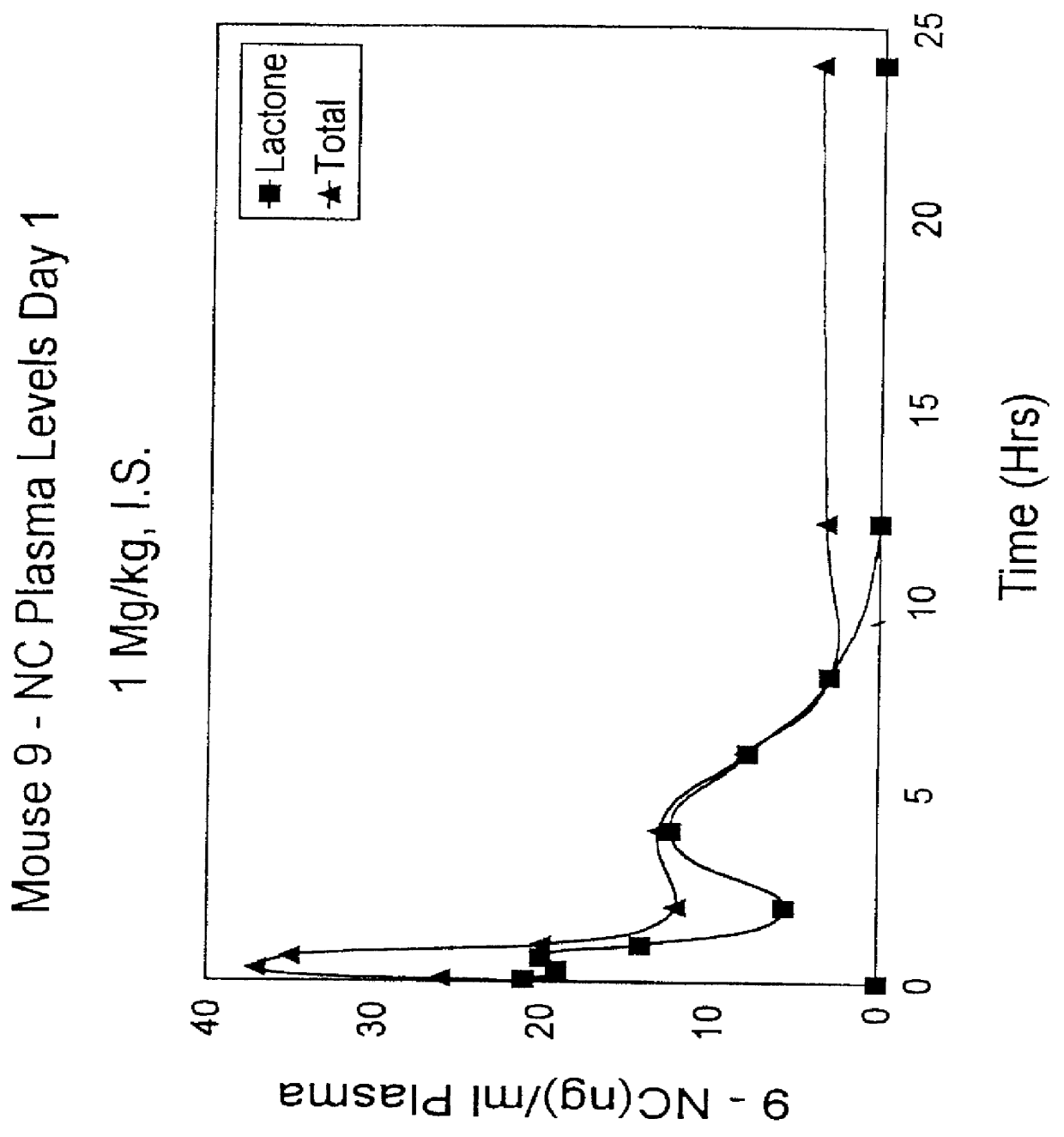
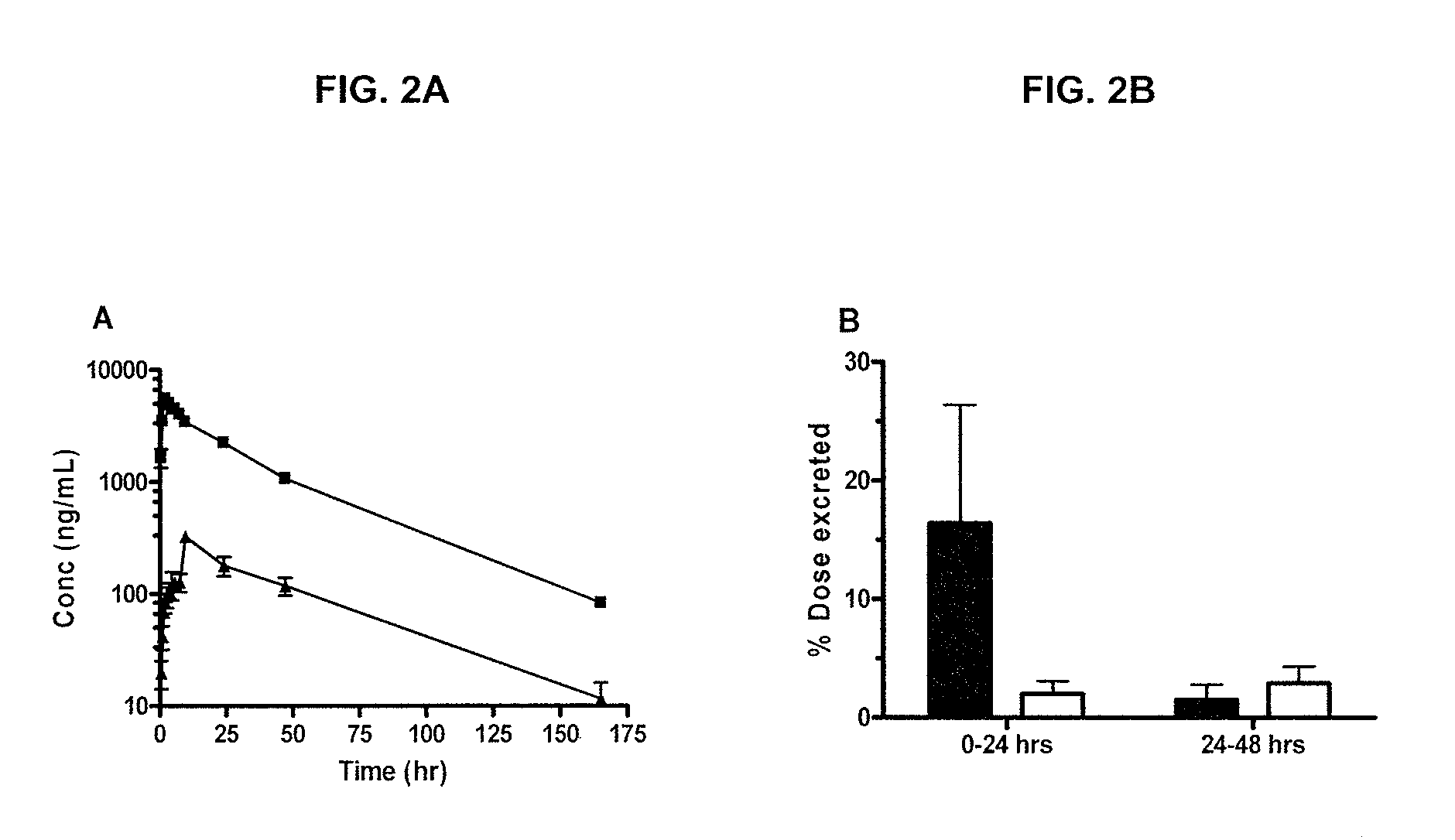
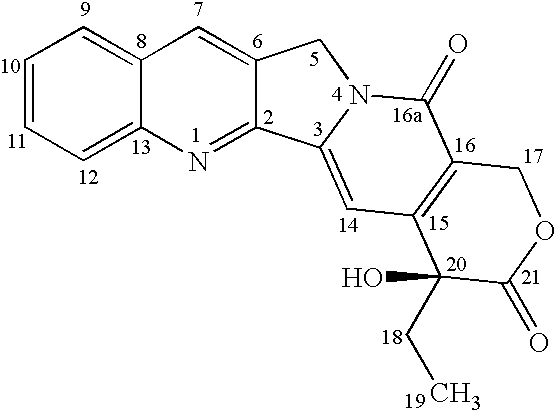
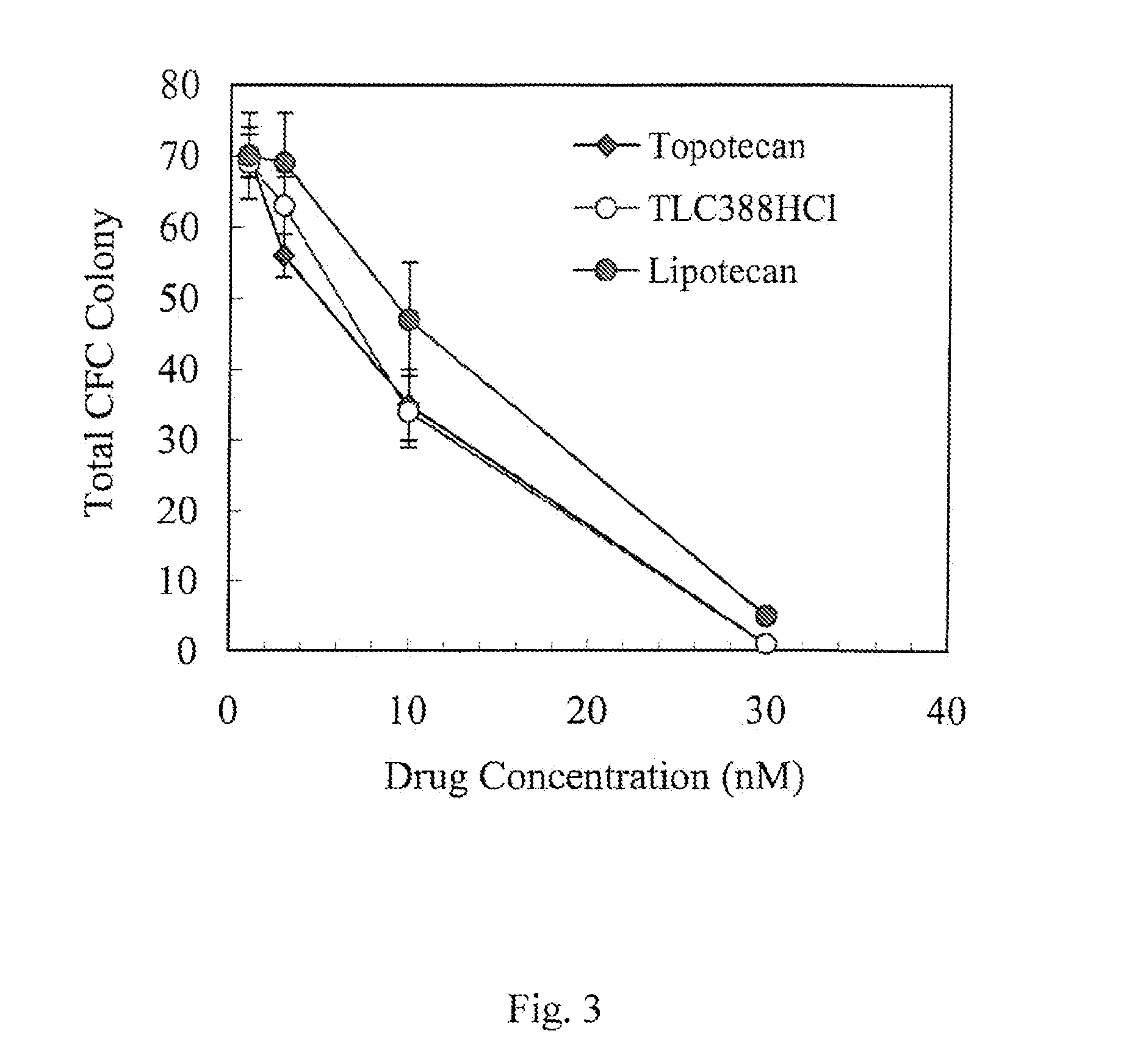
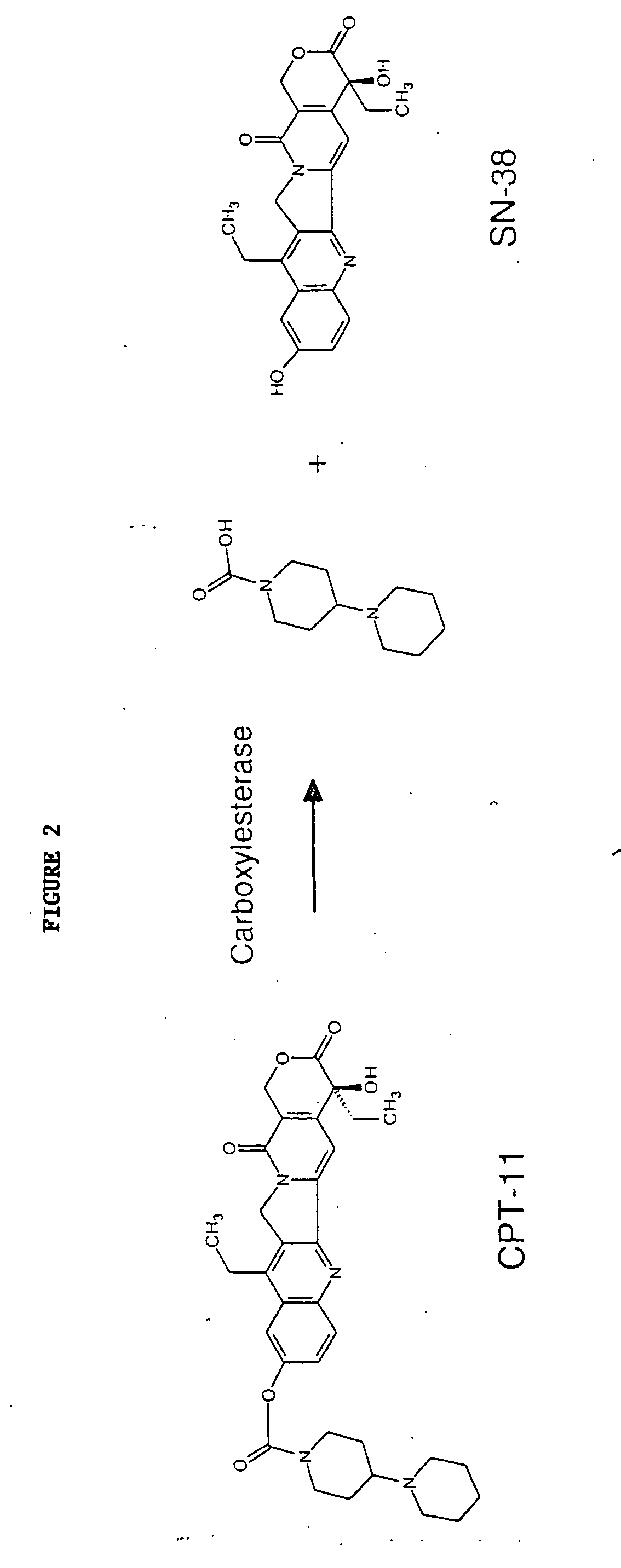
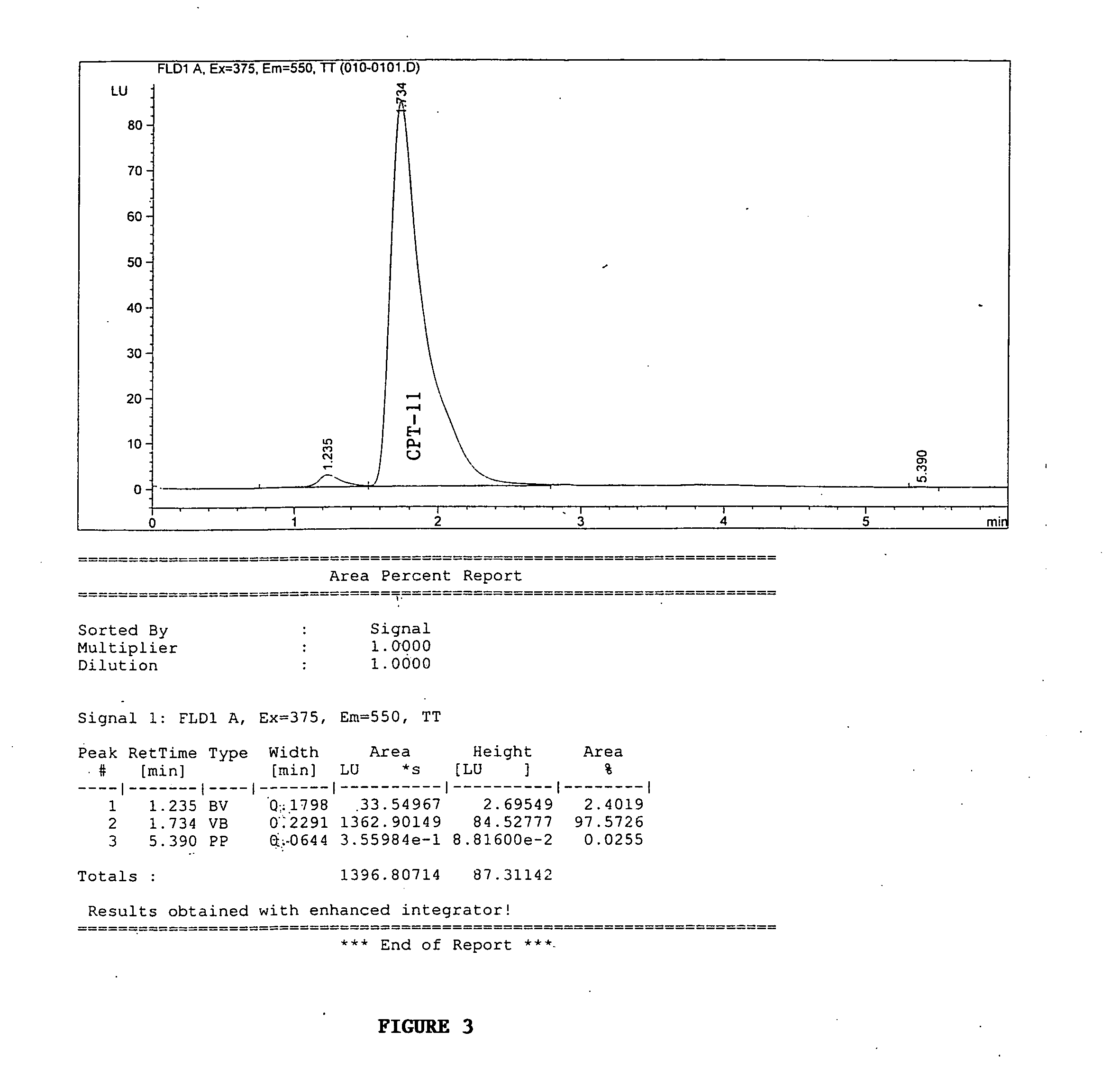
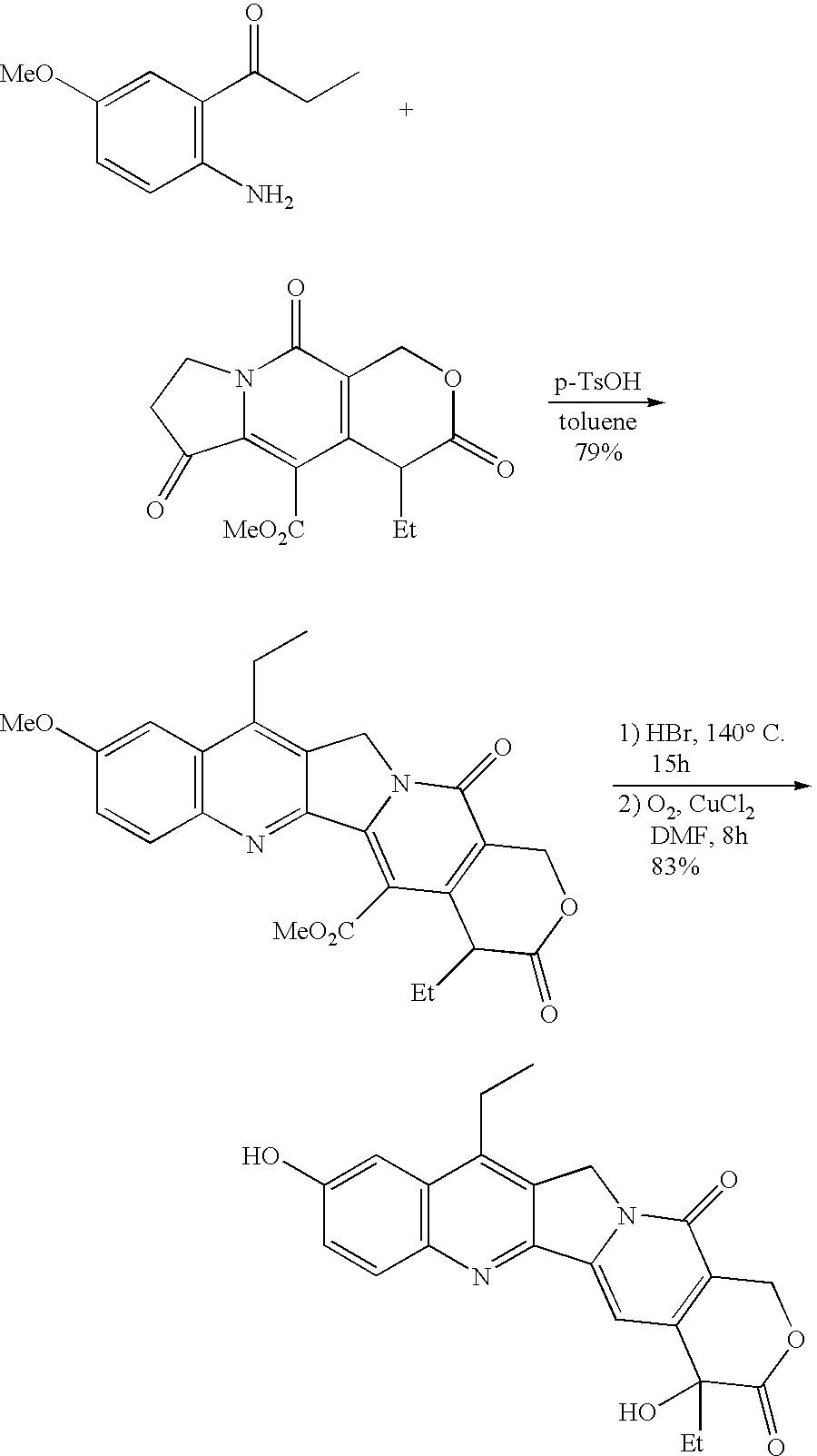
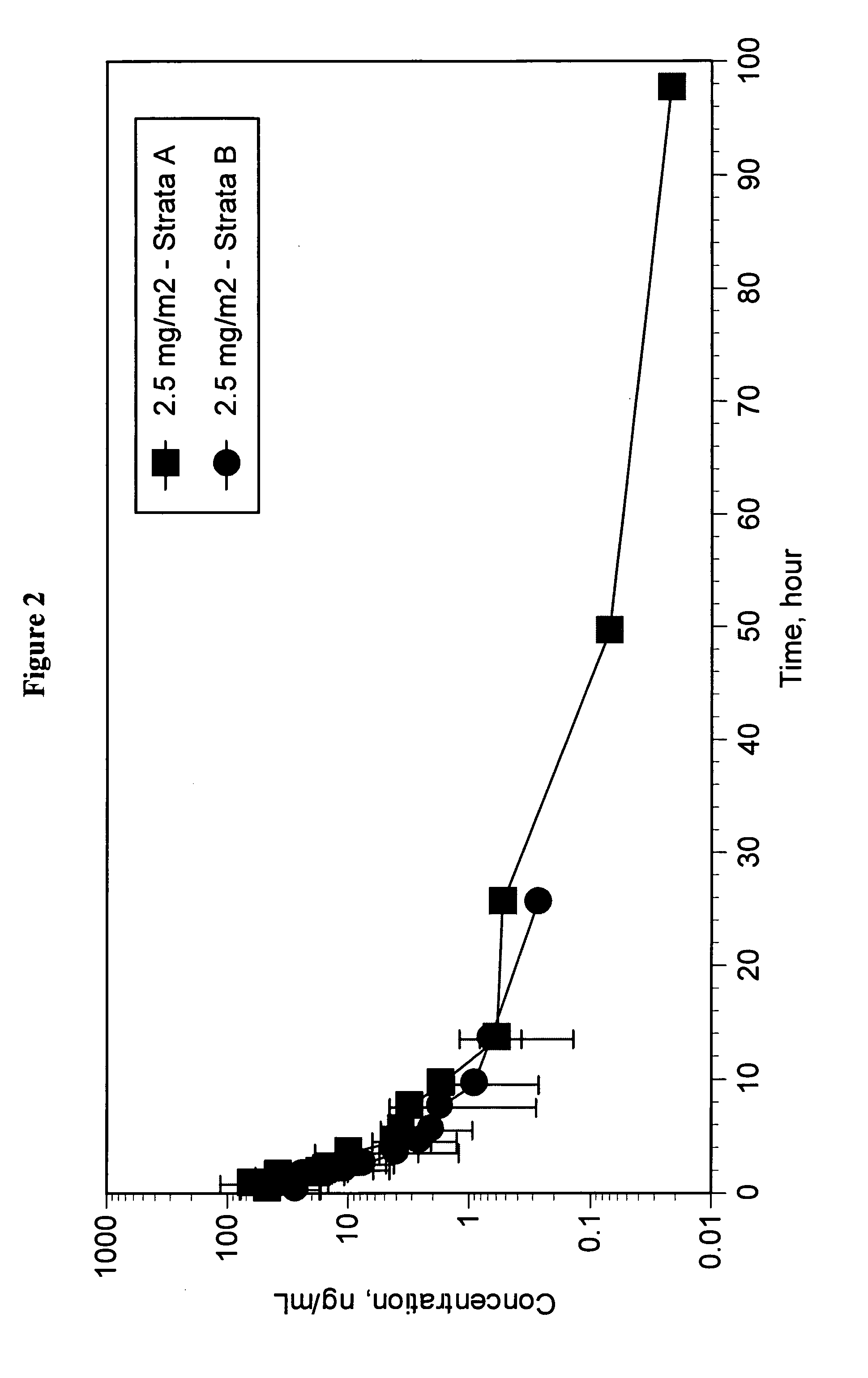
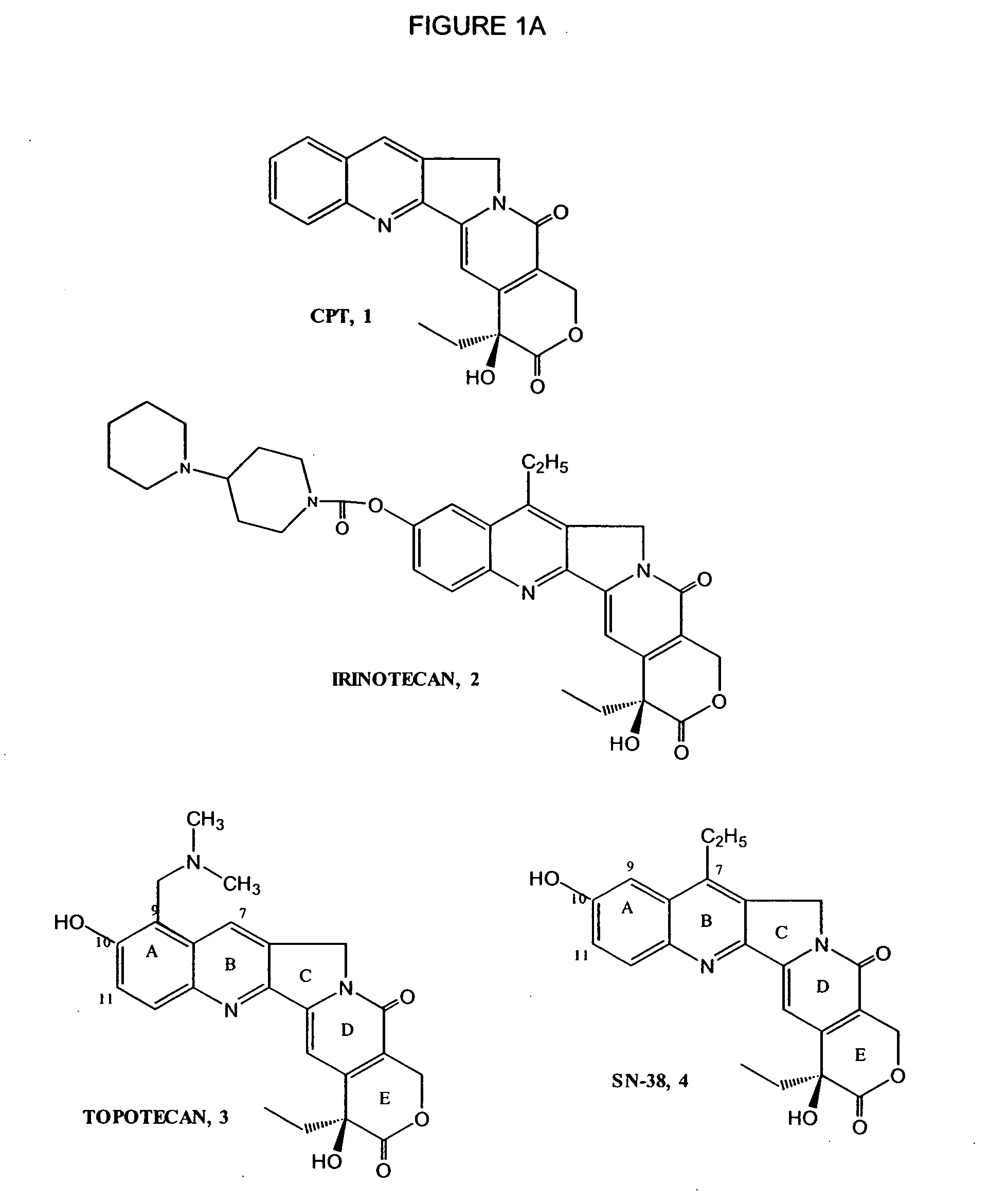
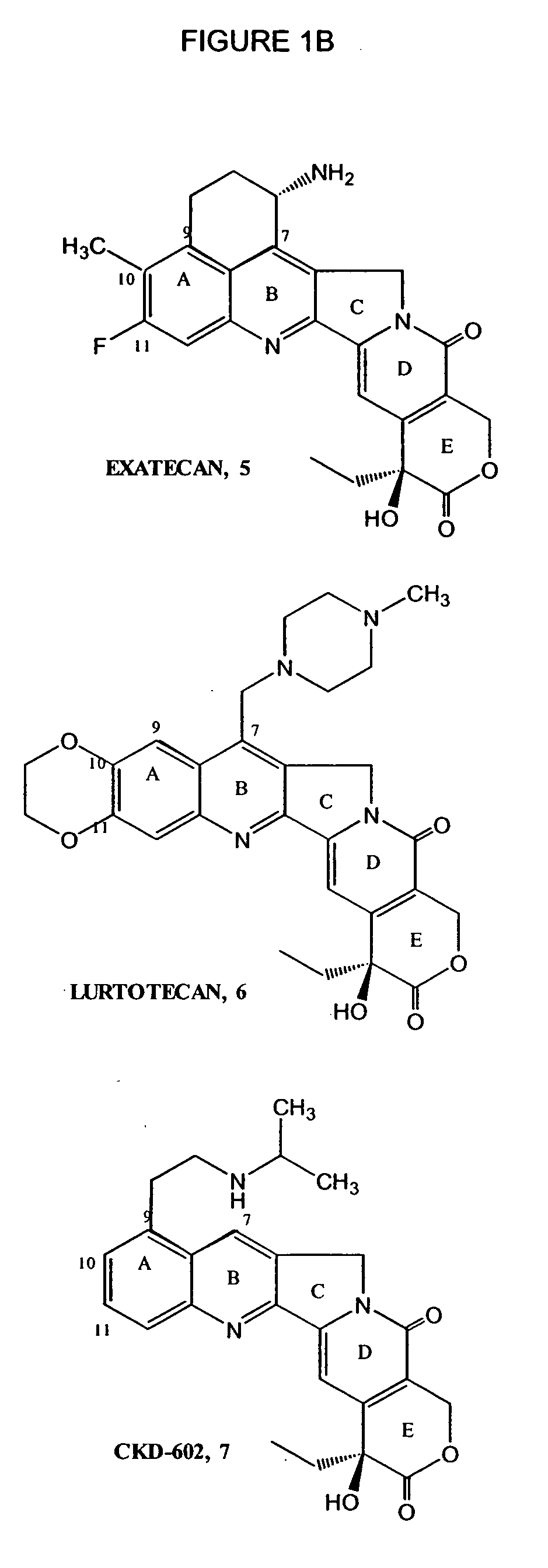
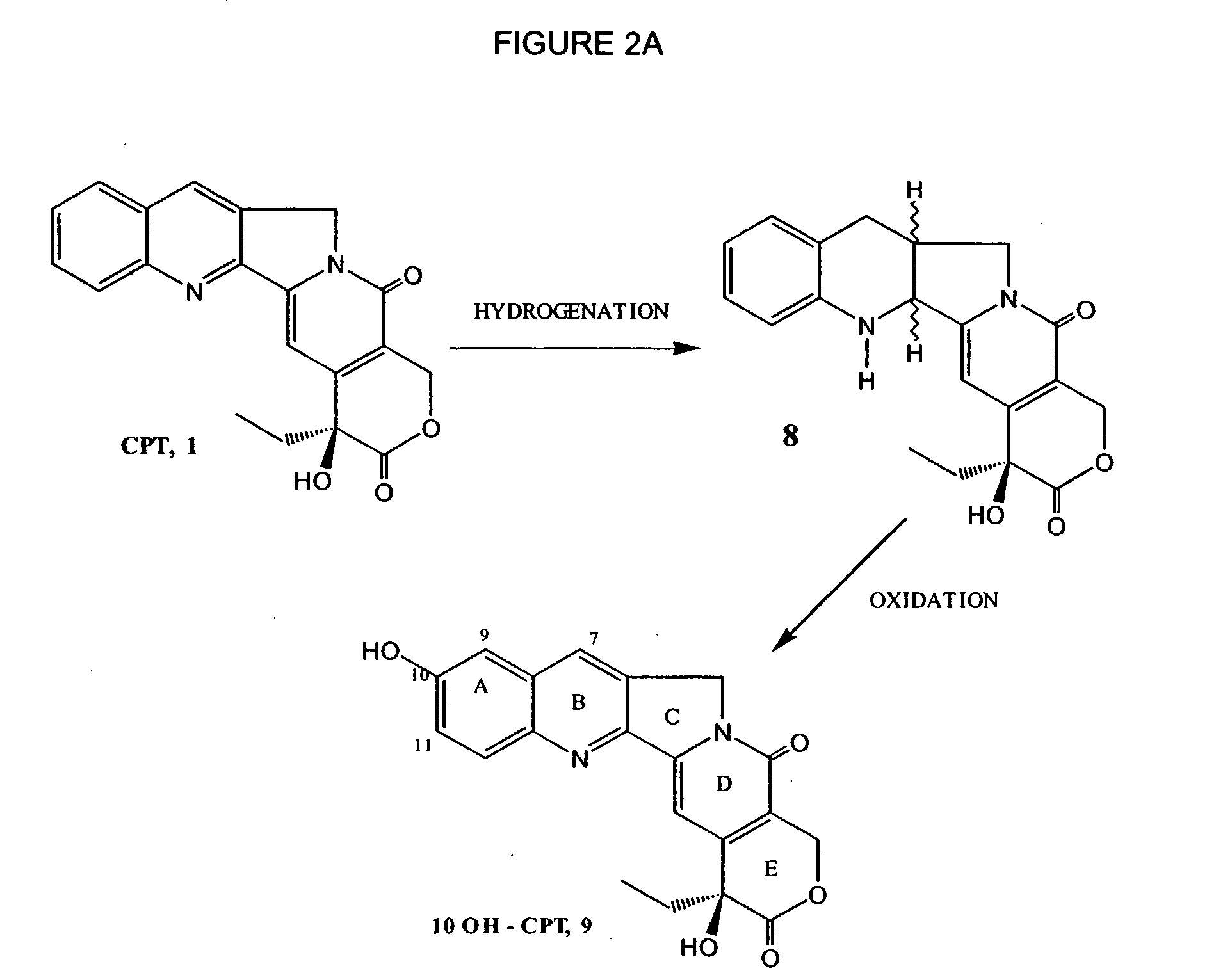
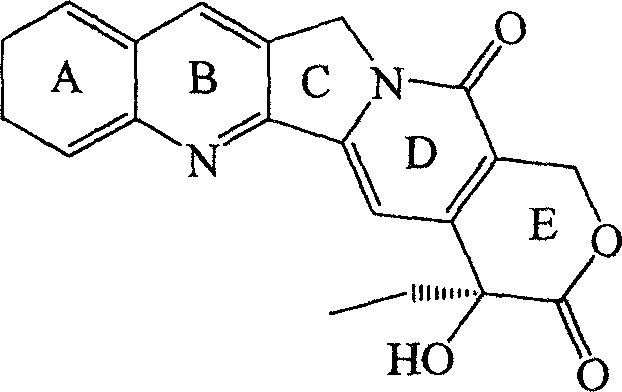
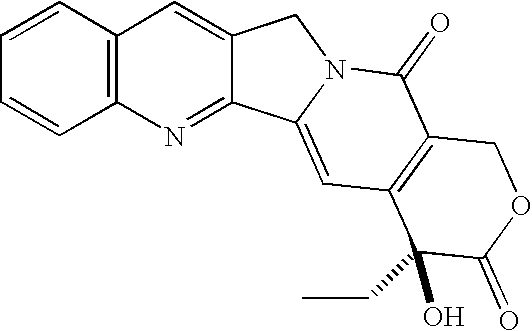
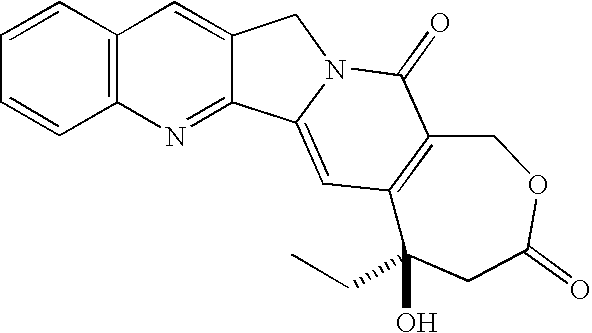
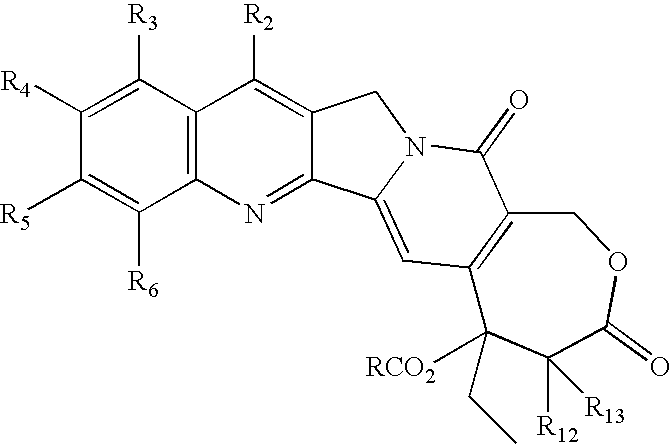
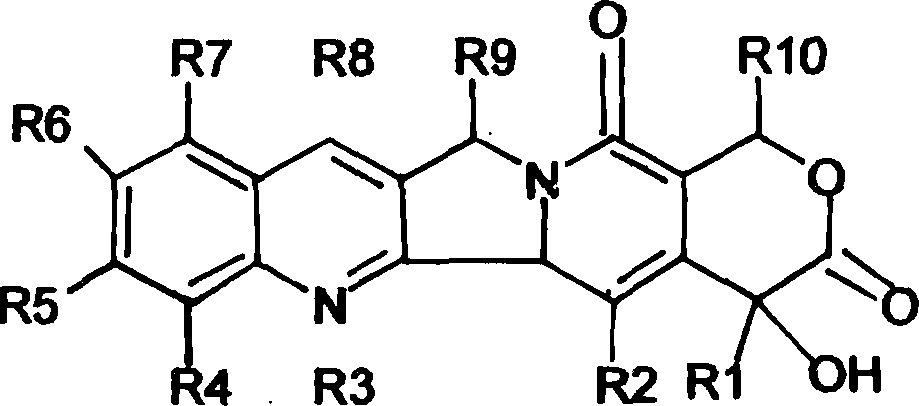
Camptothecin derivatives. Camptothecin is the active agent derived from the bark extract of the Camptotheca acuminata tree. Two analogs of camptothecin have been developed that are clinically active and less toxic than the parent compound: irinotecan (CPT-11) and topotecan (see Figs.
Liposomal prodrugs comprising derivatives of camptothecin and methods of treating cancer using these prodrugs
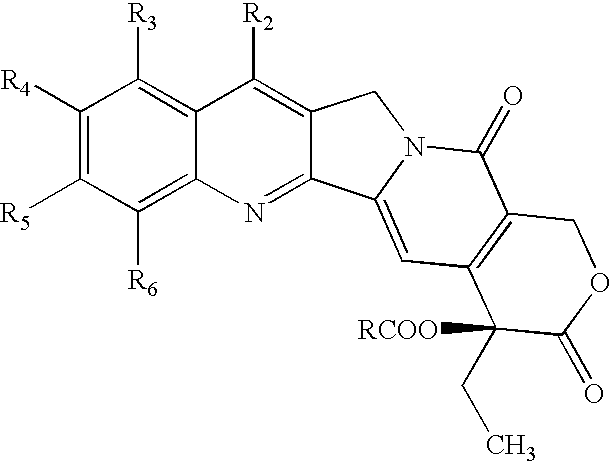
Liposomal prodrugs include specific derivatives of camptothecin restrained by a liposomal delivery system. The derivatives of camptothecin are represented by the general formula: ##STR1## wherein