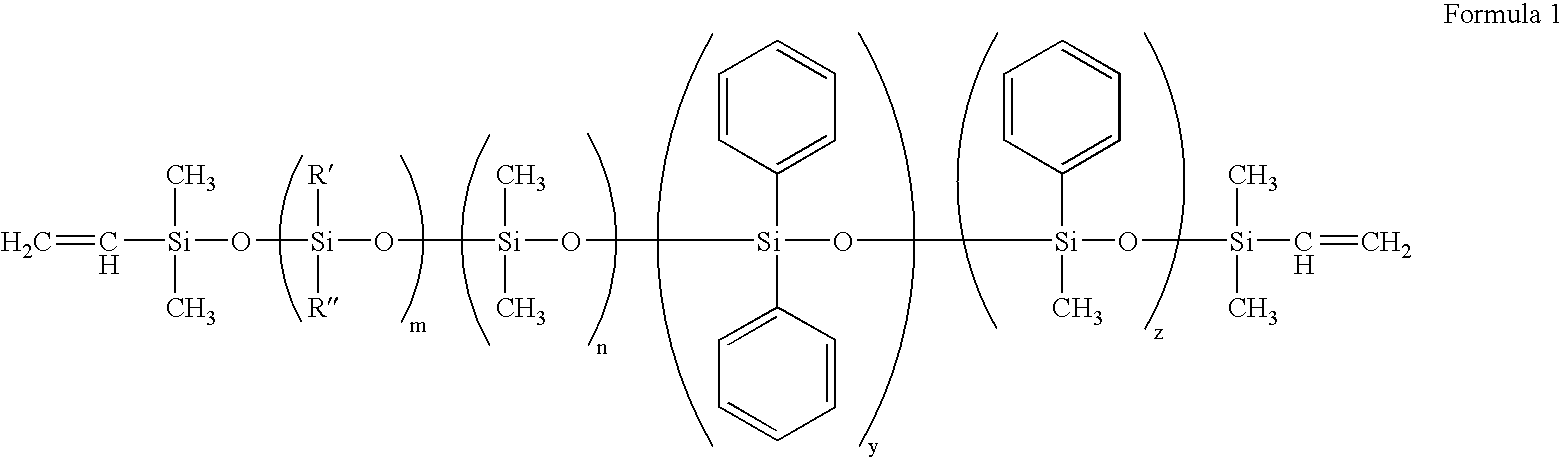
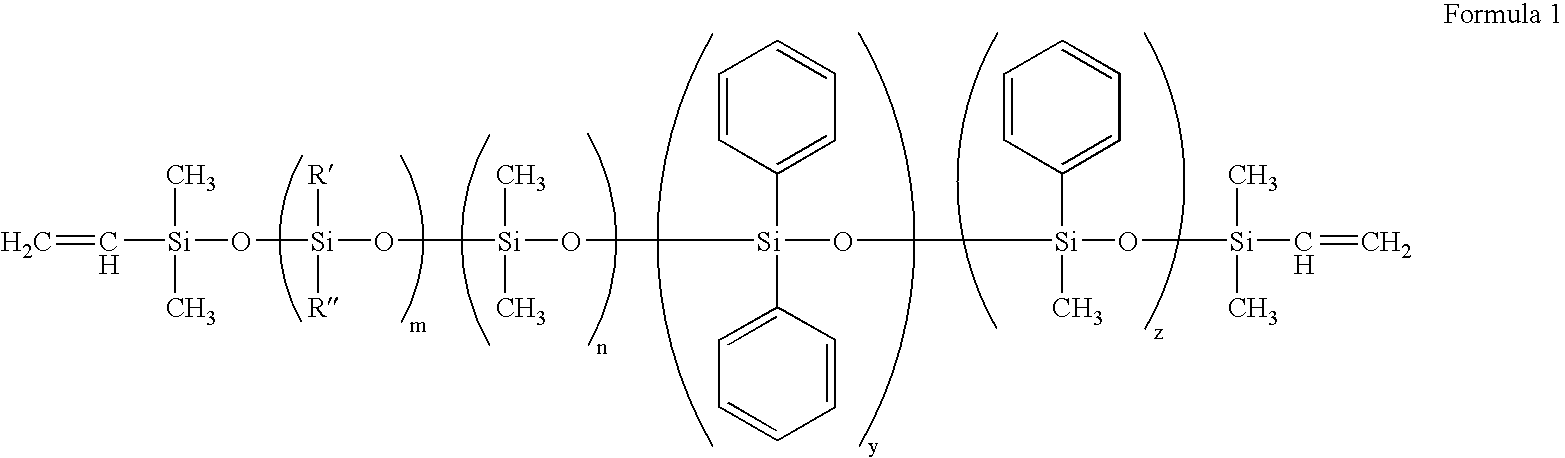
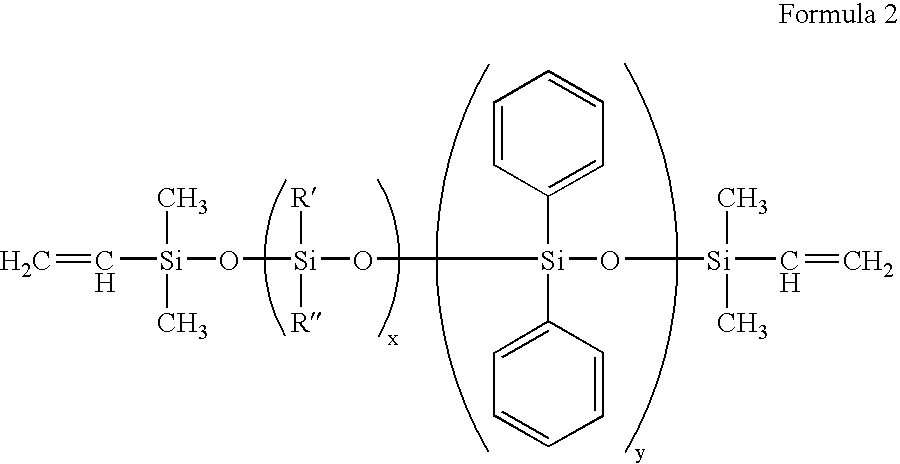
Patents
Literature
328results about How to "Suitable for manufacture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and apparatus for additive manufacturing
ActiveUS20150306819A1Easy to manufactureSuitable for manufactureManufacturing driving meansElectric discharge tubesAdditive layer manufacturingMaterials science
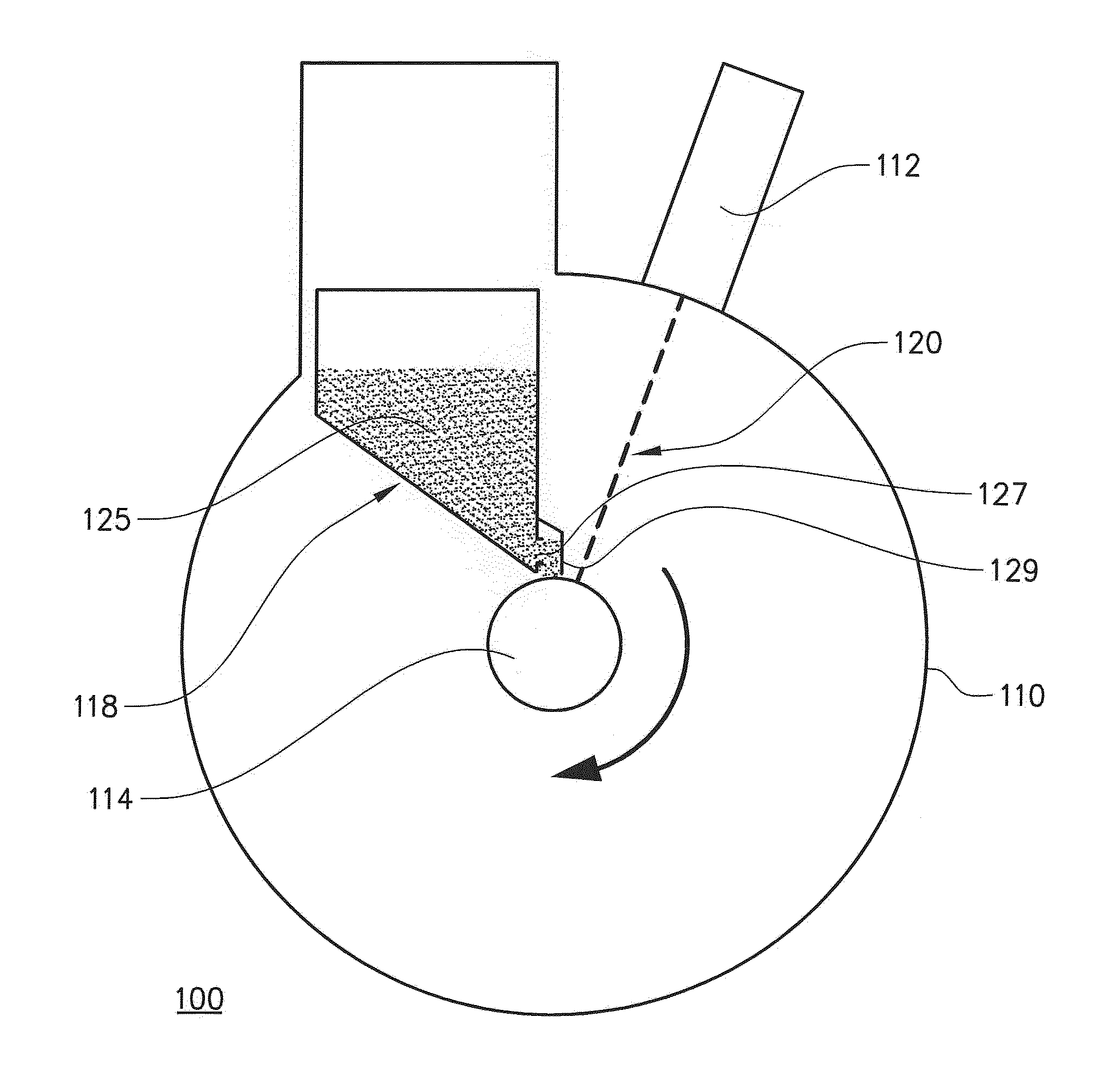
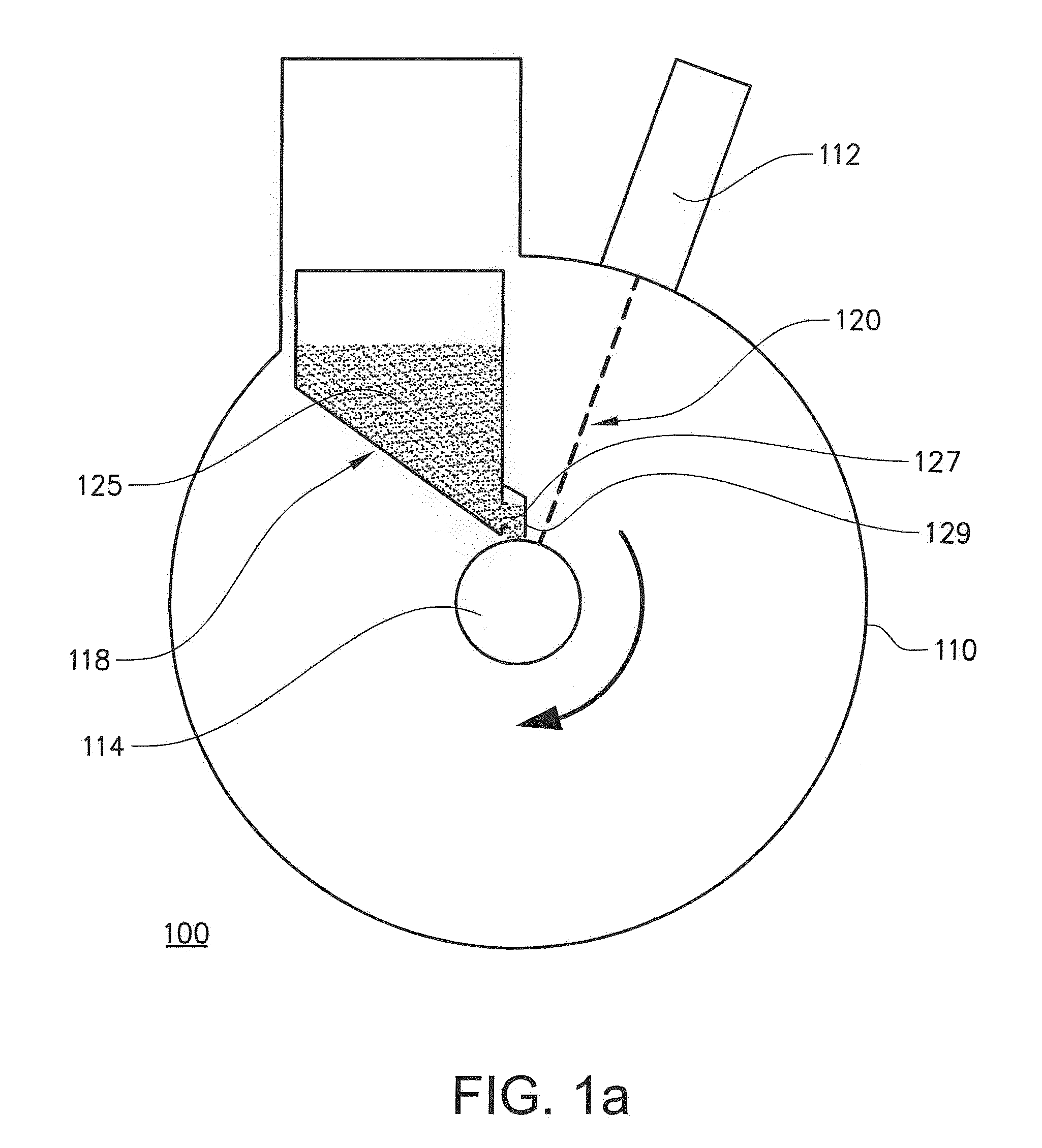
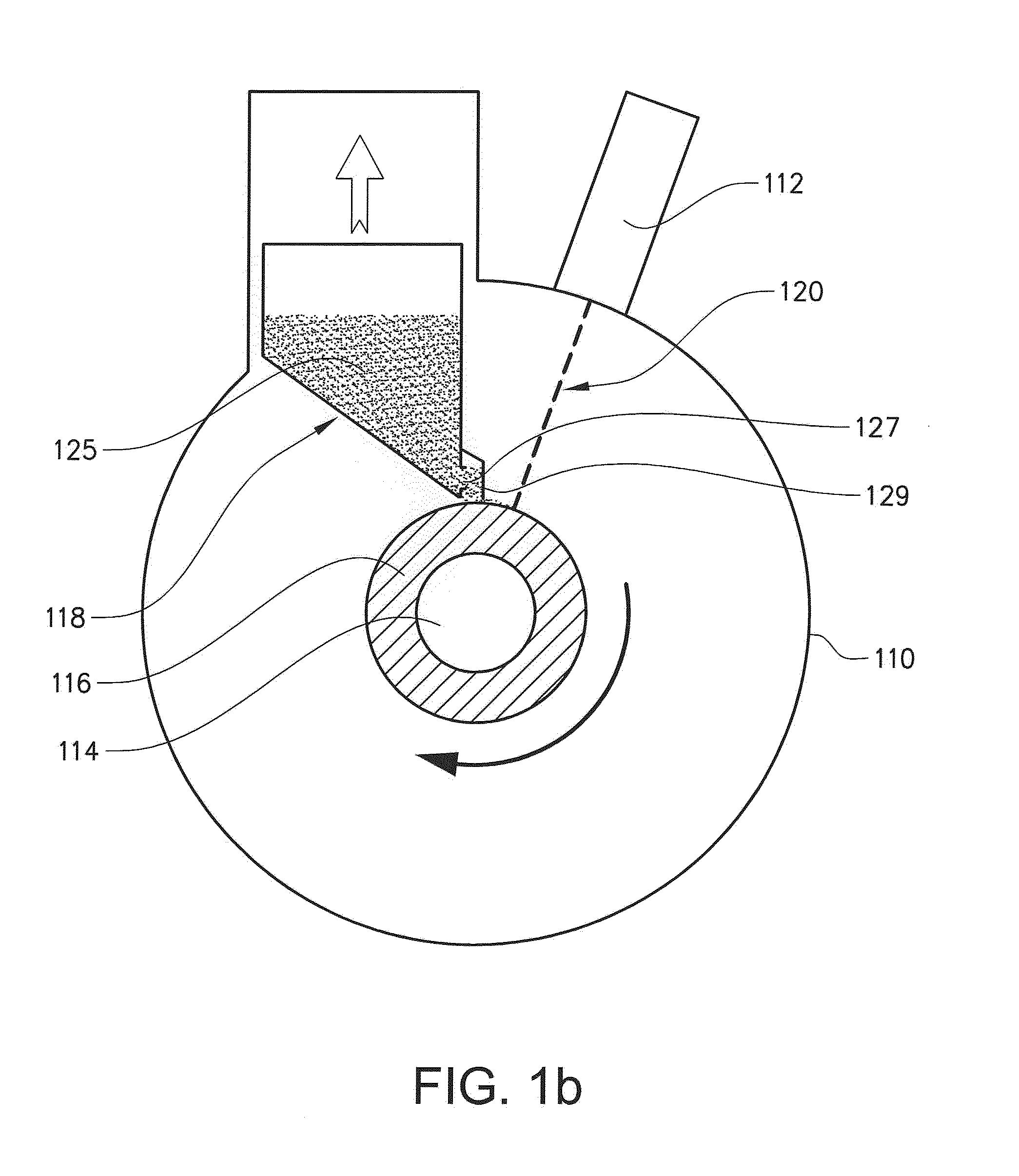
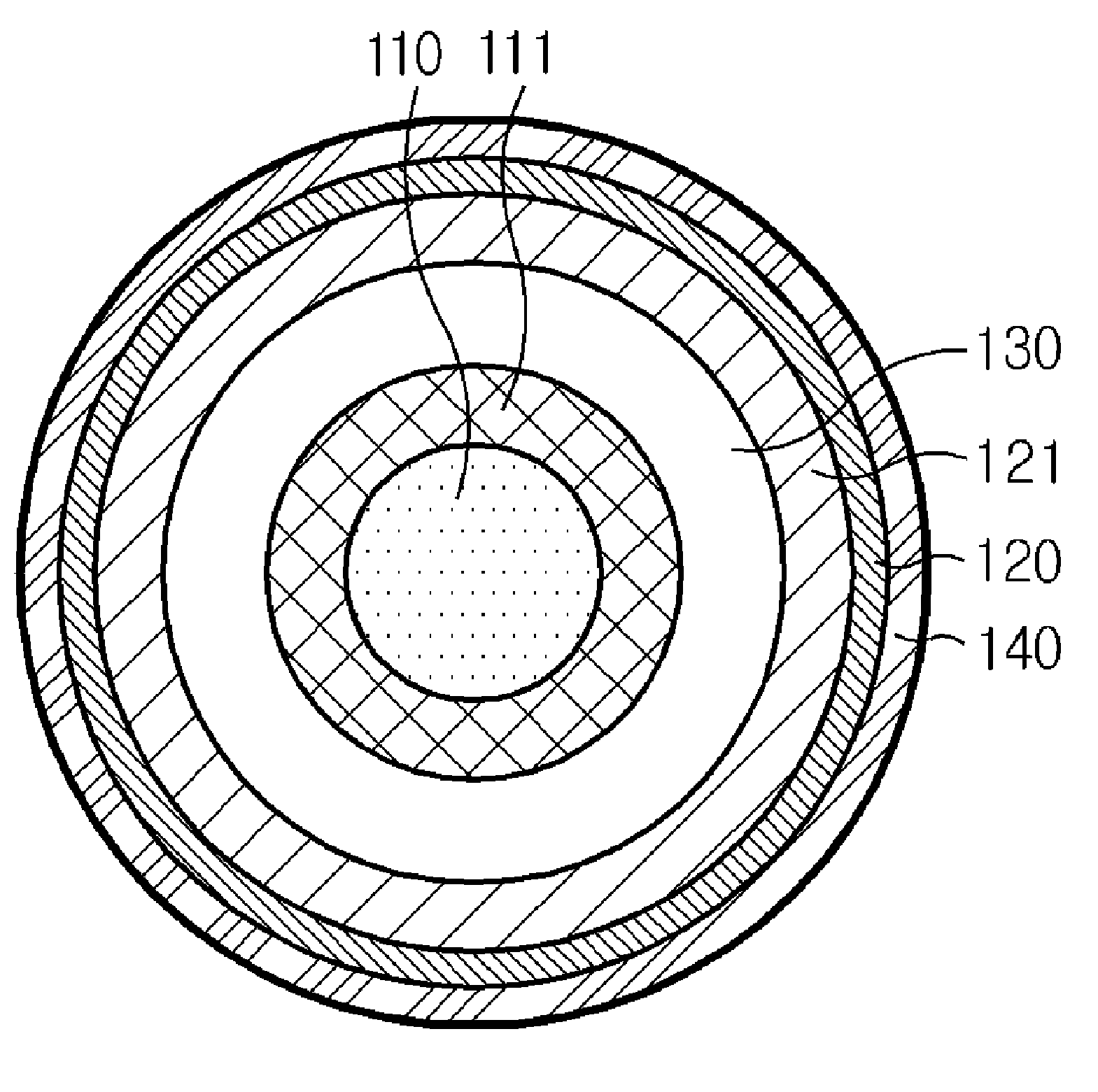
The present invention relates to a method for forming a three-dimensional article through successive fusion of applied powder. Said method comprising the steps of: providing at least one powder hopper comprising powder to be used for forming said three-dimensional article, providing a predetermined amount of powder at a build