Combination of mPEG-PLA-tree alkali medicament
A technology of conjugates and camptothecin, which is applied in the field of camptothecin prodrugs, can solve problems such as high toxicity and side effects, unsatisfactory clinical trial results, and severe side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
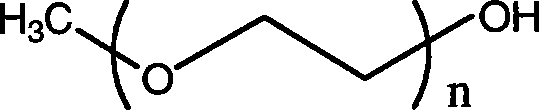
[0033] Preparation of Methoxypolyethylene Glycol Polylactic Acid
[0034]
[0035] 6 g of methoxypolyethylene glycol and 0.13 g of succinic anhydride (the molar ratio of the two is 1:1.1) were dissolved in pyridine, and reacted at 50° C. for 5 hours. After the reaction was completed, pyridine was removed by rotary evaporation, recrystallized in isopropanol three times, and vacuum-dried at 40°C.
[0036] Dissolve 1.0 g of stannous octoate in 25 ml of toluene to obtain a 0.04 g / mL catalyst-toluene solution. Weigh 2.33g of lactide and 1.33g of succinylated methoxypolyethylene glycol into a dry polymerization tube, and add 70ul of toluene-stannous octoate solution. Vacuumize to remove toluene, then flush with nitrogen protection. Then place the polymer tube in an oil bath and heat it to 140-160°C, the lactide crystals will melt, shake and mix thoroughly, N 2 Under the protection, keep warm and polymerize for 5 hours. After natural cooling, the polymer ...
Embodiment 2
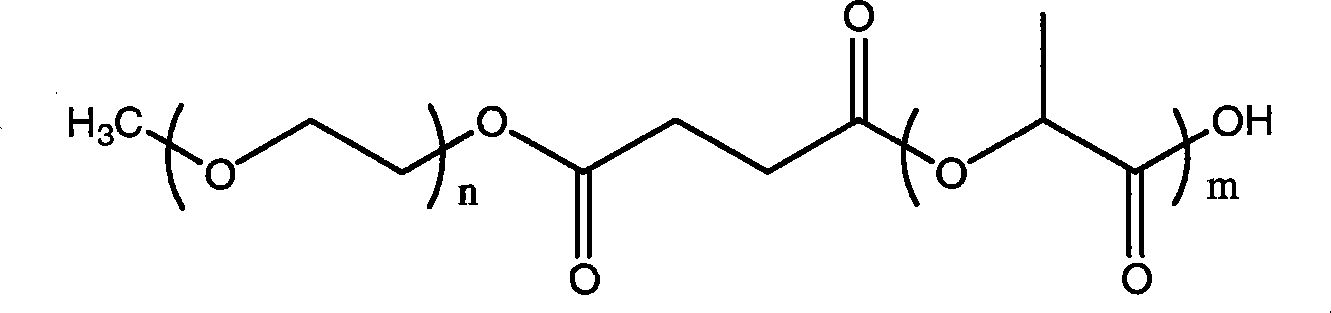
[0038] Preparation of methoxypolyethylene glycol-polylactic acid-camptothecin conjugate
[0039]
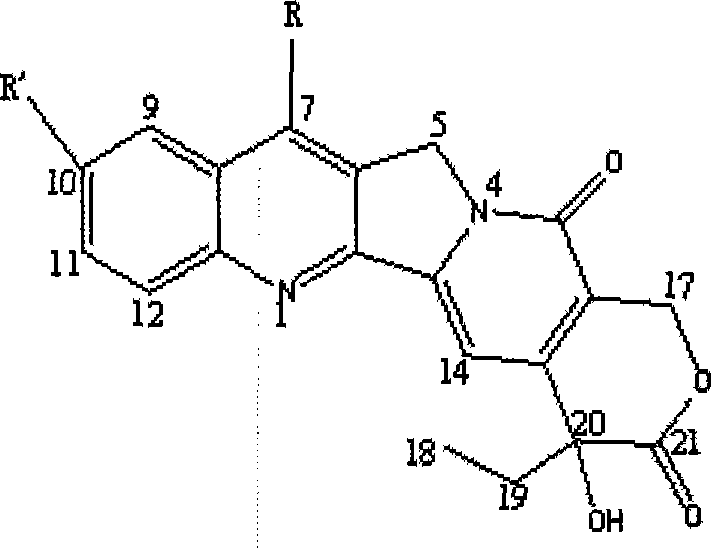
[0040] Take 0.5g mPEG-PLA-COOH and 30mg camptothecin and pre-lyophilize for 2 hours. Under the protection of nitrogen, put it into a 50ml round bottom flask, dissolve it with 20ml of chloroform, then add 0.6ml of pyridine and 0.3ml of phenoxyphosphoric acid chloride. The reaction was carried out under magnetic stirring at room temperature for 24 hours. After the reaction is complete, wash with 1N hydrochloric acid, 25ml each time, twice. Anhydrous sulfuric acid was used to remove residual moisture, and concentrated under reduced pressure to obtain a yellow product. Wash off the unreacted drug camptothecin with excess isopropanol, and then wash off the yellow color with anhydrous ether to obtain a pale white solid product.
Embodiment 3
[0042] Preparation of Methoxypolyethylene Glycol Polylactic Acid-10 Hydroxycamptothecin Conjugate
[0043]
[0044] Take 0.5g mPEG-PLA-COOH and 30mg 10-hydroxycamptothecin (10-HCPT) and pre-lyophilize for 2 hours. Under the protection of nitrogen, put it into a 50ml round bottom flask, dissolve it with 20ml of chloroform, then add 0.6ml of pyridine and 0.3ml of phenoxyphosphoric acid chloride. The reaction was carried out under magnetic stirring at room temperature for 24 hours. After the reaction is complete, wash with 1N hydrochloric acid, 25ml each time, twice. Anhydrous sulfuric acid was used to remove residual moisture, and concentrated under reduced pressure to obtain a yellow product. Wash off the unreacted drug 10-hydroxycamptothecin with excess isopropanol, and then wash off the yellow color with anhydrous ether to obtain a light yellow solid product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com