when R.sub.2 is H, R.sub.1 is a C.sub.2 -C.sub.4 alkyl group, a C.sub.6 -C.sub.15 alkyl group, a C.sub.3 -C.sub.8 cycloalkyl group, a C.sub.2 -C.sub.15 alkenyl group or a C.sub.2 -C.sub.15 epoxy group; and when R.sub.2 is a nitro group, R.sub.1 is a C.sub.1 -C.sub.15 alkyl group, a C.sub.2 -C.sub.15 alkenyl group, a C.sub.3 -C.sub.8 cycloalkyl group, or an epoxy group. Processes for making these prodrugs and for using them in cancer treatment are also disclosed.
Owner:THE STEHLIN FOUND FOR CANCER RES
Method for treating abnormal cell growth
InactiveUS20050272755A1Ease of detectabilityEasy to prepareBiocideAnimal repellantsAbnormal tissue growthMetabolite
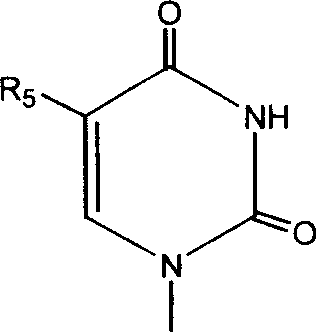
The present Invention relates to a method of treating abnormal cell growth in a subject, comprising administering to said subject having abnormal cell growth: (a) a compound selected from the group consisting of a camptothecin, a camptothecin derivative, or a pharmaceutically acceptable salt, solvate or prodrug of said compounds; (b) a pyrimidine derivative or a pharmaceutically acceptable salt, solvate or prodrug of said pyrimidine derivative; and (c) an anti-tumor agent selected from the group consisting of antiproliferative agents, kinase inhibitors, angiogenesis inhibitors, growth