support, directing an energy beam over said build support causing at least a portion of said powder to sinter and causing at least a portion of said powder to bond to said build support, directing an energy beam over said build support causing said powder to fuse in selected locations according to a model to form a first portion of said three-dimensional article, rotating said build support around an axis of rotation for creating said three-dimensional article, which three-dimensional article is build up layer by layer in a radial direction with respect to said axis of rotation.
Owner:ARCAM AB
Method For Manufacturing Cable-Type Secondary Battery
ActiveUS20120009331A1Thickness of coating is limitedSuitable for manufactureFinal product manufactureVehicular energy storageElectrical batteryCurrent collector
The present invention relates to a method for manufacturing a cable-type secondary battery comprising an electrode that extends longitudinally in a parallel arrangement and that includes a current collector having a horizontal cross section of a predetermined shape and an active material layer formed on the current collector, and the electrode is formed by putting an electrode slurry including an active material, a polymer binder, and a solvent into an extruder, by extrusion-coating the electrode slurry on the current collector while continuously providing the current collector to the extruder, and by drying the current collector coated with the electrode slurry to form an active material layer.
Owner:LG ENERGY SOLUTION LTD
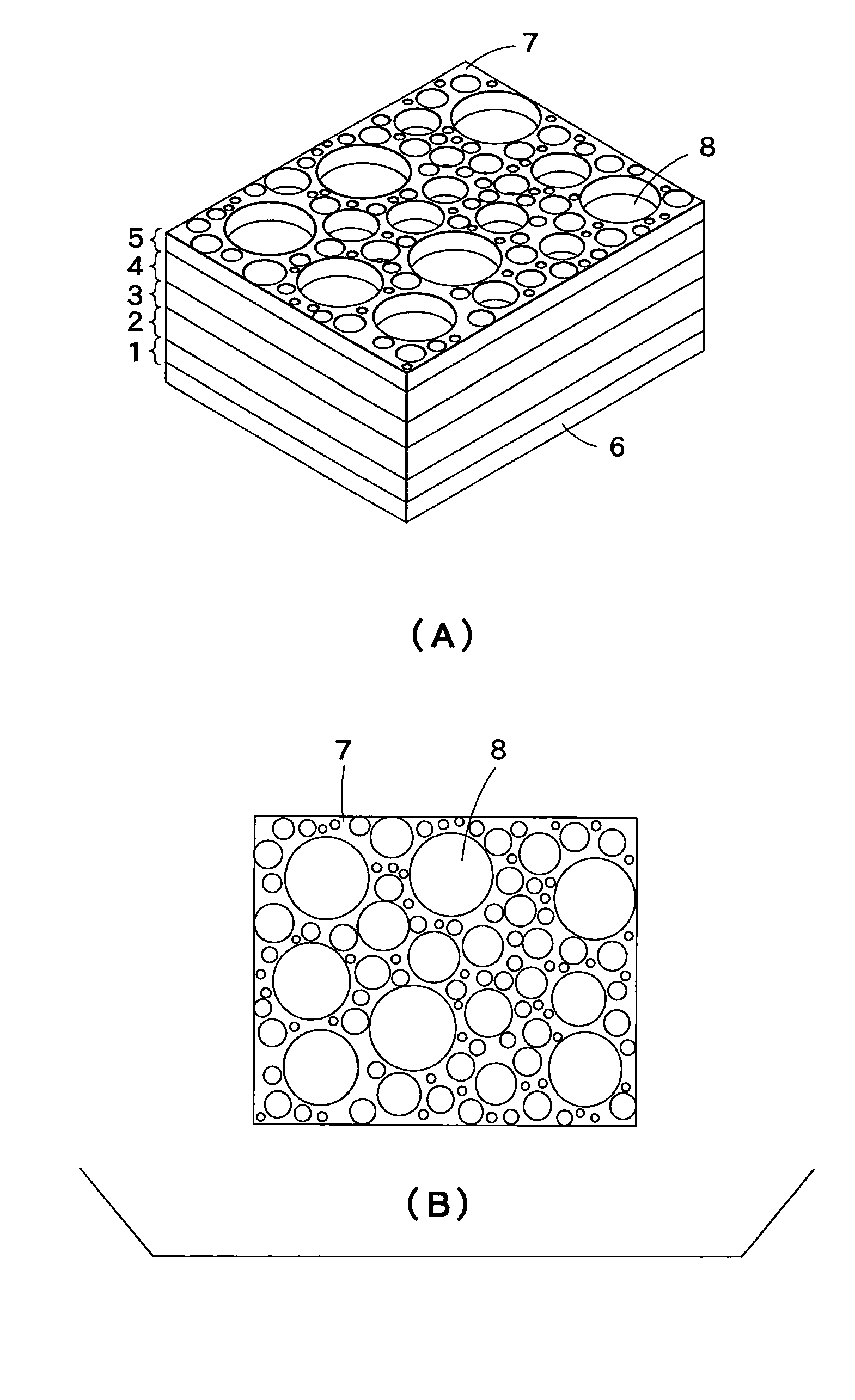
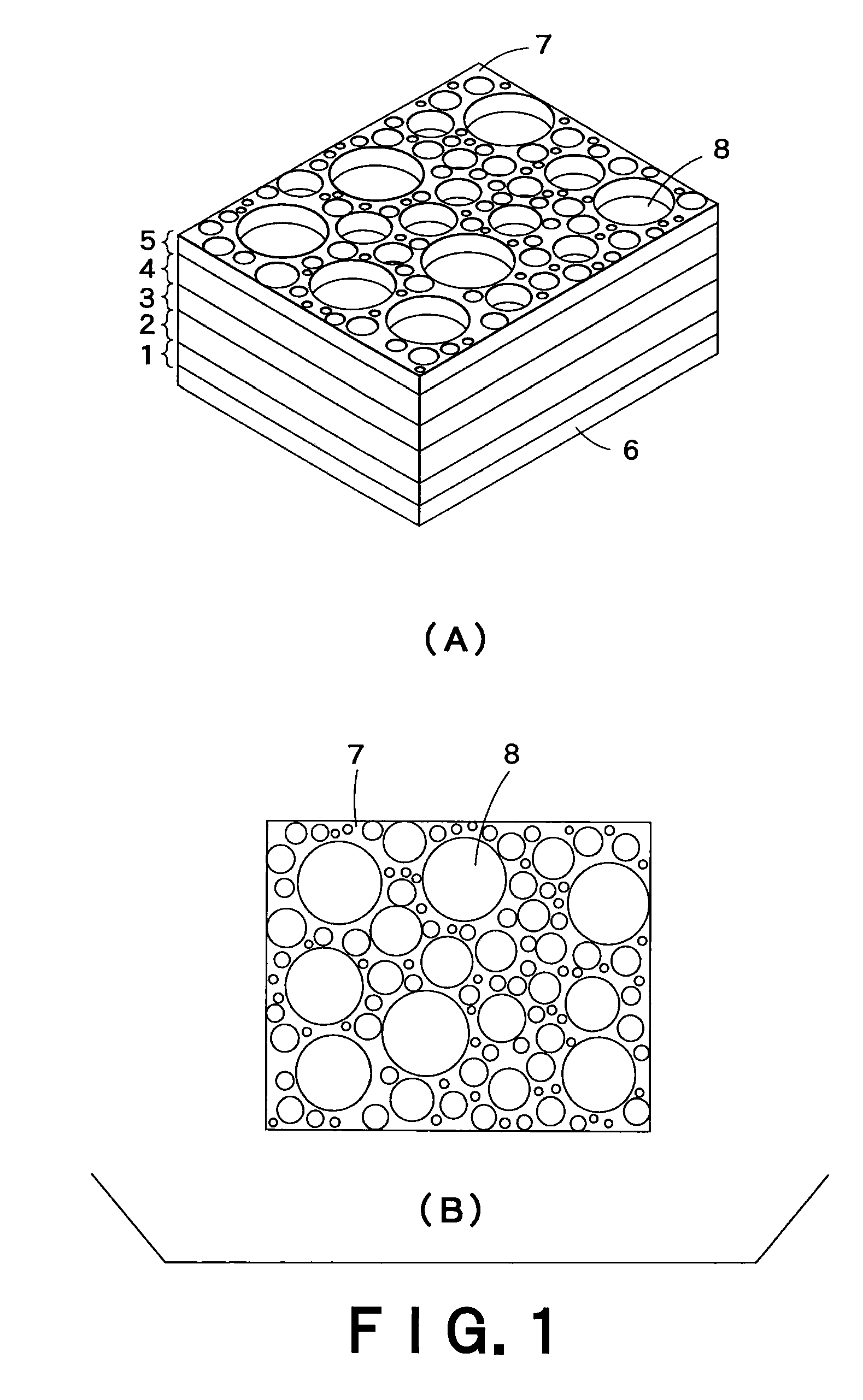
Pore forming material for porous body, manufacturing method of pore forming material for porous body, manufacturing method of porous body, porous body, and honeycomb structural body
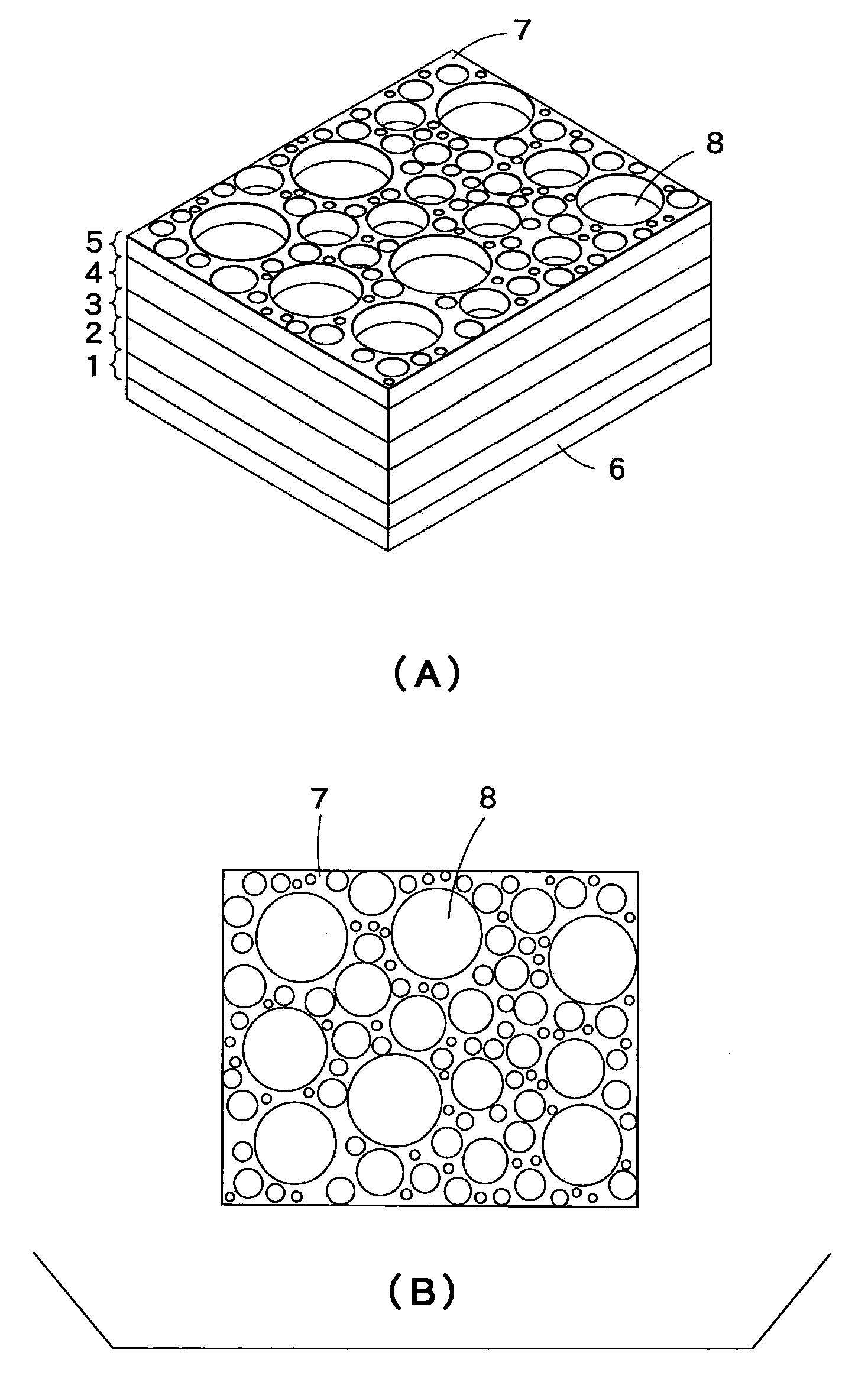
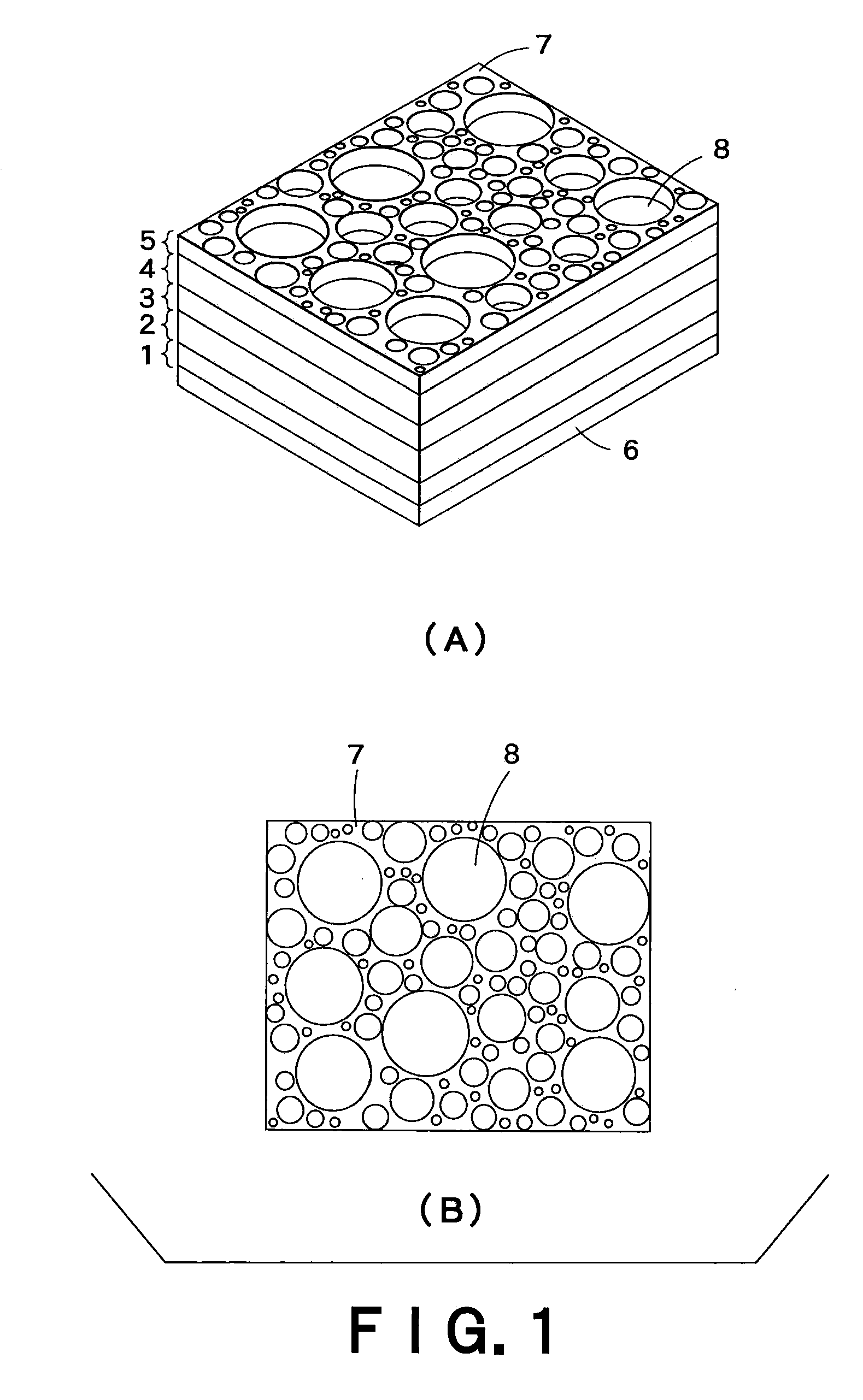