factor inhibitors, cox-I inhibitors, cox-II inhibitors, mitotic inhibitors, alkylating agents, anti-metabolites, intercalating antibiotics, growth factor inhibitors, radiation, cell cycle inhibitors, enzymes, topoisomerase inhibitors, biological response modifiers, antibodies, cytotoxics, anti-hormones, anti-androgens and combinations thereof.
Owner:PFIZER INC
Treatment of cancer
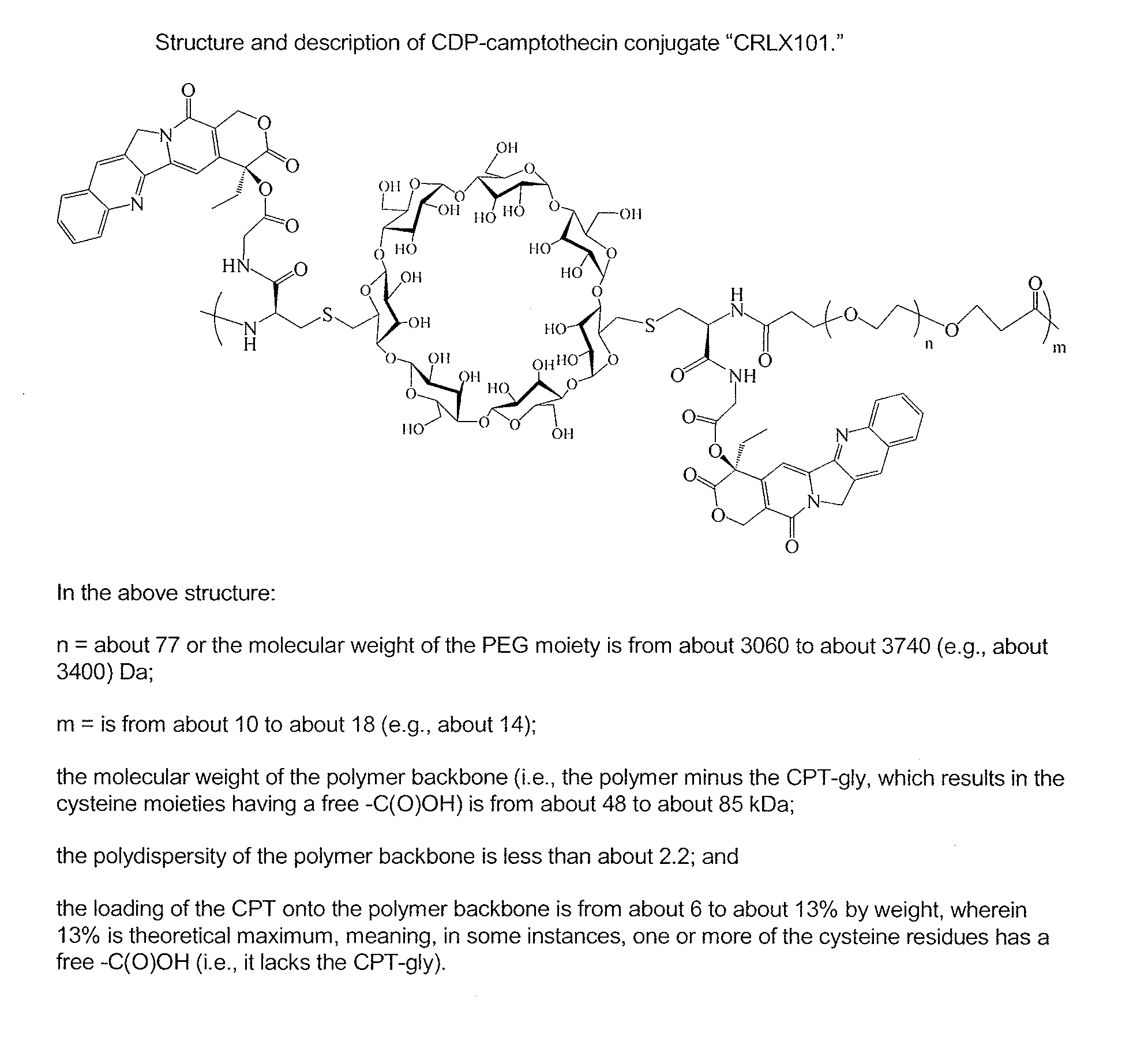
InactiveUS20110160159A1Efficient productionSufficient abilityBiocideOrganic active ingredientsMedicineCRLX101
Provided are methods relating to compositions that include a CDP-topoisomerase inhibitor, e.g., a CDP-camptothecin or camptothecin derivative conjugate, e.g., CRLX101.
Owner:CERULEAN PHARMA +1
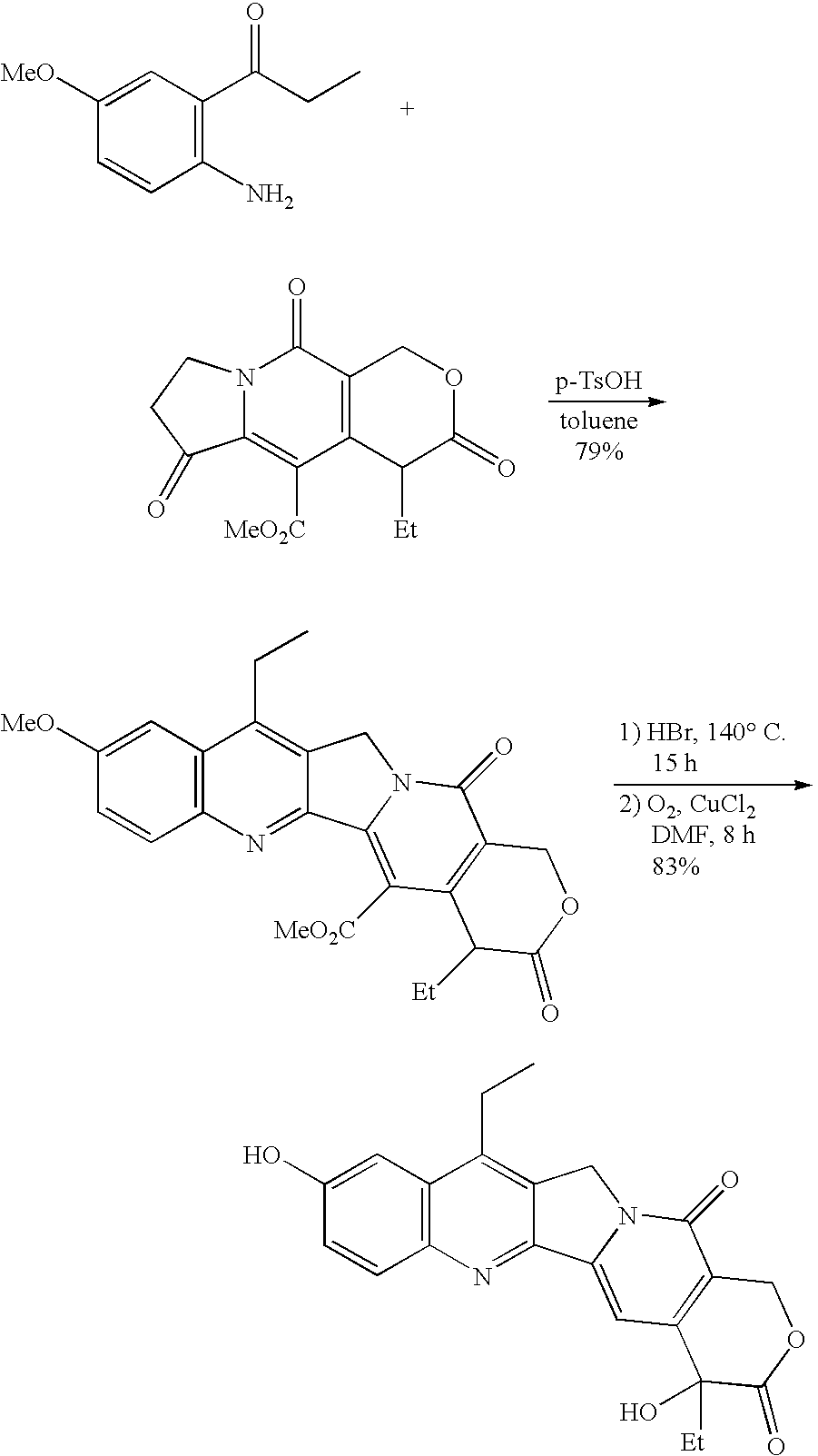
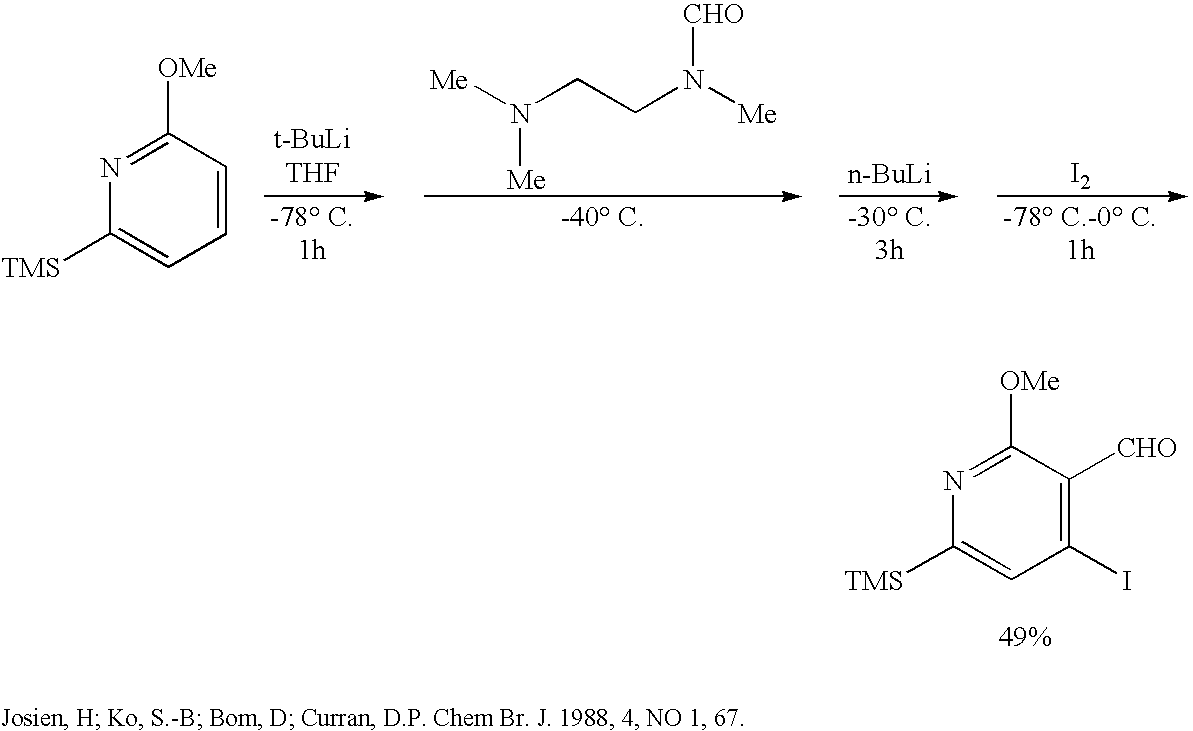
Method of synthesizing camptothecin-relating compounds
The present invention is to prepare efficiently 2′-amino-5′-hydroxypropiophenone corresponding to the AB-ring part of camptothecin (CPT) skeleton and a tricyclic ketone corresponding to the CDE-ring part in order to provide efficiently CPT by the total synthesis, which is a starting material for irinotecan hydrochloride and various kinds of camptothecin derivatives, and to provide stably CPT and its derivatives.