InactiveUS7396586B2High porosityAvoid crackingSolid waste managementExhaust apparatusInorganic particleMicrosphere
The pore forming material for a porous body of the present invention comprises organic polymer particles and inorganic particles. As the embodiment thereof, there may be mentioned one having a structure in which the inorganic particles are contained in the organic polymer particles, and an aggregate body of the organic polymer particles and the inorganic particles. The inorganic particles may be inorganic micro-balloons, and the organic polymer particles may be organic micro-balloons.
Owner:IBIDEN CO LTD
Ceramic filter for exhaust gas emission control
ActiveUS7393376B2High strengthSuitable for manufactureCombination devicesAuxillary pretreatmentMetallurgyExhaust gas emissions
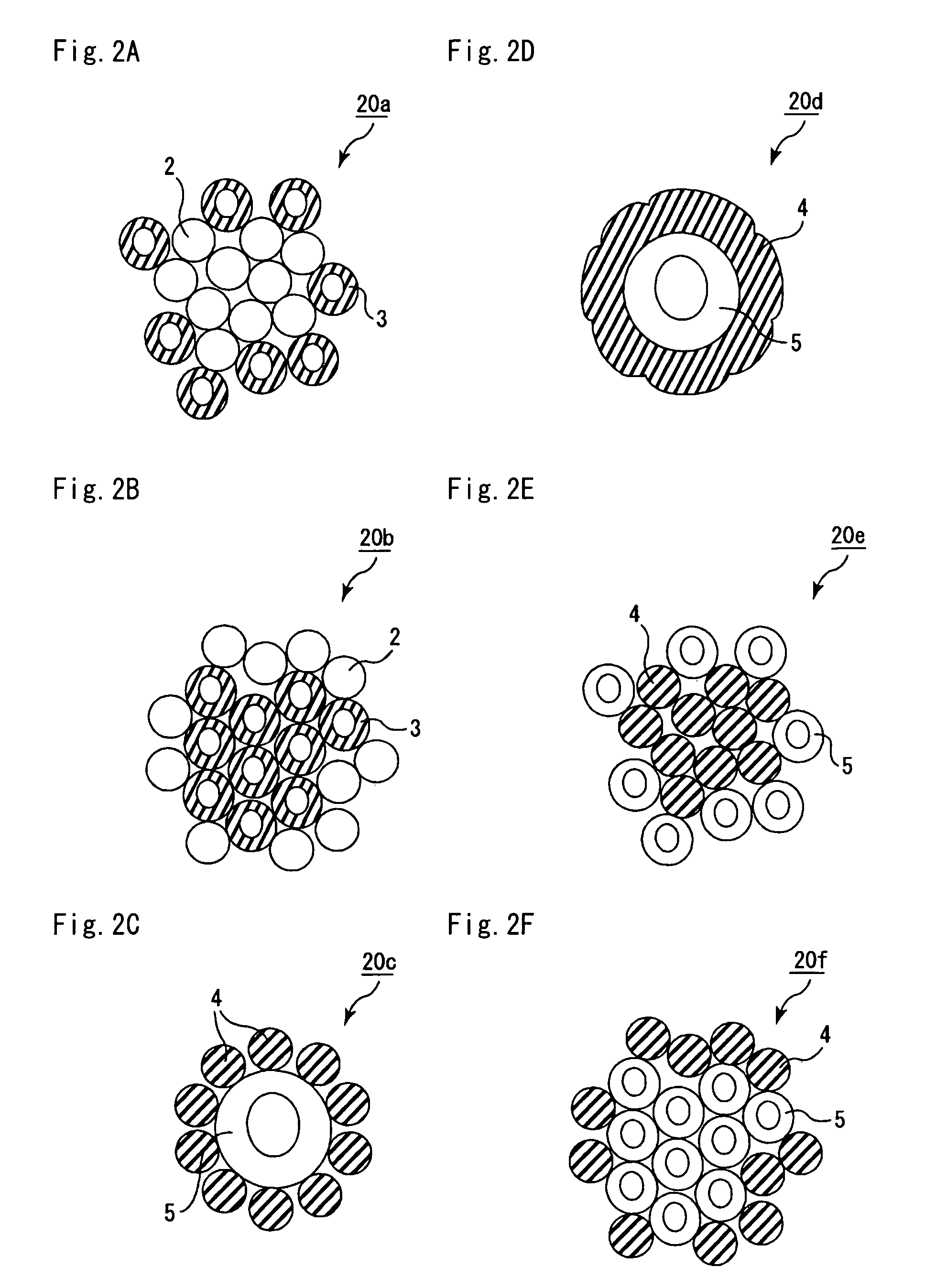
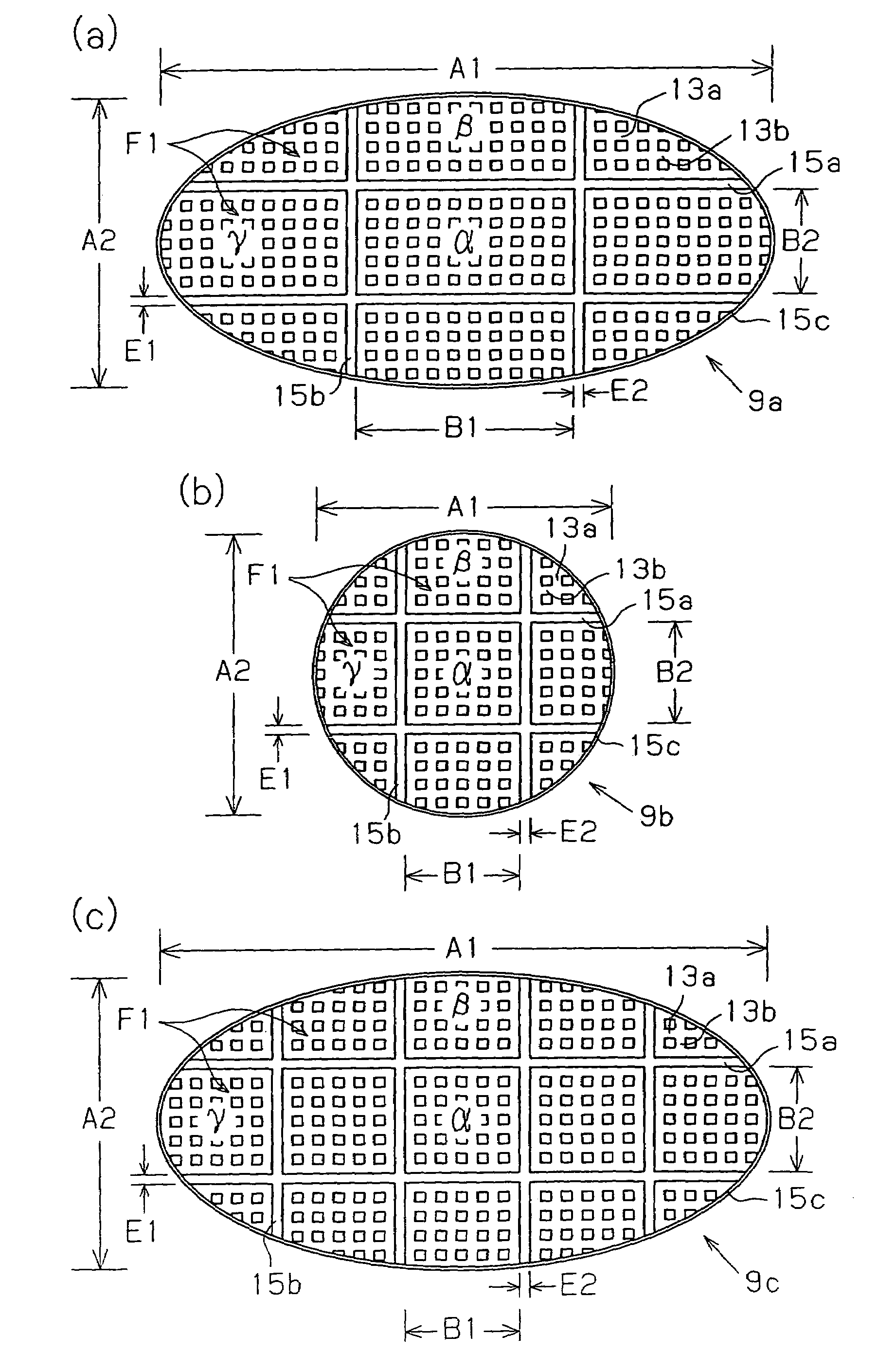
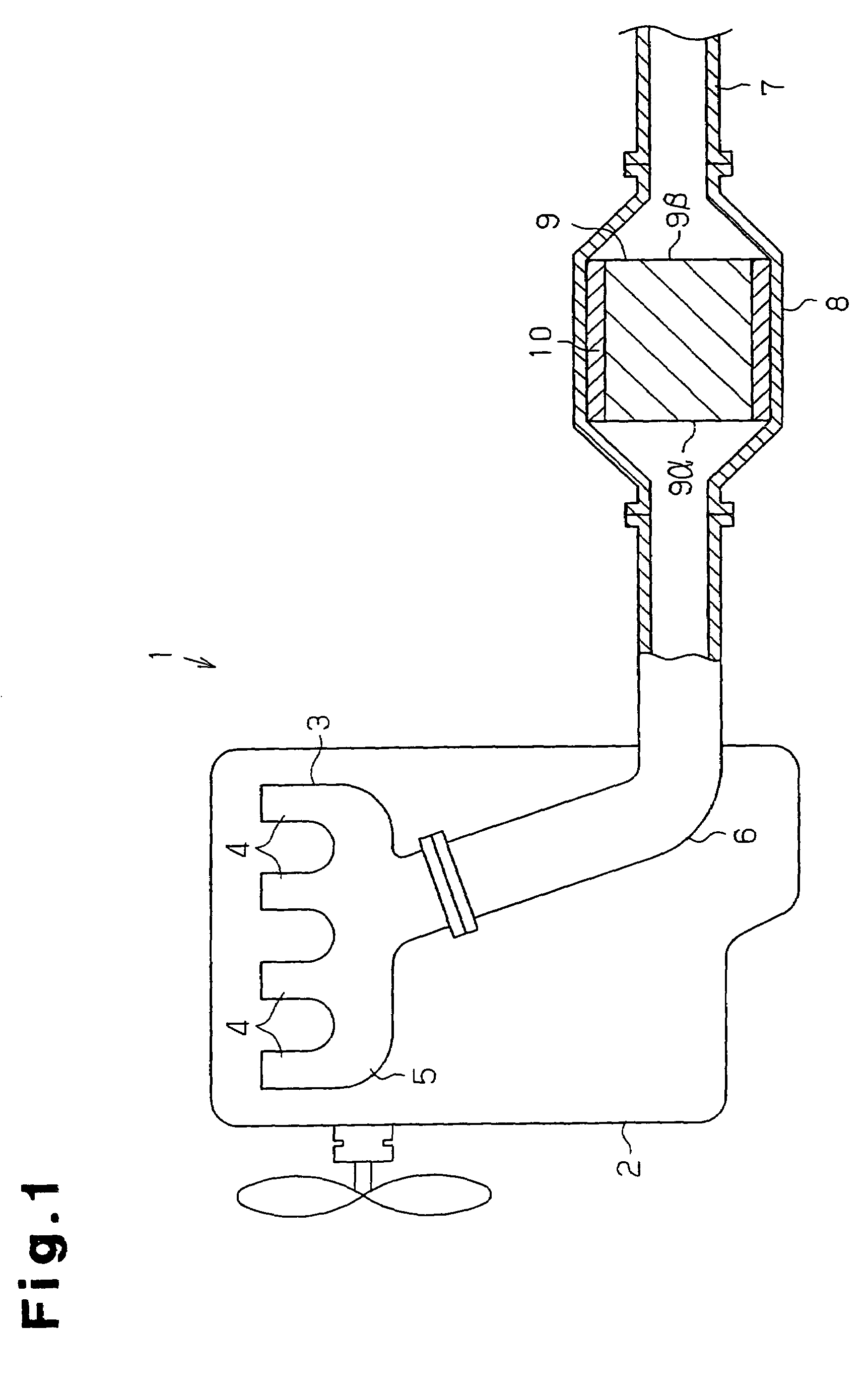
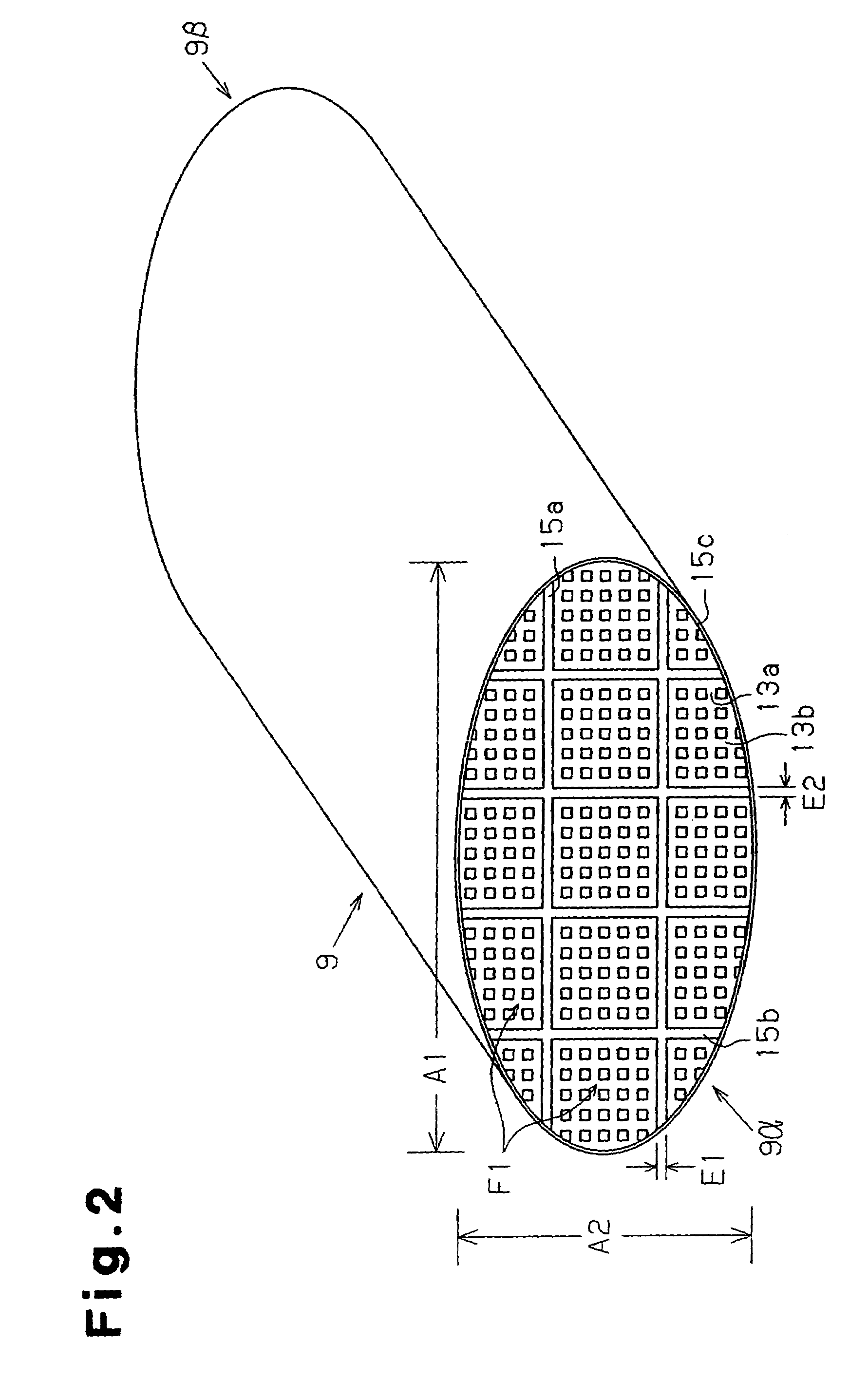
A ceramic filter assembly that resists cracking. The ceramic filter assembly is formed by integrating a plurality of columnar honeycomb filters made of a porous ceramic sintered material with a ceramic sealing material layer and formed to have a substantially elliptical cross sectional shape. The honeycomb filters includes square columnar honeycomb filters in which the ratio between the lengths of their long sides and short sides is between 1.1 and 3.0. The honeycomb filters are arranged so that the long sides and the short sides are respectively parallel to the major axis and the minor axis of the assembly.
Owner:IBIDEN CO LTD
Light extraction from a semiconductor light emitting device via chip shaping
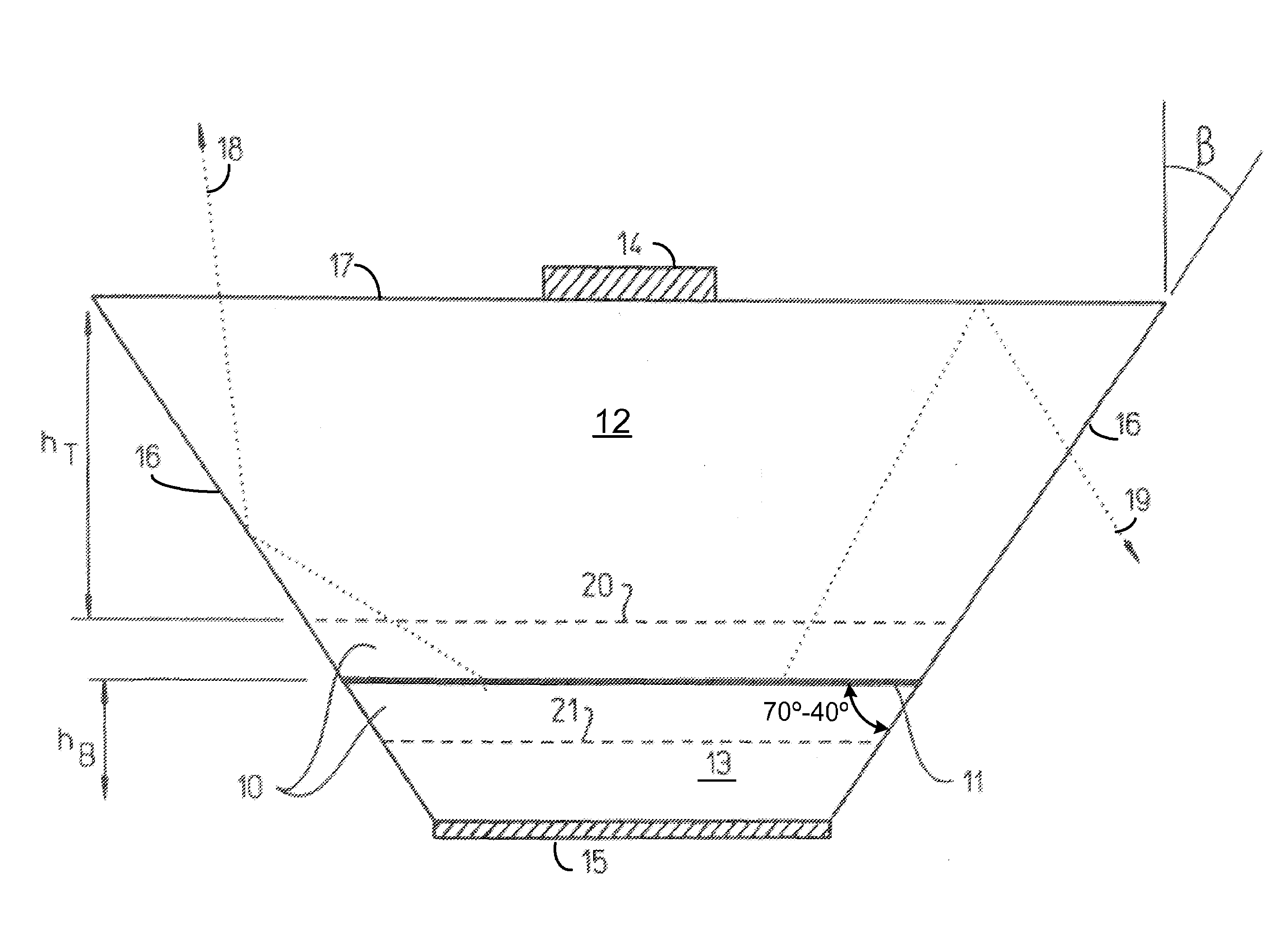
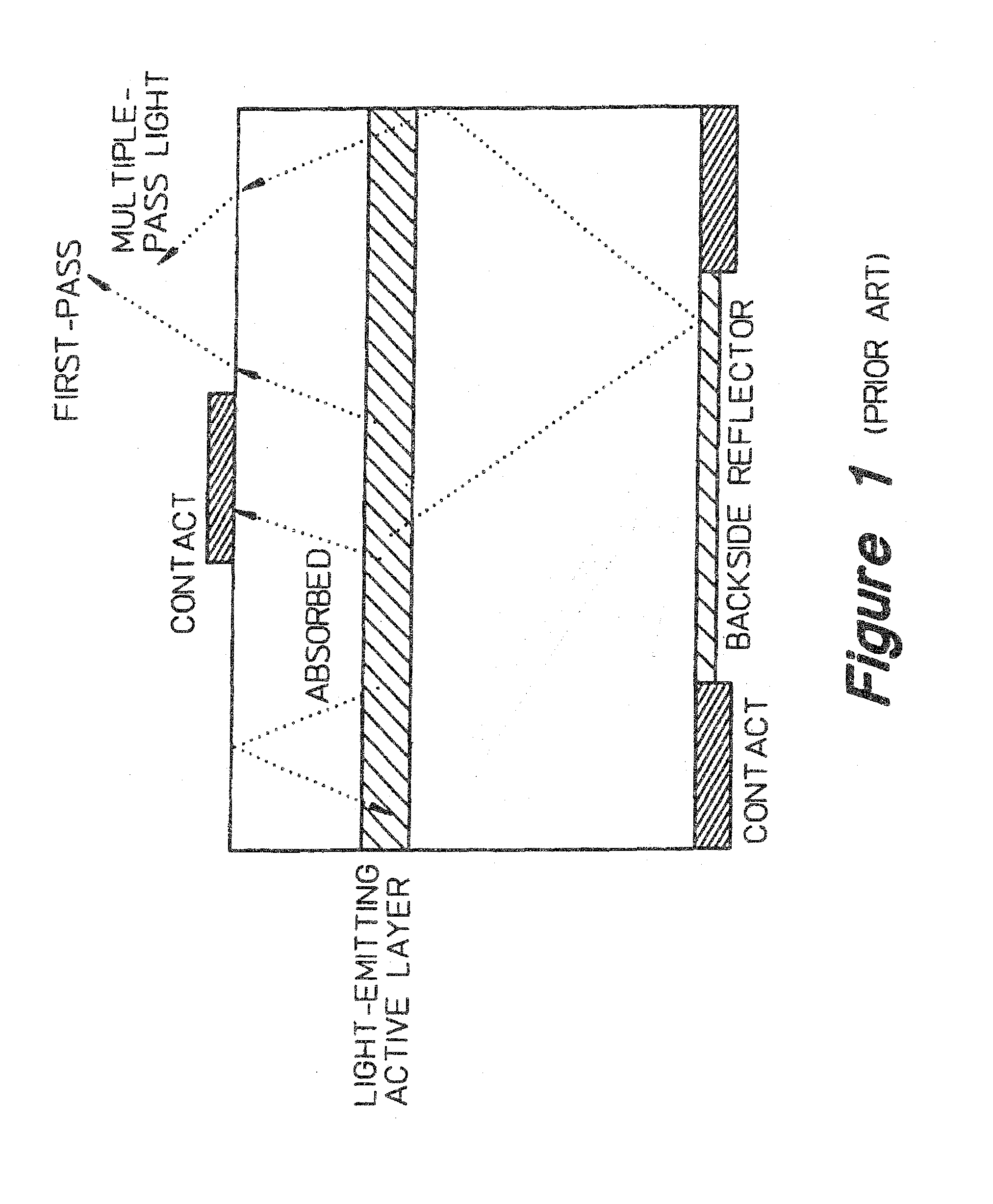
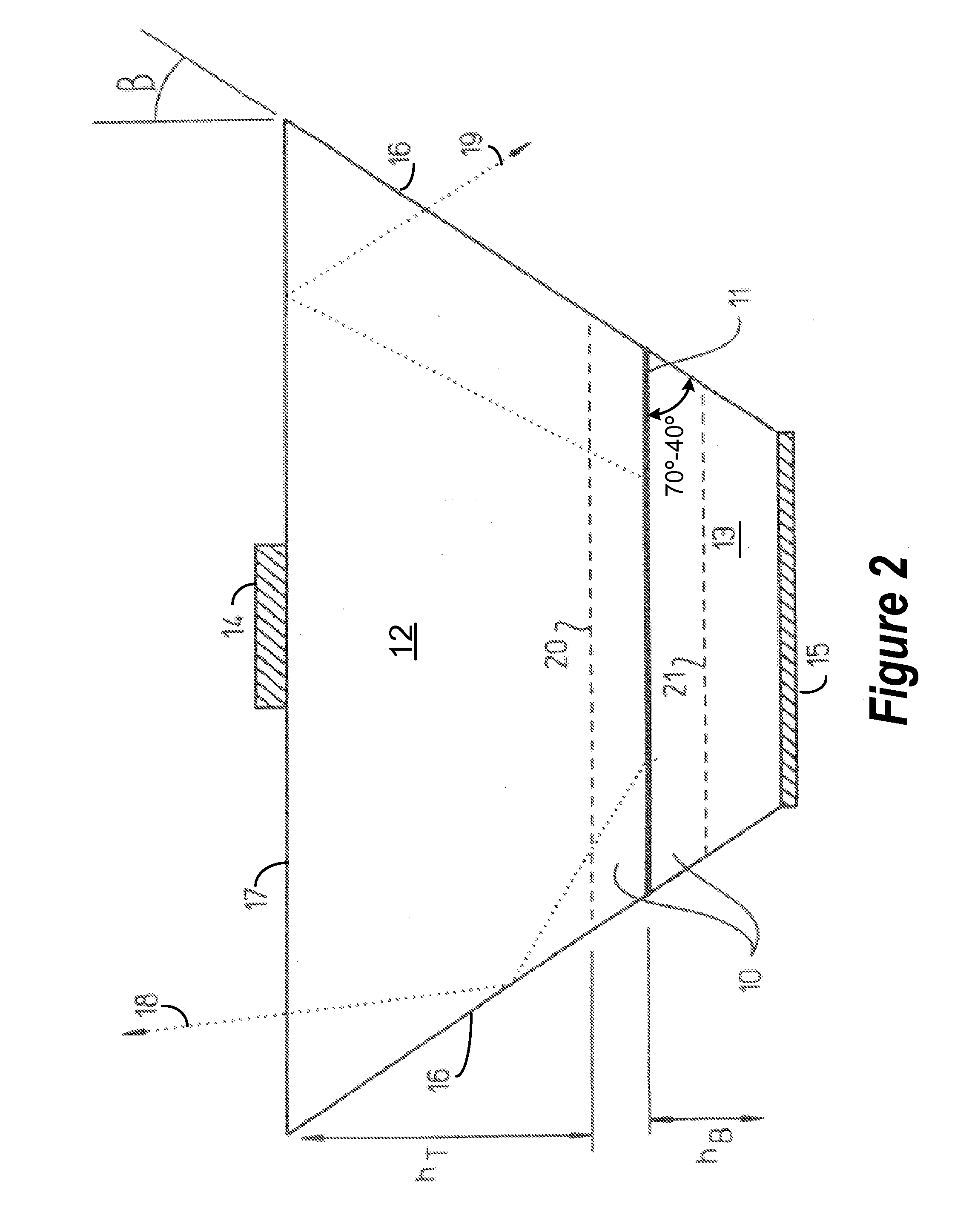