Owner:YAKULT HONSHA KK
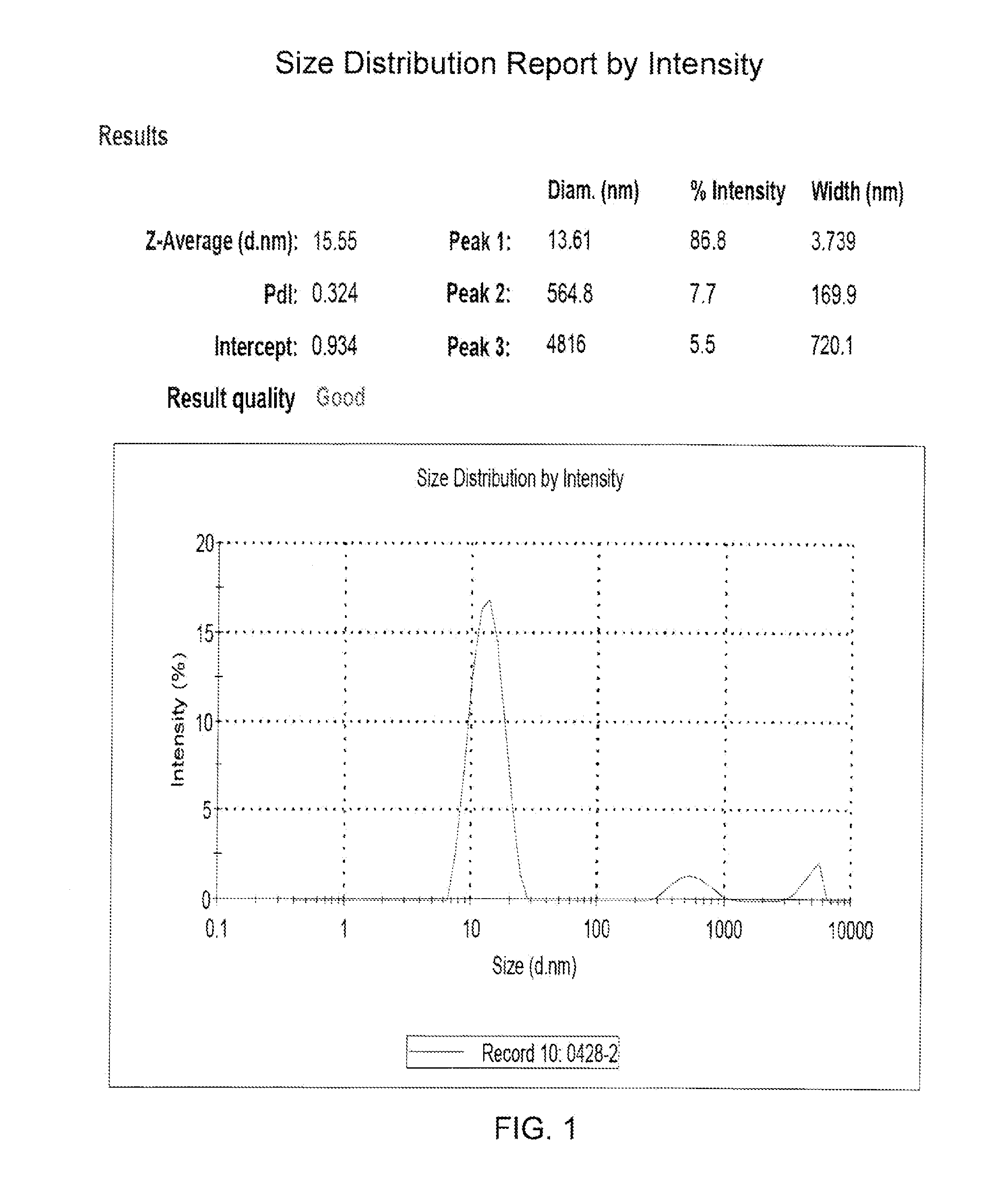
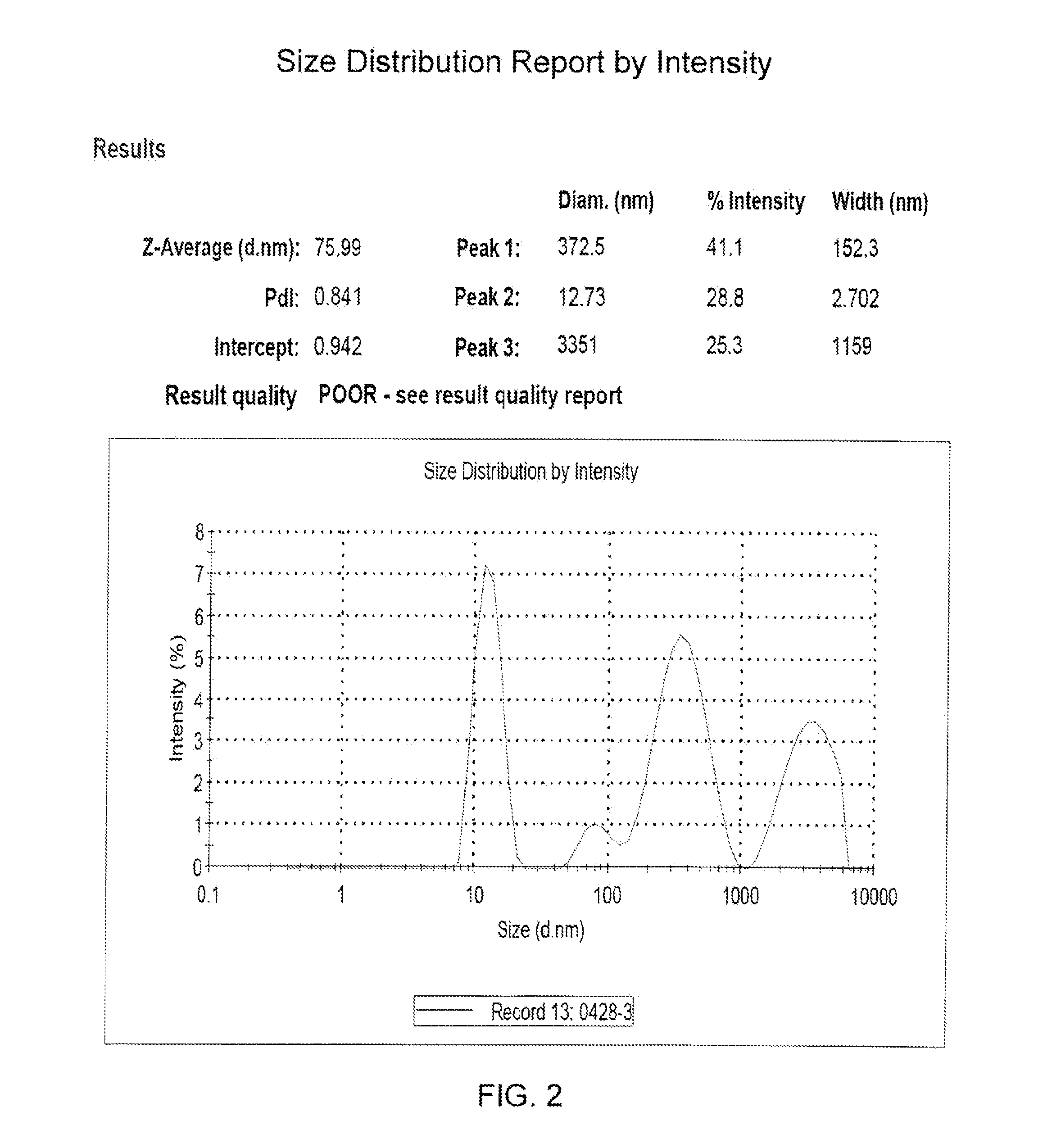
Submicron nanoparticle of poorly water soluble camptothecin derivatives and process for preparation thereof
InactiveUS20100008998A1Efficient productionExcellent lactone stabilityBiocidePowder deliveryPolymer scienceNanoparticle
The present invention relates to a nanoparticle composition comprising a camptothecin derivative, solid polyethyleneglycol and an anti-associative agent, and the process for preparing the same. Specifically, the present invention provides a composition comprising a nanoparticle of the camptothecin derivative, which is prepared by solid-dispersing the poorly water soluble camptothecin derivative in polyethyleneglycol and dissolving the solid dispersions in an aqueous solution containing an anti-associative agent. The composition of the present invention stabilizes the camptothecin derivative lactone form in body fluid for effective anticancer activity.
Owner:SAMYANG BIOPHARMLS CORP
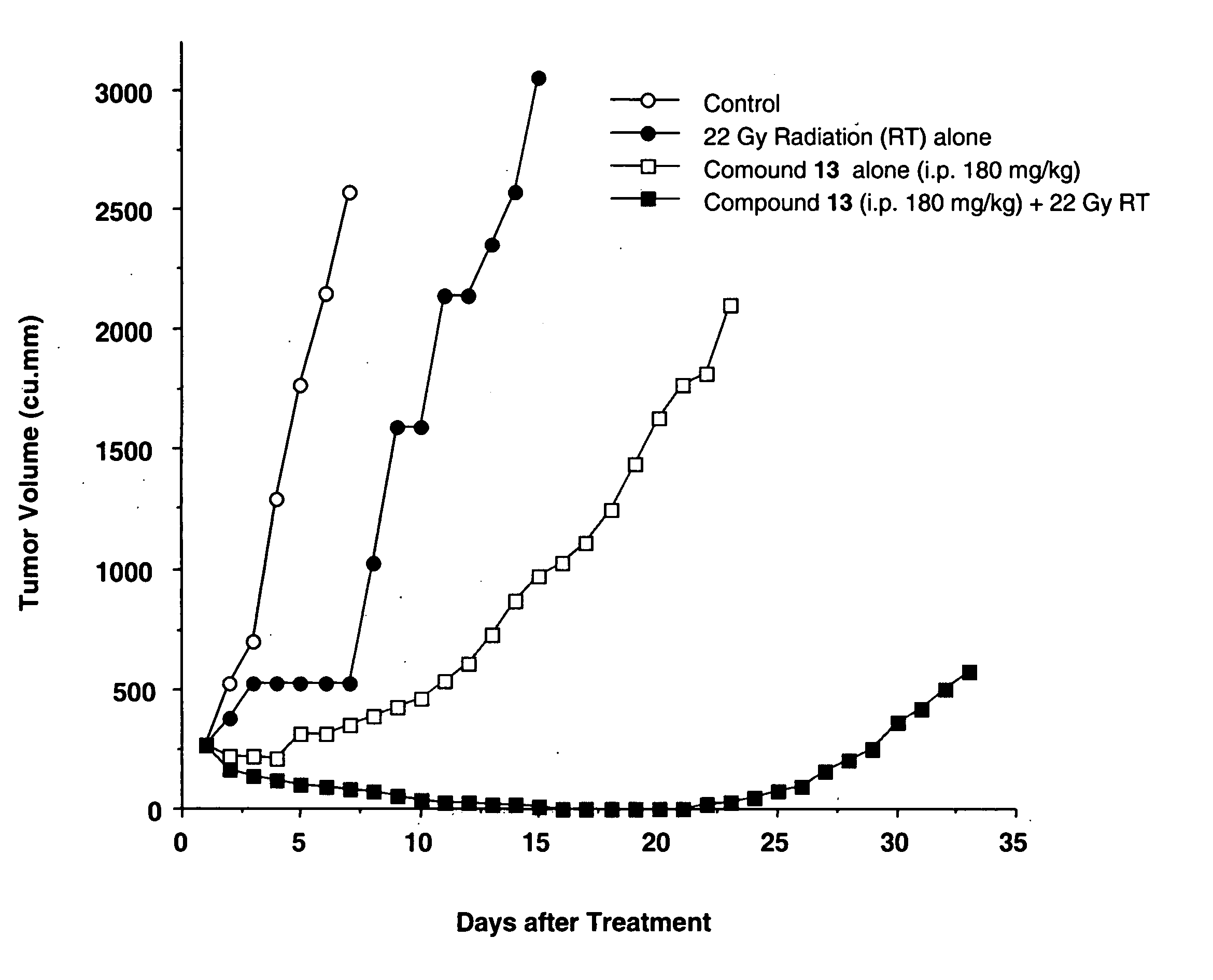
Camptothecin derivatives as chemoradiosensitizing agents
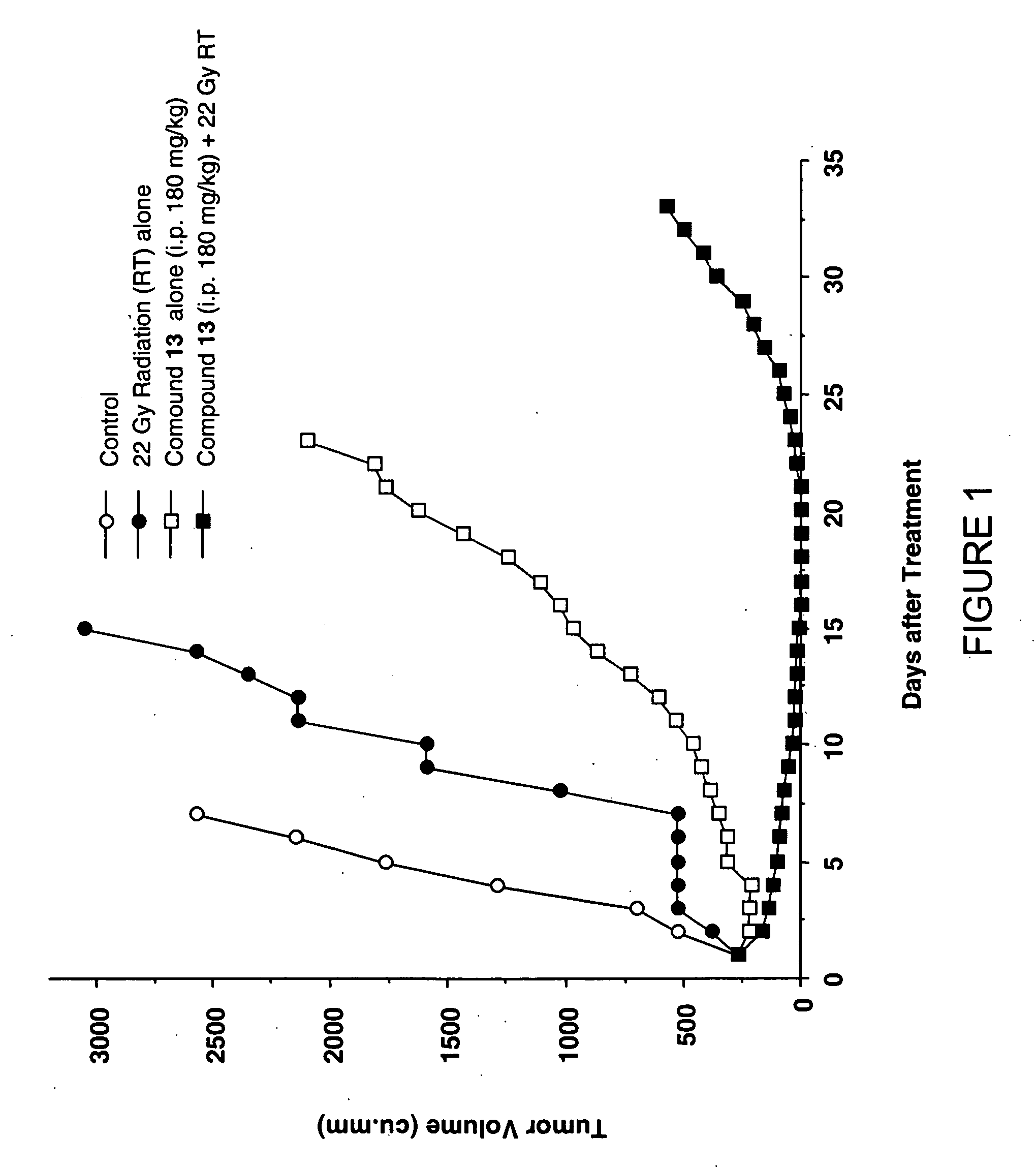
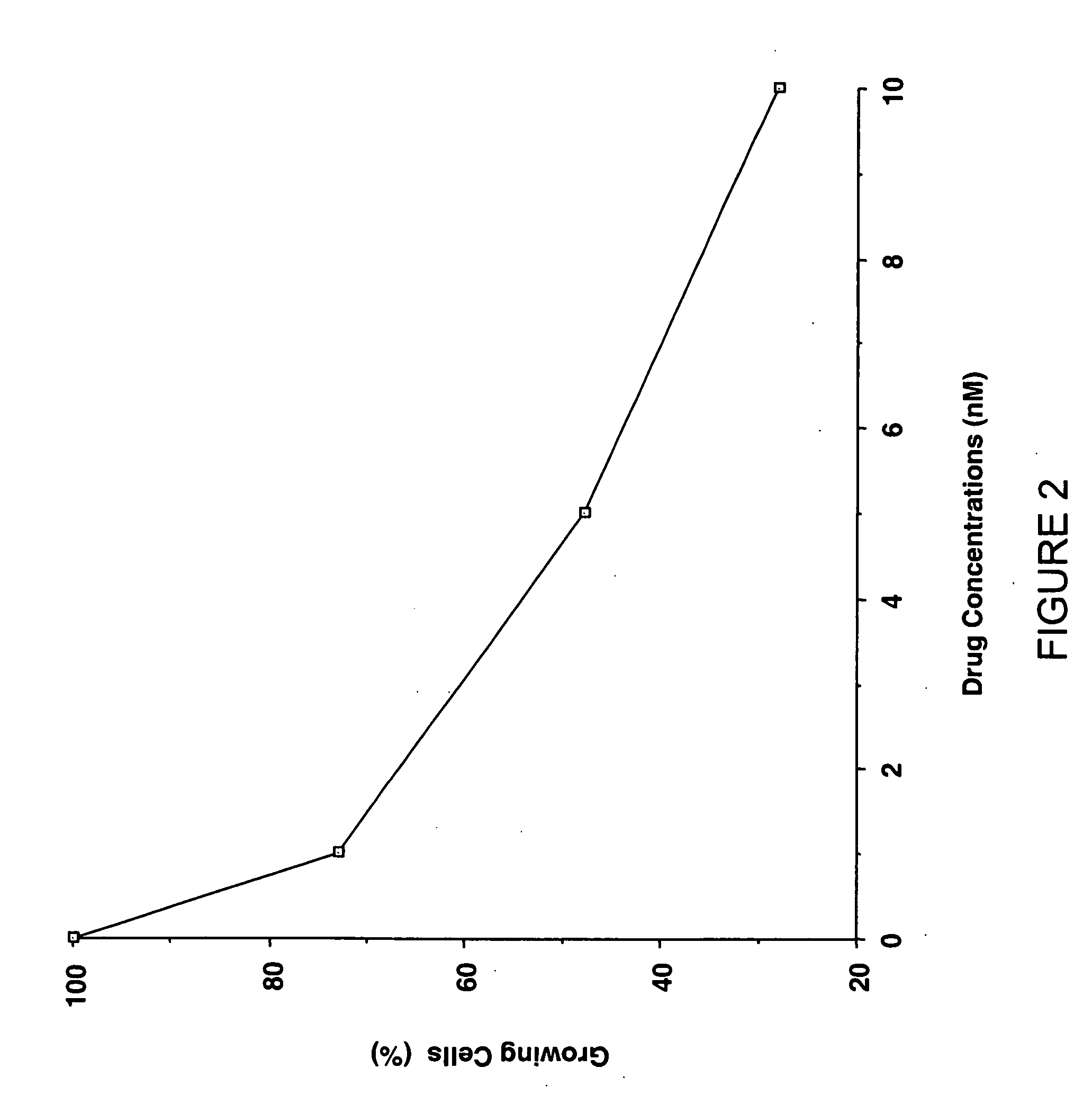
Camptothecin-based compounds are useful for treating a neoplasm in mammalian subjects by administering such compound to the subjects in combination with radiotherapy, i.e., the treatment of tumors with radioactive substances or radiation from a source external to the subject. Camptothecin-based compounds are modified by positioning at least one electron-affinic group around the camptothecin structure to enhance their value in combination with radiotherapy. New Camptothecin-based compounds are disclosed that are useful for treating cancer by administering the novel compounds alone or in combination with radiotherapy.
Owner:SUTTER WEST BAY HOSPITALS +1
Pharmaceutical compositions containing dds compounds
InactiveUS20030148931A1Simple contentEnsure storage stabilityBiocidePowder deliveryCompound aAlcohol sugars
A pharmaceutical composition having an ensured preservation stability, which contains a compound, wherein a polysaccharide derivative having a carboxyl group is bonded to a camptothecin derivative via a spacer or without mediated by any spacer, and a sugar or a sugar alcohol optionally together with a pH-adjusting substance.
Owner:DAIICHI PHARMA CO LTD
20-bit esterified camptothecine derivate, its preparation method and drug composite and use
InactiveCN1955183AOrganic active ingredientsOrganic chemistryCamptothecin derivativeAnti-Tumor Drugs
This invention discloses a new camptothecin derivate of 20-position esterification, its preparation, containing their medicine combination, and its usage of using it as medicine, especially as antitumor drug.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
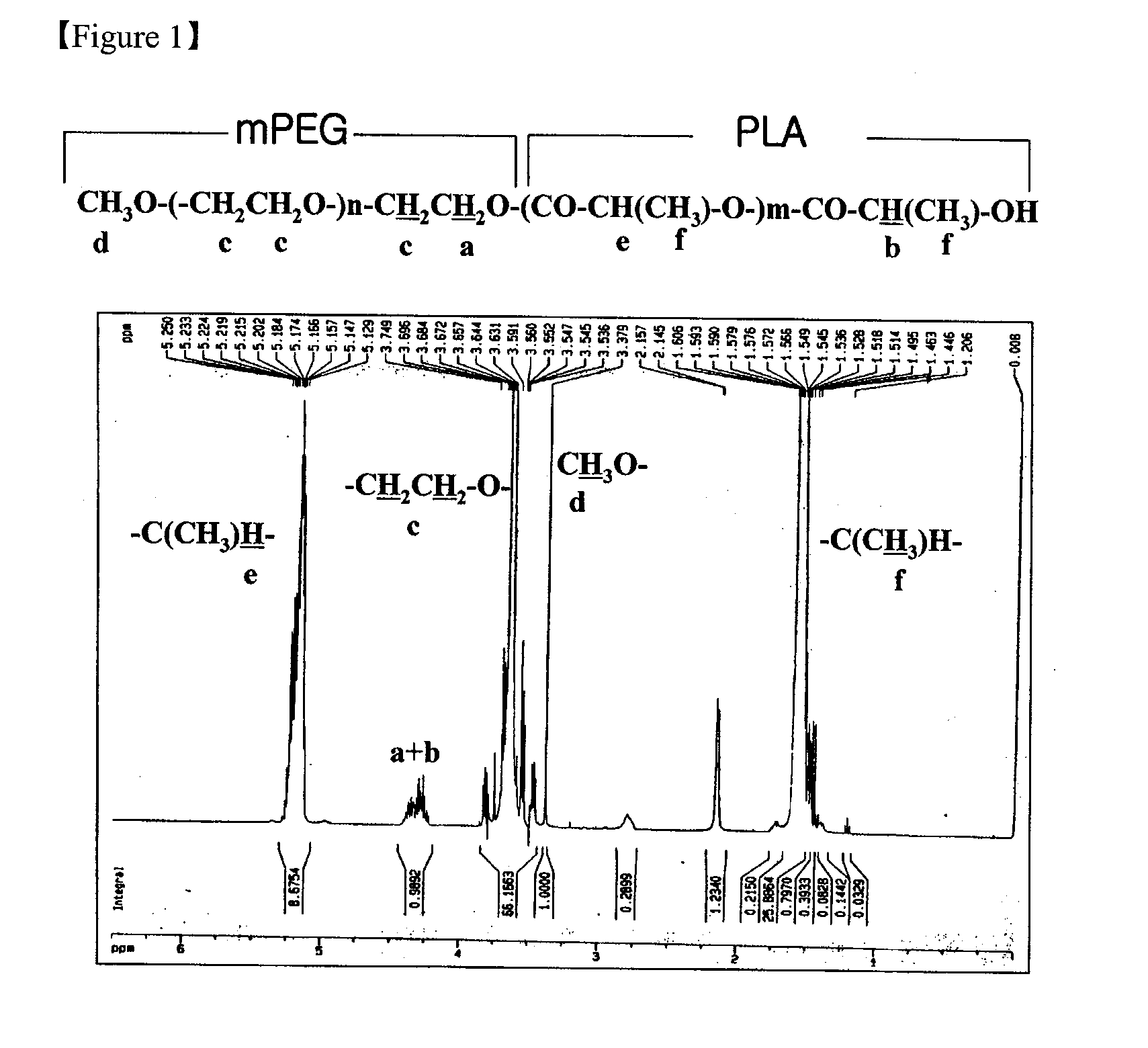
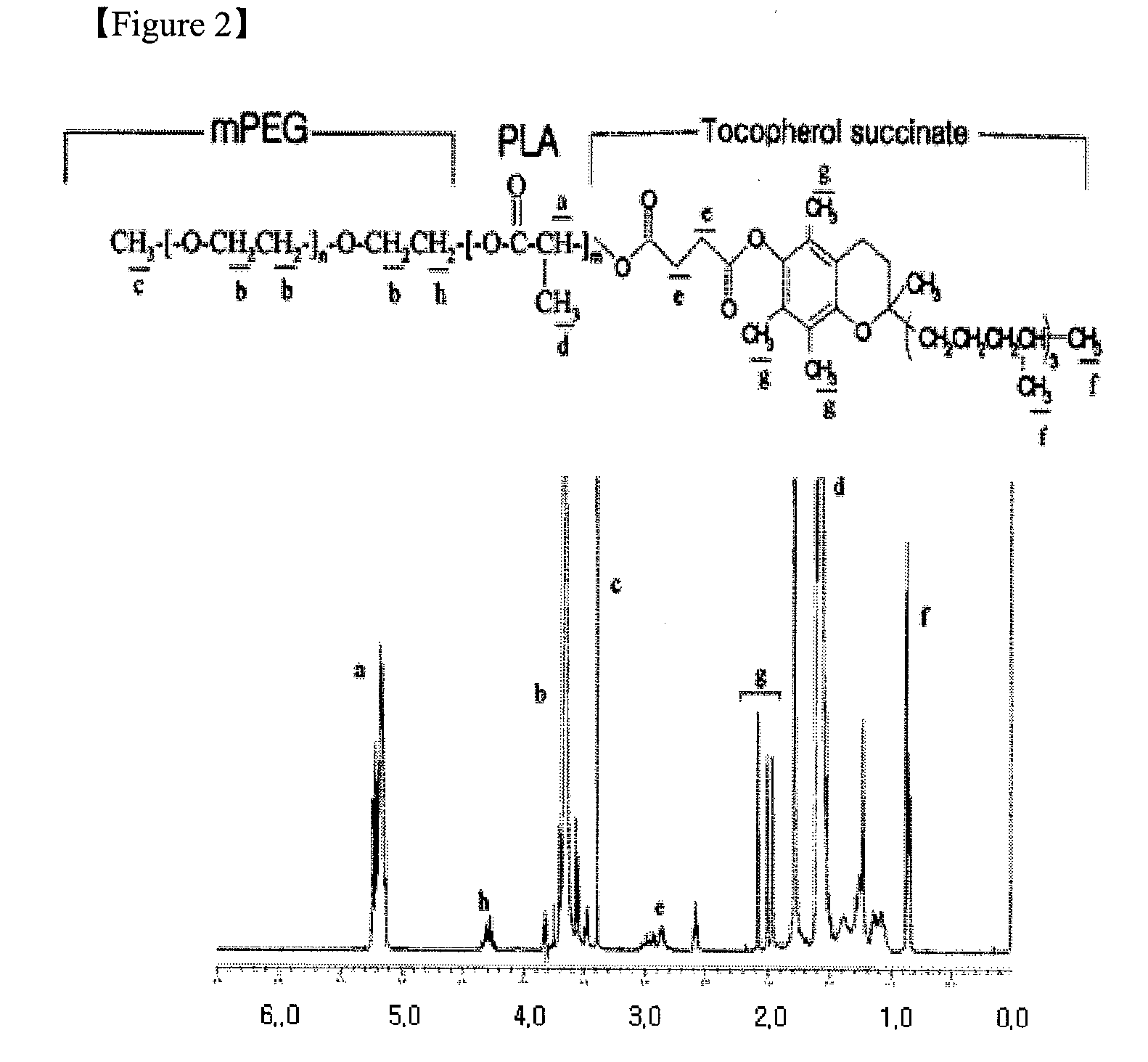
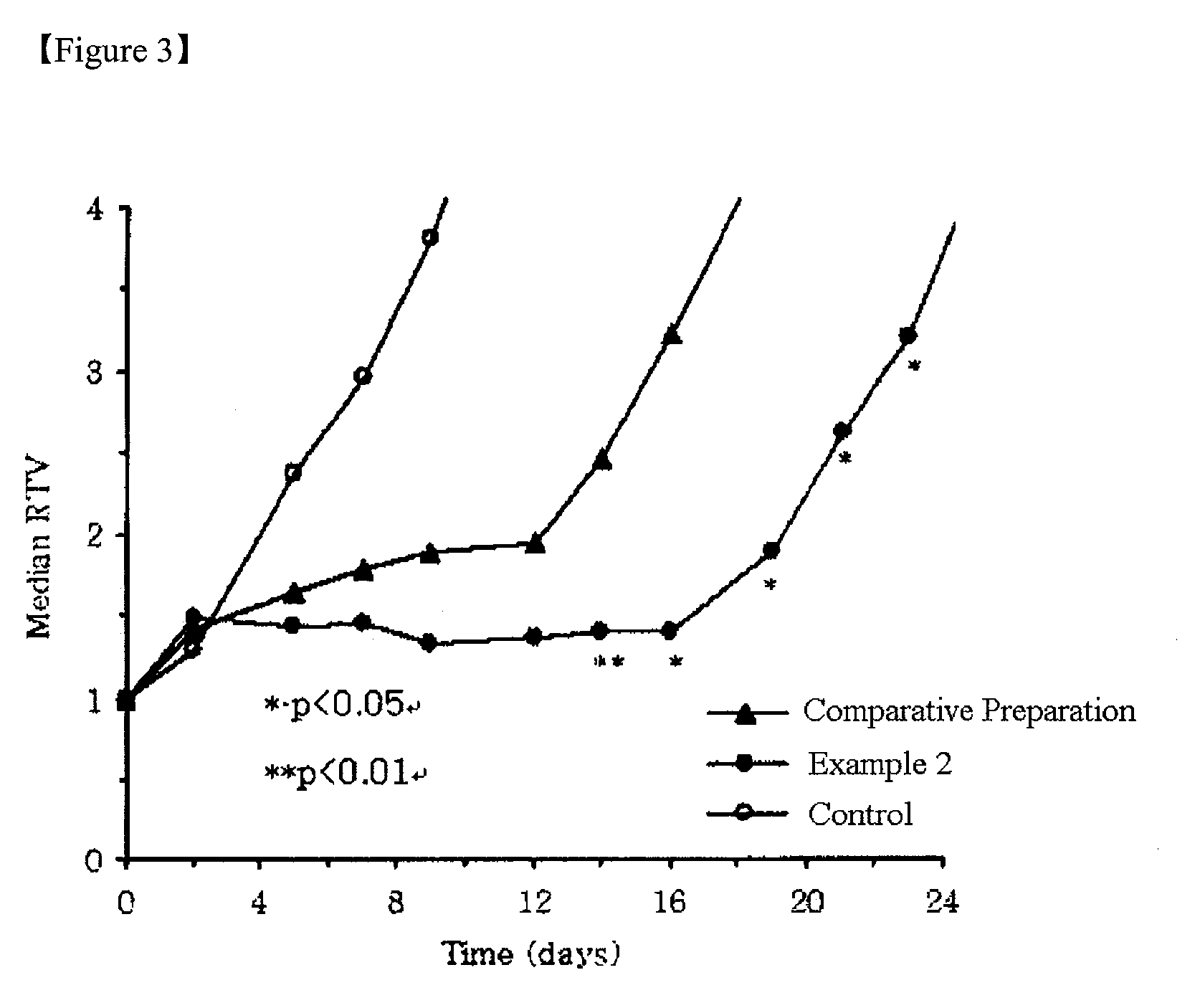
Combination of mPEG-PLA-tree alkali medicament
ActiveCN101199857AOrganic active ingredientsPharmaceutical non-active ingredientsButanedioic acidMedicine
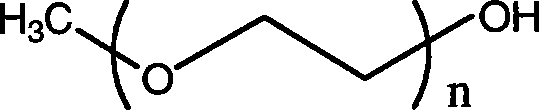
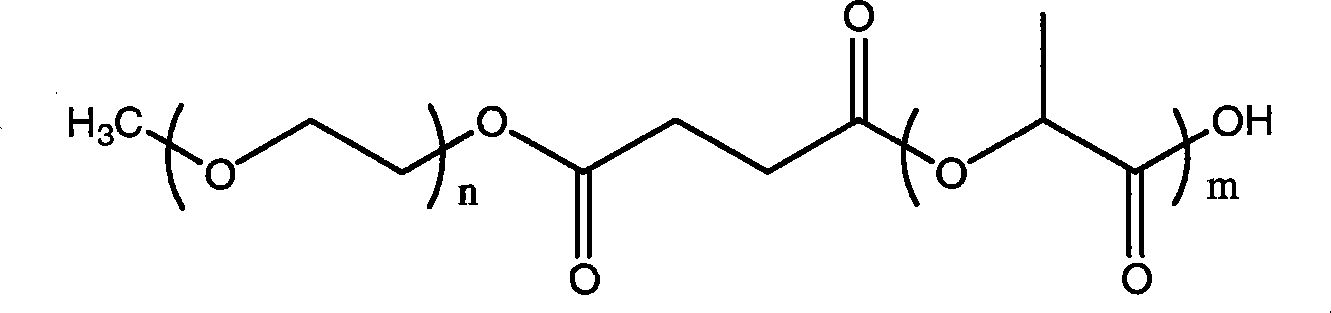
The invention relates to a novel amphiphilic-block-copolymer-based pro-camptothecin drug, which is a compound of methoxy polyethylene glycol- polylactic acid and camptothecin derivatives (mPEG-X-PLA-T), wherein, mPEG refers to methoxy polyethylene glycol; X refers to linking group, for example, succinic acid; PLA refers to polylatic acid; T refers to drug molecule, that is, camptothecin derivatives such as, camptothecin, 10-hydroxycamptothecine, 7-ethyl-10- hydroxycamptothecine. Polylactic acid is connected with camptothecin derivatives through ester bond.
Owner:CHINA PHARM UNIV
Oral formulations for anti-tumor compounds
The present invention relates to a semi-solid filling medium which comprises a camptothecin derivative; a pharmaceutically acceptable carrier matrix which is a polyglycolized glyceride; and an effective thickening-reducing and stabilizing-promoting amount of one or more pharmaceutically acceptable excipients.
Owner:PHARMACIA ITAL SPA
Camptothecin, self-emulsifying medicine precursor of derivative thereof and application thereof
InactiveCN101628919AImprove the delivery effectGood biocompatibilitySugar derivativesGroup 5/15 element organic compoundsAlcoholMatrine
The invention discloses camptothecin and a self-emulsifying medicine precursor of a derivative thereof. The self-emulsifying medicine precursor is prepared by the covalent union of medicine molecules and hydrophilic radicals, wherein the medicine molecules are camptothecin molecules or camptothecin derivative molecules, and the medicine carrying quantity of the precursor is as high as more than 50%. The invention also discloses application of the self-emulsifying medicine precursor. The self-emulsifying medicine precursor can form micelles or vesicles with nano size in water, can be used as a medicine carrier used for loading one or a plurality of other anticancer medicines, such as CPT derivatives, yew alcohol, turmeric essence, methotrexate, irinotecan, danshinolic acid, matrine, doxorubicine and the like, can form a nano medicine carrying a plurality of medicines and can realize the synergic treatment of the medicines.
Owner:ZHEJIANG UNIV
Nitrogen-based camptothecin derivatives
(20S) esters of camptothecin analogs are provided. The compounds are (20S) esters of an aminoalkanoic acid or an imidoalkanoic acid and camptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the camptothecin ring. The compounds are useful for treating cancer.
Owner:SUTTER WEST BAY HOSPITALS +1
Pharmaceutical compositions of hydrophobic camptothecin derivatives
The present invention provides a pharmaceutical composition comprising at least one hydrophobic camptothecin derivative or a pharmaceutically acceptable salt of said derivative and a polyethylene glycol (PEG) conjugated phospholipid. Also provided is a method to inhibit cancer cells in a subject in need thereof by administering the pharmaceutical composition of the present invention.
Owner:TAIWAN LIPOSOME CO LTD +1
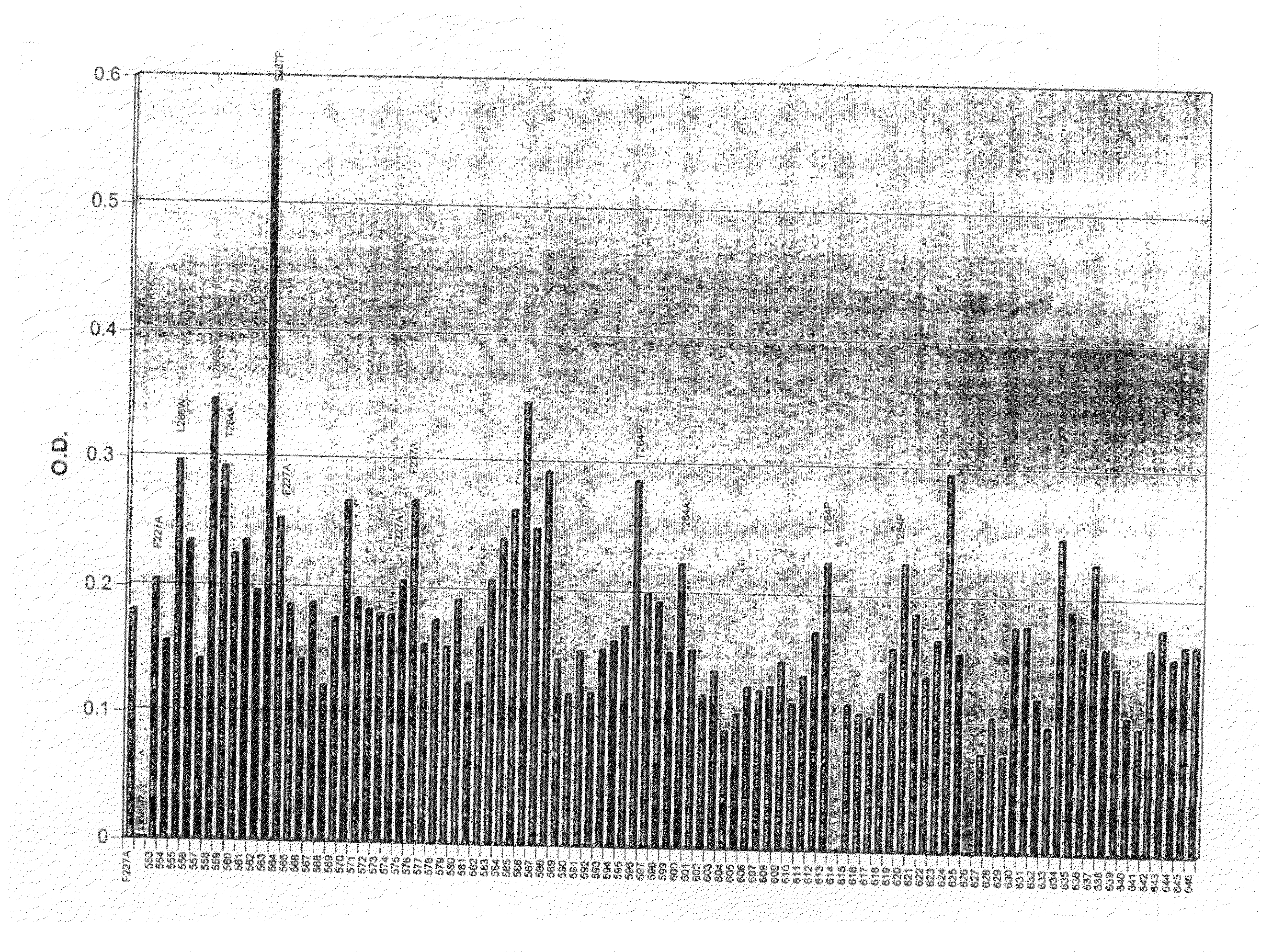
Butyrylcholinesterase variants that alter the activity of chemotherapeutic agents
The invention provides a butyrylcholinesterase variant having the amino acid sequence selected from SEQ ID NOS: 4, 6, 8, 10, 12, 14, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72, 74, 76, 78, 80, 82, 84, 86, 88, 90, 92, 94, 96, 98, 100, 102, 104, 106, 108, 110, 112, 114, 116, 118, 120, 122, 124, 126, 128, 130, 132, 134, 136, 138, 140, 142, 144, 146, 148, 150, 152, 154, 156, 158, 160, 162, 164, 166, 168, 170, 172, 174, 176, 178, 180, 182, 184, 186, 188, 190, 192, 194, and 196, or functional fragment thereof. In addition, the invention provides a method of converting a camptothecin derivative to a topoisomerase inhibitor by contacting the camptothecin derivative with a butyrylcholinesterase variant selected from SEQ ID NOS: 2, 4, 6, 8, 10, 12, 14, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72, 74, 76, 78, 80, 82, 84, 86, 88, 90, 92, 94, 96, 98, 100, 102, 104, 106, 108, 110, 112, 114, 116, 118, 120, 122, 124, 126, 128, 130, 132, 134, 136, 138, 140, 142, 144, 146, 148, 150, 152, 154, 156, 158, 160, 162, 164, 166, 168, 170, 172, 174, 176, 178, 180, 182, 184, 186, 188, 190, 192, 194, and 196, or functional fragment thereof, under conditions that allow conversion of a camptothecin derivative to a topoisomerase inhibitor. Further, the invention provides a method of treating cancer by administering to an individual an effective amount of a butyrylcholinesterase variant selected from SEQ ID NO: 2, 4, 6, 8, 10, 12, 14, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72, 74, 76, 78, 80, 82, 84, 86, 88, 90, 92, 94, 96, 98, 100, 102, 104, 106, 108, 110, 112, 114, 116, 118, 120, 122, 124, 126, 128, 130, 132, 134, 136, 138, 140, 142, 144, 146, 148, 150, 152, 154, 156, 158, 160, 162, 164, 166, 168, 170, 172, 174, 176, 178, 180, 182, 184, 186, 188, 190, 192, 194, and 196, or functional fragment thereof, exhibiting increased capability to convert a camptothecin derivative to a topoisomerase inhibitor compared to butyrylcholinesterase.
Owner:APPLIED MOLECULAR EVOLUTION
Method of synthesizing camptothecin-relating compounds
InactiveUS20040106830A1Efficiently provideOrganic compound preparationAmino-carboxyl compound preparationKetoneCamptothecin derivative
The present invention is to prepare efficiently 2'-amino-5'-hydroxypropiophenone corresponding to the AB-ring part of camptothecin (CPT) skeleton and a tricyclic ketone corresponding to the CDE-ring part in order to provide efficiently CPT by the total synthesis, which is a starting material for irinotecan hydrochloride and various kinds of camptothecin derivatives, and to provide stably CPT and its derivatives.
Owner:YAKULT HONSHA KK
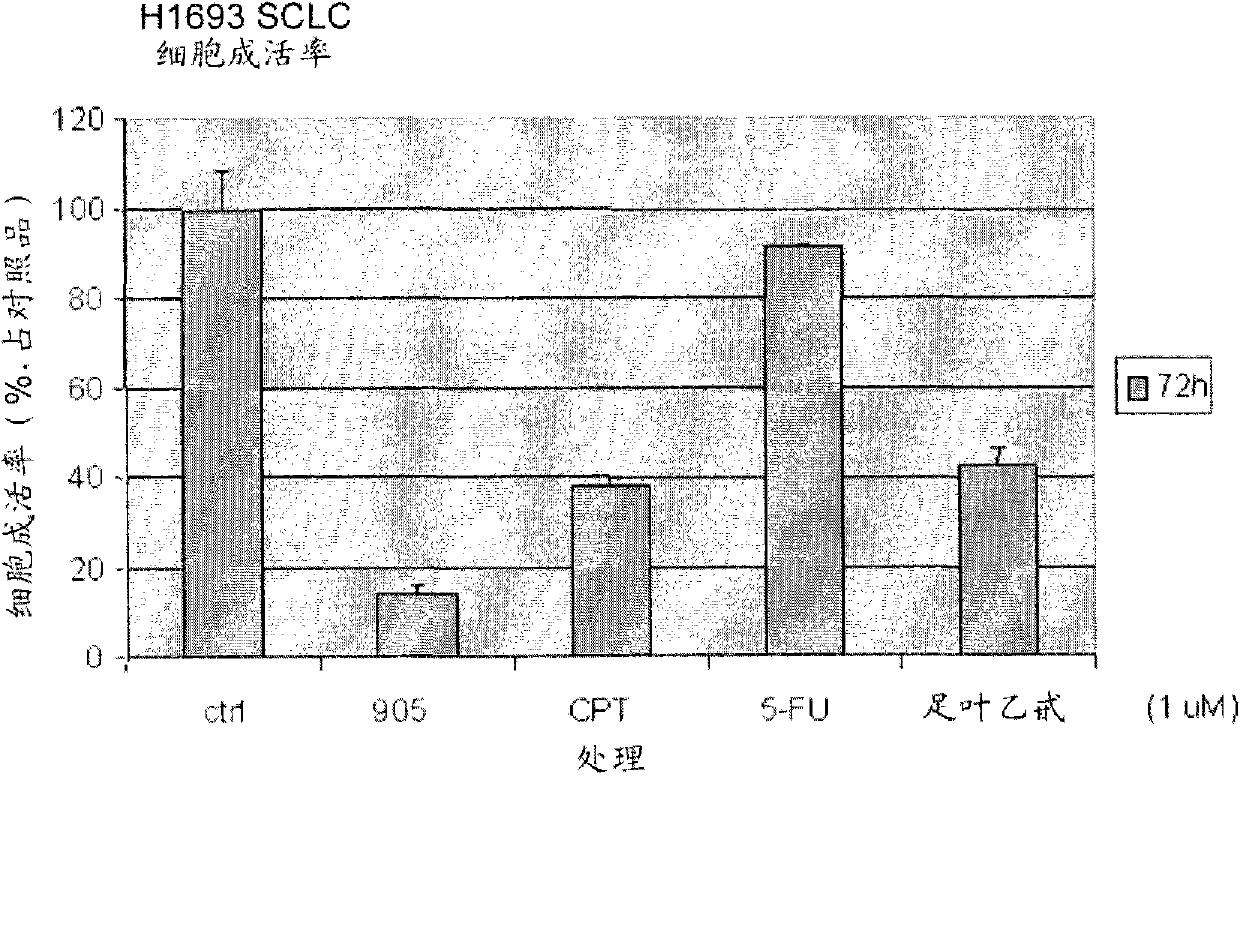
Inhibition of abnormal cell proliferation with camptothecin and combinations including the same
InactiveUS20050118180A1Enhance immune responseReduce inhibitionBiocideGenetic material ingredientsLeukemiaAbnormal cells
A method for treating diseases associated with abnormal cell proliferation comprises delivering to a patient in need of treatment a compound selected from the group consisting of 20(S)-camptothecin, analog of 20(S)-camptothecin, derivative of 20(S)-camptothecin, prodrug of 20(S)-camptothecin, and pharmaceutically active metabolite of 20(S)-camptothecin, in combination with an effective amount of one or more agents selected from the group consisting of alkylating agent, antibiotic agent, an alkylating agent, antibiotic agent, antimetabolic agent, hormonal agent, plant-derived agent, anti-angiogenesis agent and biologic agent. The method can be used to treat benign tumors, malignant or metastatic tumors, leukemia and diseases associated with abnormal angiogenesis.
Owner:SUPERGEN
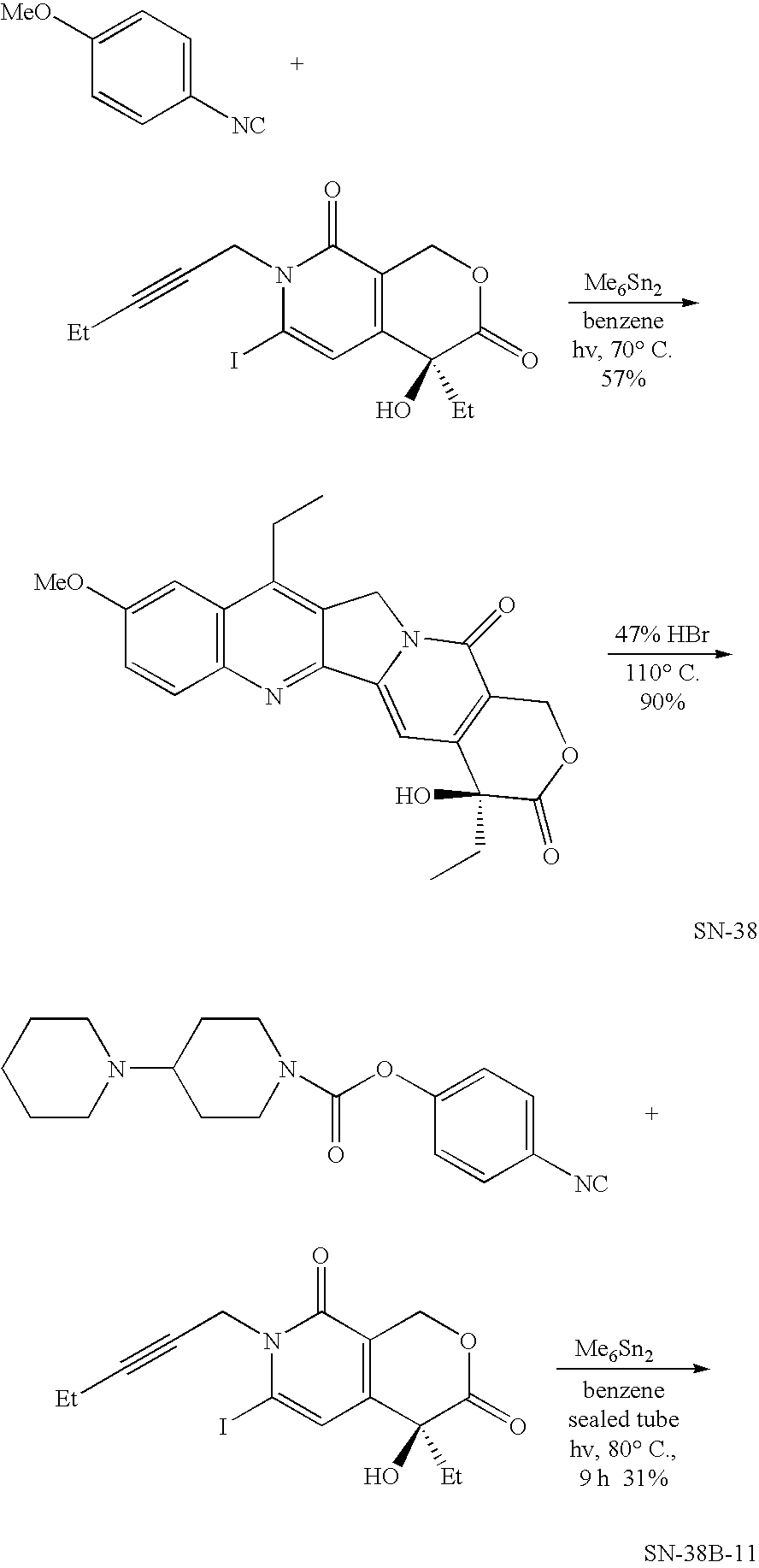
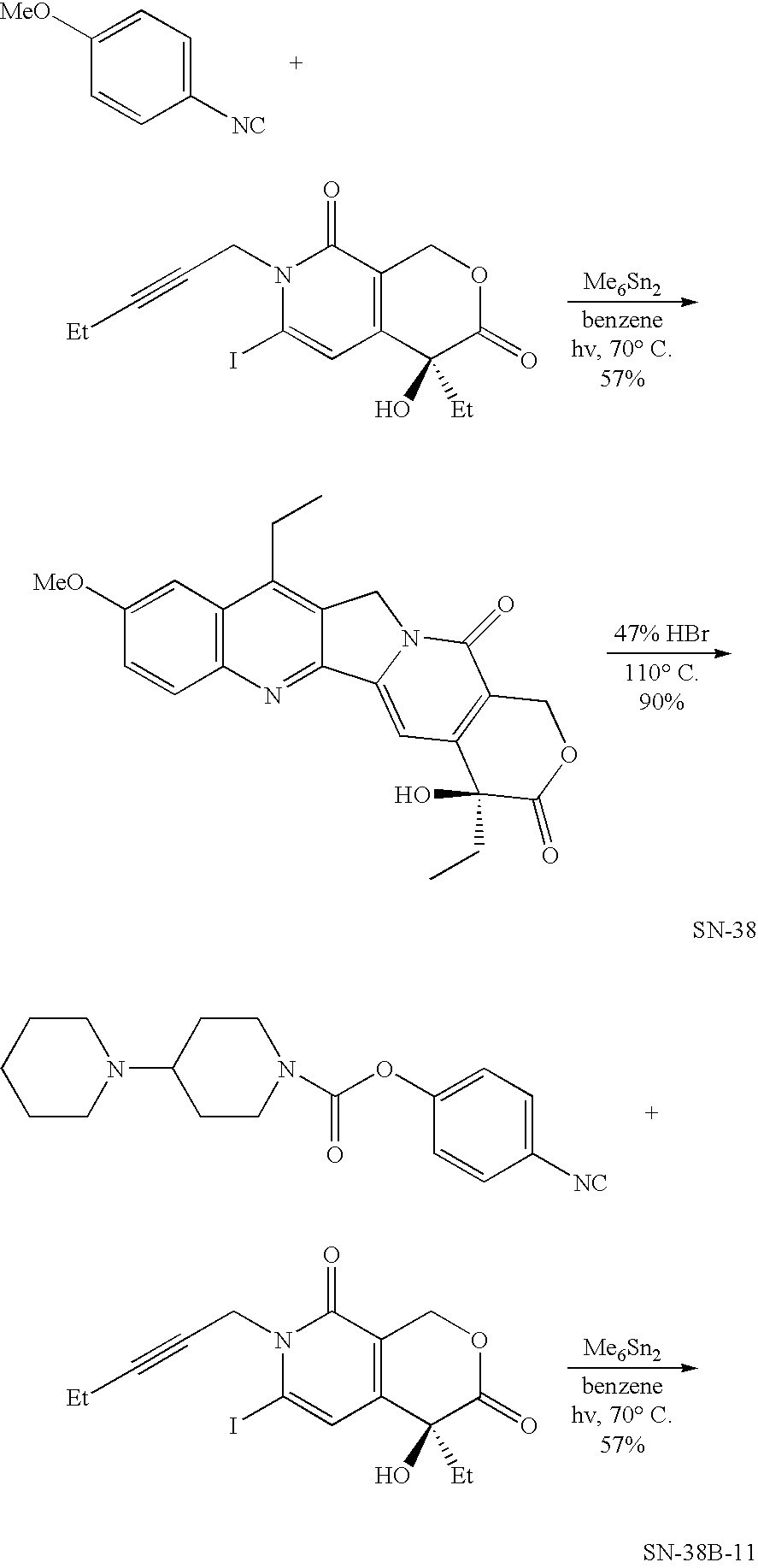
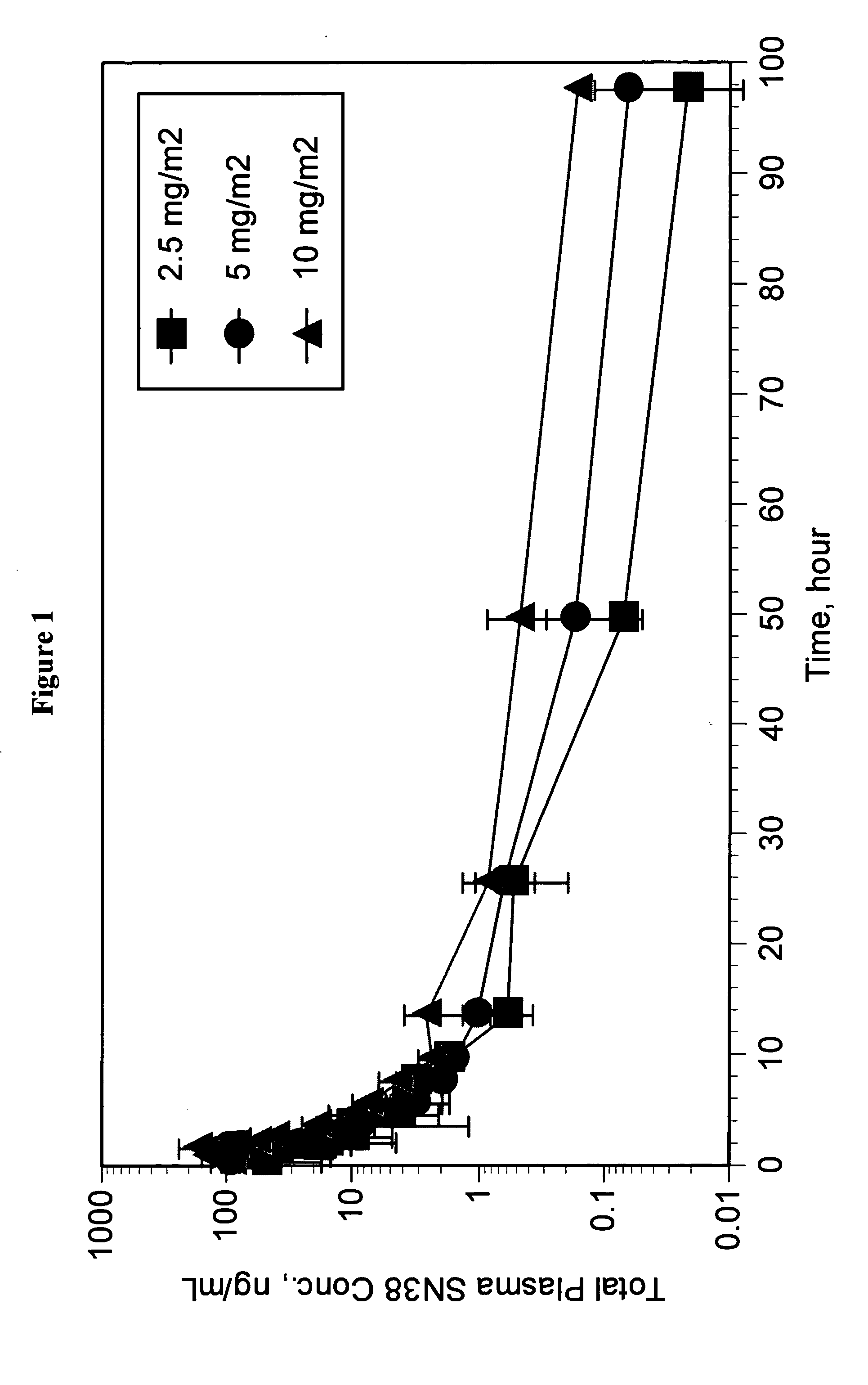
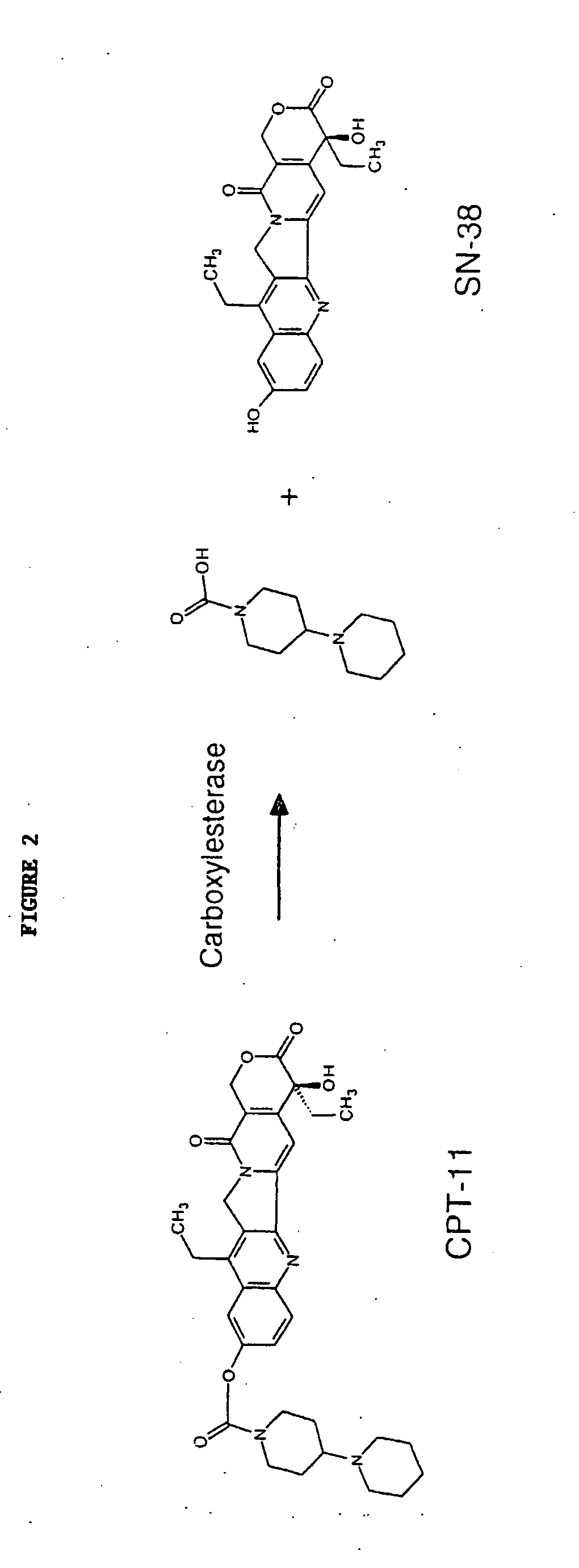
Pharmaceutically active lipid based formulation of SN-38
SN38, camptothecin derivatives are poorly water soluble, highly lipophilic camptothecin derivatives and are very active against a variety of human cancers. Because of their very poor water solubility, SN38 has not been used to treat human patients with cancer due to the inability to administer sufficient quantities of dissolved in a pharmaceutical formulation. This invention overcomes these limitations by teaching novel pharmaceutically acceptable SN38 liposome complex formulation for the direct administration of the formulation to human patients with cancer. The claimed invention also describes the methods to prepare liposomal SN38 complexes and antitumor compositions of liposomal SN38 complexes to allow the administration in sufficient amounts to treat various types of cancer and as antiviral agents. This invention is also directed to injectable sterile solutions, antitumor compositions, liposomes. The present invention is for novel compositions and methods for treating diseases caused by cellular proliferation, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include SN38 lipid complexes in which the complexes can contain any of a variety of neutral or charged lipids and, desirably, cardiolipin. The compositions are capable of efficiently incorporating SN38 into complexes and are capable of solubilizing relatively high concentrations of SN38.
Owner:NEOPHARMA INC
Pesticide containing comptothecin or dreivates of camptothecin, and preparation method
ActiveCN1759675ABroad insecticidal spectrumGood effectBiocideAnimal repellantsOrganic solventOrder Lepidoptera
An insecticide for the pests in homopteran, thysanoptera, lepidoptera and coleoptera is prepared from camptothecine or its derivative, emulsifier, penetrant and organic solvent through proportional mixing.
Owner:YIFAN AGRI CHEM PLANT ZHEJIANG PROV
Camptothecin derivatives with antitumor activity
Novel camptothecin derivatives having antitumor activity, the processes for the preparation thereof, the use thereof as antitumor drugs and pharmaceutical compositions containing them.
Owner:INDENA SPA
Camptothecin derivative and conjugate thereof
The invention discloses a camptothecin derivative for an antitumor drug and an antibody-drug conjugate of the camptothecin derivative. According to the invention, through a series of molecular structure modification, a better camptothecin antitumor drug is obtained, so that the camptothecin antitumor drug is more suitable for being used as an antibody coupling drug.
Owner:SICHUAN BAILI PHARM CO LTD +1
Camptothecin derivatives
InactiveUS6617456B1Improve anti-tumor activityLess side effectsSaccharide peptide ingredientsImmunoglobulinsCarboxyl radicalCamptothecin derivative
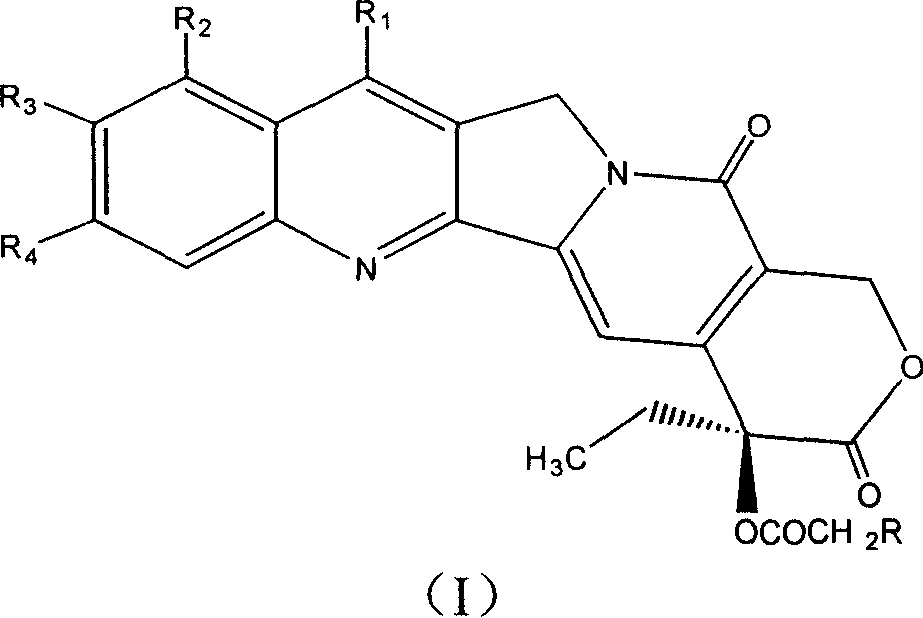
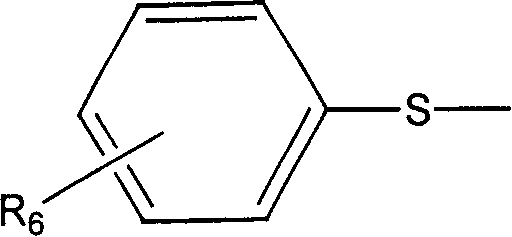
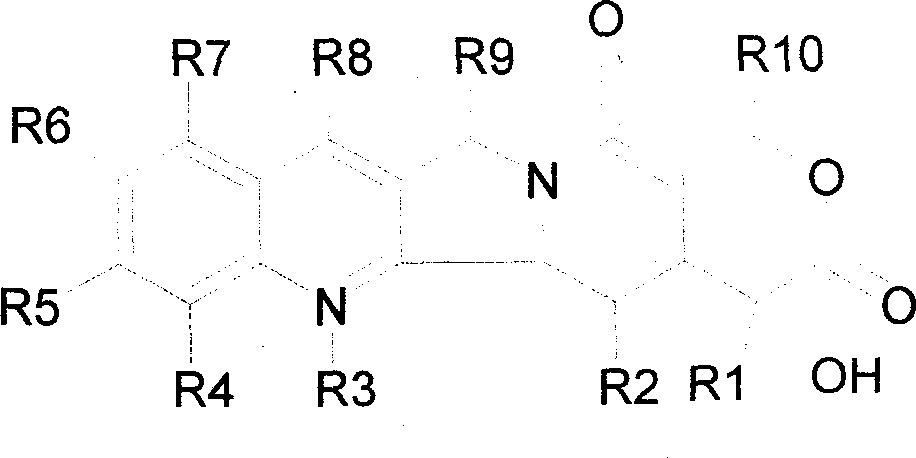
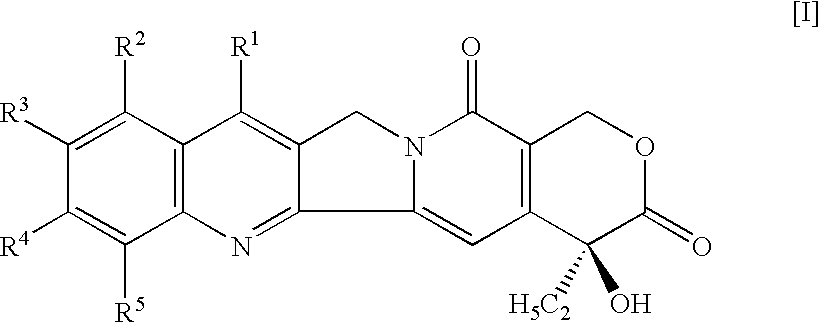
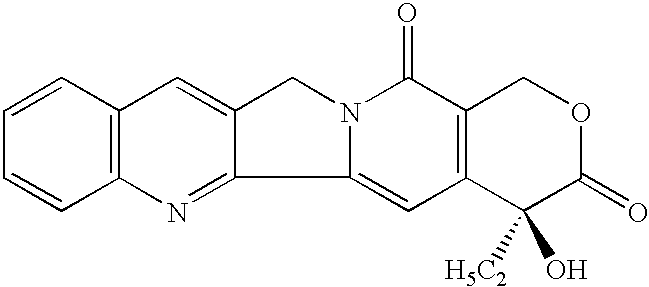
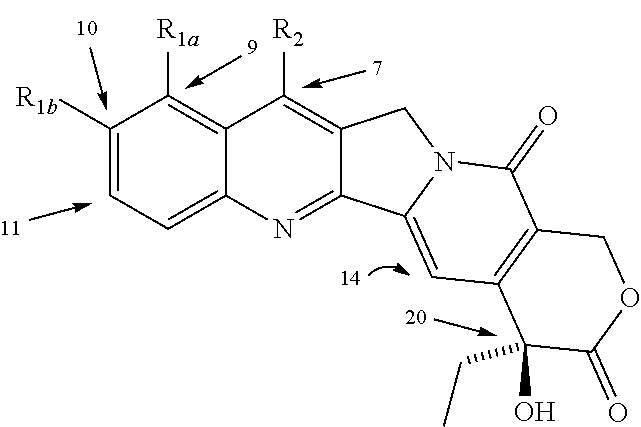
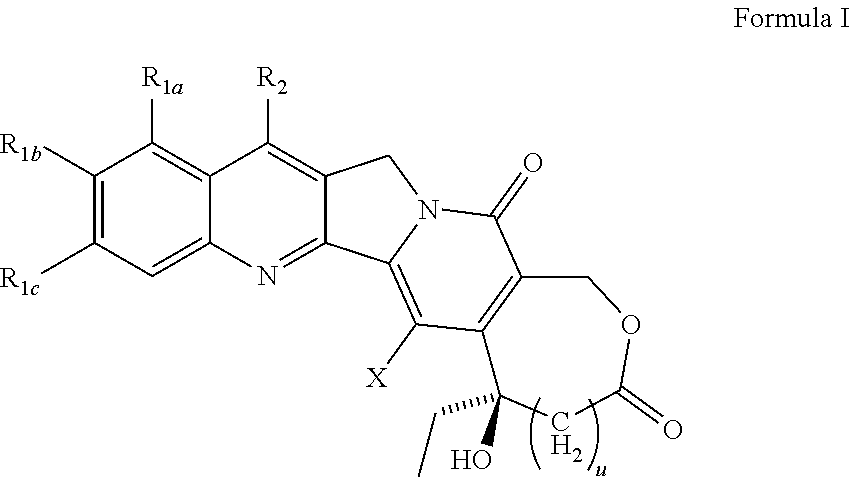
A camptothecin derivative comprising a compound of the formula [I]:wherein R1, R2, R3, R4 and R5 are (A) adjacent two groups combine to form alkylene, or both are H, and one of the remaining three groups is -Xn-Alkm-R6, and the other two are H, alkyl or halogen, or (B) adjacent two groups combine to form alkylene, and one of the carbon atoms of said alkylene group is substituted by -Xn-Alkm-R6, and the remaining three groups are H, alkyl or a halogen, and one or two -CH2- of the alkylene in (A) or (B) may optionally be replaced by -O-, -S- or -NH-, X is -O- or -NH-, Alk is alkylene,or -OH, m and n are both 0 or 1, or m is 1 and n is 0, which camptothecin compound is bound to a polysaccharide having carboxyl groups via an amino acid or a peptide, or a pharmaceutically acceptable salt thereof. Said camptothecin derivatives show enhanced antitumor activities but few side effects and are useful as a medicament.
Owner:MITSUBISHI TANABE PHARMA CORP
Camptothecin derivatives having antitumor activity
InactiveUS20010008939A1Better indexEasy to handleOrganic active ingredientsOrganic chemistryMalarial parasitePharmaceutical drug
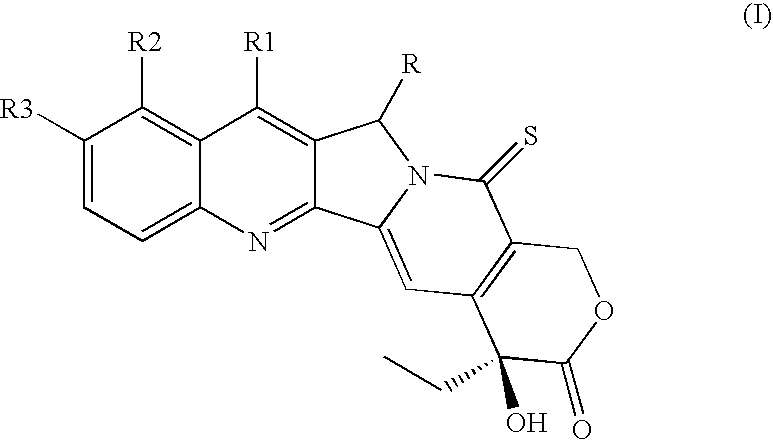
Camptothecin derivatives of camptothecin of formula (I) wherein the groups R1, R2 and R3 are as defined in the description are disclosed. The compounds of formula (I) are endowed with antitumor activity and show a good therapeutic index. Processes for the preparation of the compounds of formula (I) and their use in the preparation of medicaments useful in the treatment of tumors, viral infections and antiplasmodium falciparum are also disclosed.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
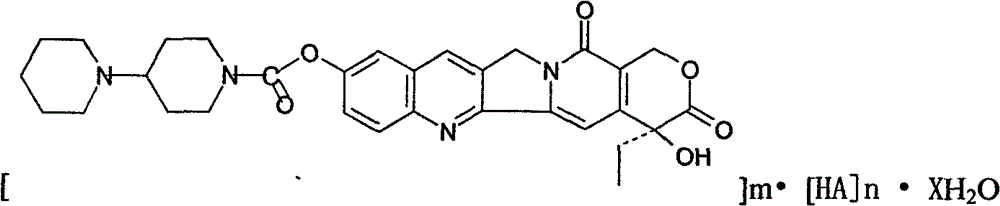
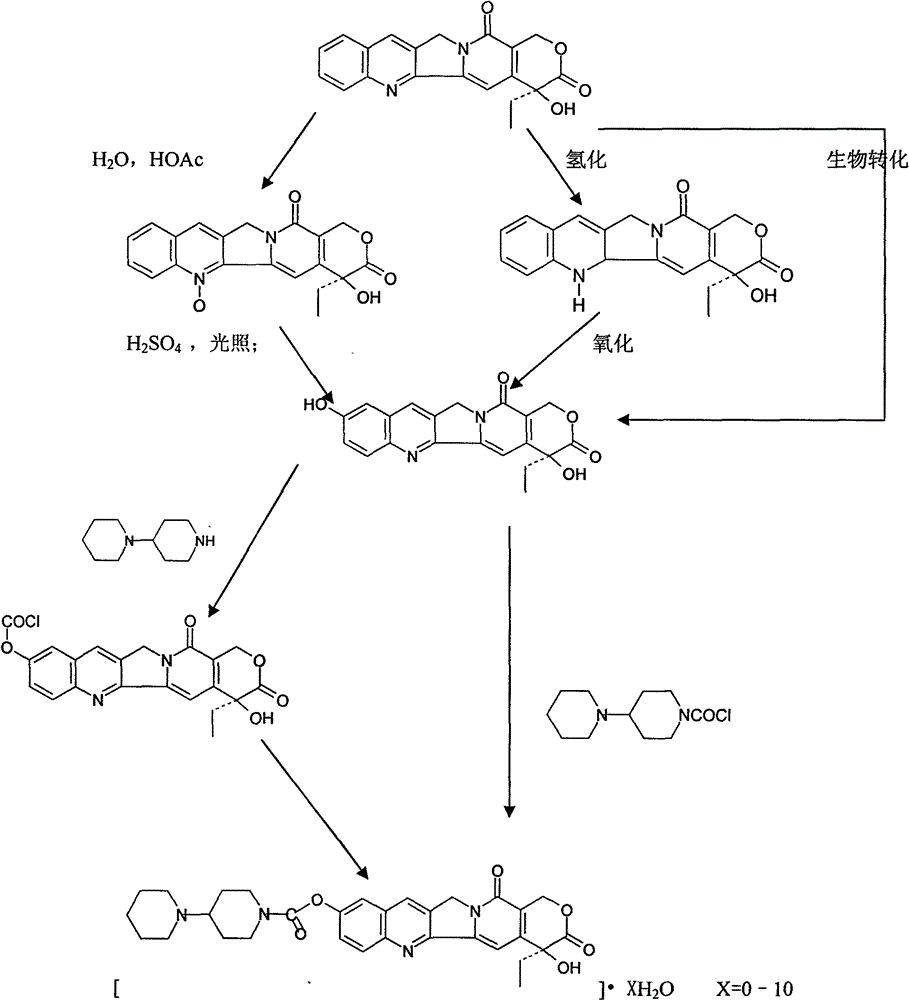
Preparation and clinic application of hydroxycamptothecin derivative and preparation thereof
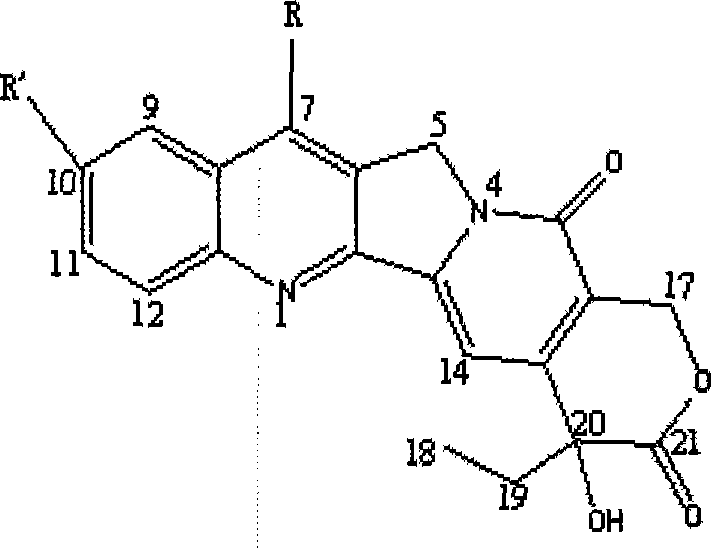
A product of the invention is a variety of hydroxycamptothecin derivative referred to as bipiperidine hydroxycamptothecin {chemical name: (+)-(4S)-4-ethyl-4-hydroxy-9 [ (4-piperidyl piperidine) carbonyl]-1H-pyrano [ 3 ', 4 ', 6 ', 7 '] butoprizine [1, 2-b] quinoline-3, 14-( 4H, 12H ) dione and (0-10) hydrate thereof}. The derivative is a broad-spectrum antineoplastic drug, and has curative effects on primary liver cancer, gastric cancer, head and neck adenoid epithelial cancer, leukemia, colon cancer, bladder cancer and other malignant tumors. The ipiperidine hydroxycamptothecin, salts thereof and (0-10) hydrate of the salts are prodrugs for hydroxycamptothecin and converted into closed hydroxycamptothecin in vivo, so as to overcome defects of open loop, poor effect and instability of a hydroxycamptothecin preparation (injection, lyophilized injection and oral preparation); and the derivative has good water solubility and is suitable for preparation of various types of preparations. The bipiperidine hydroxycamptothecin can be added with different salts to be prepared into different water-soluble compounds and hydrates thereof, is beneficial to preparation of various preparations, and has stable quality and curative effect obvious better than that of hydroxycamptothecin and preparation thereof. The bipiperidine hydroxycamptothecin, salts thereof, (0-10) hydrate of the salts and preparations thereof are in forms of tablet, capsule, particulate agent, oral liquid, injection, infusion solutions and lyophilized injection.
Owner:肖文辉 +1
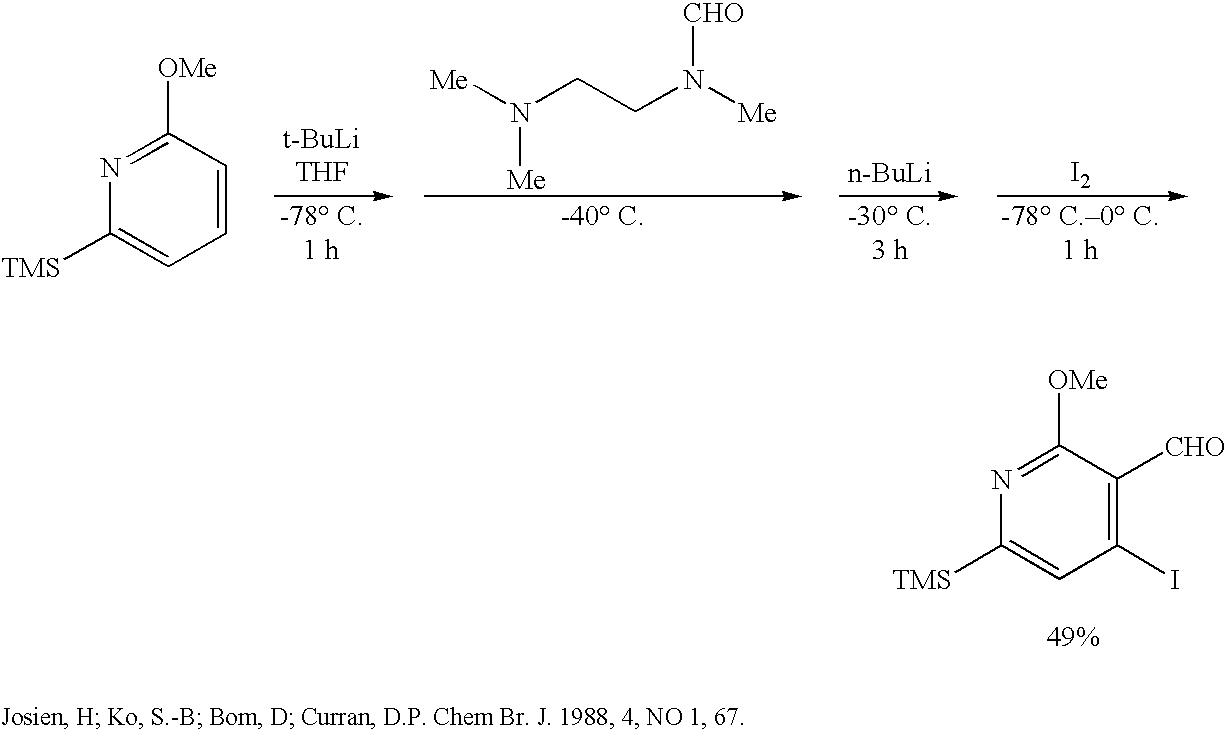
Process to prepare camptothecin derivatives and novel intermediate and compounds thereof
New processes are disclosed for the preparation of camptothecin derivatives, such as, irinotecan and topotecan, as well as new intermediates and compounds related thereof.
Owner:INNOVATIONAL HLDG LLC
Butyrylcholinesterase Variants that Alter the Activity of Chemotherapeutic Agents
InactiveUS20080213281A1Sugar derivativesPeptide/protein ingredientsButyrylcholinesteraseTopoisomerase inhibitor
The invention provides a butyrylchinesterase variant, a method of converting a camptothecin derivative to a topoisomerase inhibitor by contacting the camptothecin derivative with a butyrylcholinesterase variant and a method of treating cancer by administering to an individual an effective amount a butyrylcholinesterase variant exhibiting increased capability to convert a camptothecin derivative to a topoisomerase inhibitor compared to butyrylcholinesterase.
Owner:APPLIED MOLECULAR EVOLUTION
Water soluble camptothecin derivative and medicinal composition containing same
ActiveCN102153607ALow toxicityImprove stabilityOrganic active ingredientsSugar derivativesWater solubleCamptothecin derivative
The invention relates to a water soluble derivative of camptothecin and a preparation method thereof. The derivative has a structure shown in a formula (I). The derivative of the camptothecin has improved anticancer activity.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Camptothecine derivative phosphatide composite liposome nano-preparation and its making method
ActiveCN101028251AIncrease fat solubilityInhibition of free rotationOrganic active ingredientsAntineoplastic agentsLiposomeCamptothecin derivative
A lipoid nanoparticle of camptothecine-phosphatide composition is prepared from phosphatide, 10-hydroxy camptothecine or its derivative and lipoid. Its preparing process is also disclosed.
Owner:SICHUAN UNIV
Camptothecin derivatives
Various 14-Nitro, 14-amino, and 14-substituted amino camptothecin derivatives are useful in the treatment of cancer and other hyperproliferative diseases. Various 14-nitro camptothecin derivatives are conveniently prepared by reacting a camptothecin derivative with fuming nitric acid, optionally employing acetic anhydride as a solvent.
Owner:MOLECULAR TEMPLATES
Nitrogen-based homo-camptothecin derivatives
(20) esters of camptothecin analogs are provided. The compounds are (20) esters of an aminoalkanoic acid or an imidoalkanoic acid and homocamptothecin, which is optionally substituted at the 7, 9, 10, 11, and 12 positions of the homocamptothecin ring. The compounds are useful for treating cancer.
Owner:CATHOLIC HEALTHCARE WEST ST JOSEPHS HOSPITAL +1
Agricultural bactericide containing camptothecin or camptothecin derivative and application thereof
Disclosed is the agricultural pesticide containing camptothecine or camptothecine derivatives and the use thereof, wherein the pesticide comprises camptothecine or its derivatives 0.02-2.0%, emulsifying agent 1.0-20%,penetrating agent 0.5-10%, and balancing organic solvent. The pesticide can be used for controlling agronomic crop downy mildew, leaf streak, pythium blight, head blight, root and stem rot, rhizoctonia rot, damping-off, wilt,bitter rot, powdery mildew, black shank, brown spot, leaf scab, ring rot, black pox, gray mold,phytophthora rot, ulcer disease, black spot, bacterial wilt, angular leaf spot, leaf streak and leaf scar.
Owner:YIFAN AGRI CHEM PLANT ZHEJIANG PROV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com