InactiveUS7268371B2Precise positioningReasonable degreeSolid-state devicesSemiconductor devicesHigh volume manufacturingActive layer
A method for designing semiconductor light emitting devices is disclosed wherein the side surfaces (surfaces not parallel to the epitaxial layers) are formed at preferred angles relative to vertical (normal to the plane of the light-emitting active layer) to improve light extraction efficiency and increase total light output efficiency. Device designs are chosen to improve efficiency without resorting to excessive active area-yield loss due to shaping. As such, these designs are suitable for low-cost, high-volume manufacturing of semiconductor light-emitting devices with improved characteristics.
Owner:PHILIPS LUMILEDS LIGHTING CO LLC
Photochromic blue light filtering materials and ophthalmic devices
InactiveUS20050254003A1High elongationHigh refractive indexPhotosensitive materialsTissue regenerationHost materialFilter material
Polymeric compositions have photochromic and blue-light filtering ability and are useful in the manufacture of ophthalmic medical devices. The polymeric compositions comprise a photochromic material incorporated into polymeric host materials and are activatable by blue light having a first wavelength range to become photochromic, and are thereby capable of absorbing blue light in a second wavelength range. Methods of making the compositions comprise incorporating a photochromic material into a polymeric host material.
Owner:BAUSCH & LOMB INC
Crystallization-free glass frit compositions and frits made therefrom for microreactor devices
InactiveUS20070123410A1Low pour pointHigh acid resistanceLaboratory glasswaresChemical/physical/physico-chemical processesMicroreactorFrit
The invention is directed to a glass composition that can be used to make glass frits suitable for use in the manufacturing of microreactors. The glass compositions, after final sintering to produce a finished microreactor, have a surface crystalline layer of 30 μm or less, or are completely amorphous throughout. Generally, the borosilicate glasses of the invention have a composition of B2O3=12-22 mol %; SiO2=68-80 mol % and additional components selected from the group consisting of either (a) Al2O3=3-8 mol % and Li2O=1-8 mol %, or (b) K2O=0-2 mol % and Na2O=0-2 mol %, except that both K2O and Na2O cannot both equal zero at the same time. One borosilicate glass has a composition, in mole percent (mol %) of B2O3=18-22 mol %, SiO2=75-80 mol %, K2O=0-2 mol %, and Na2O=0-2 mol %, except that both K2O and Na2O cannot both equal zero at the same time.
Owner:CORNING INC
Template generating method and apparatus of the same, pattern detecting method, position detecting method and apparatus of the same, exposure apparatus and method of the same, device manufacturing method and template generating program
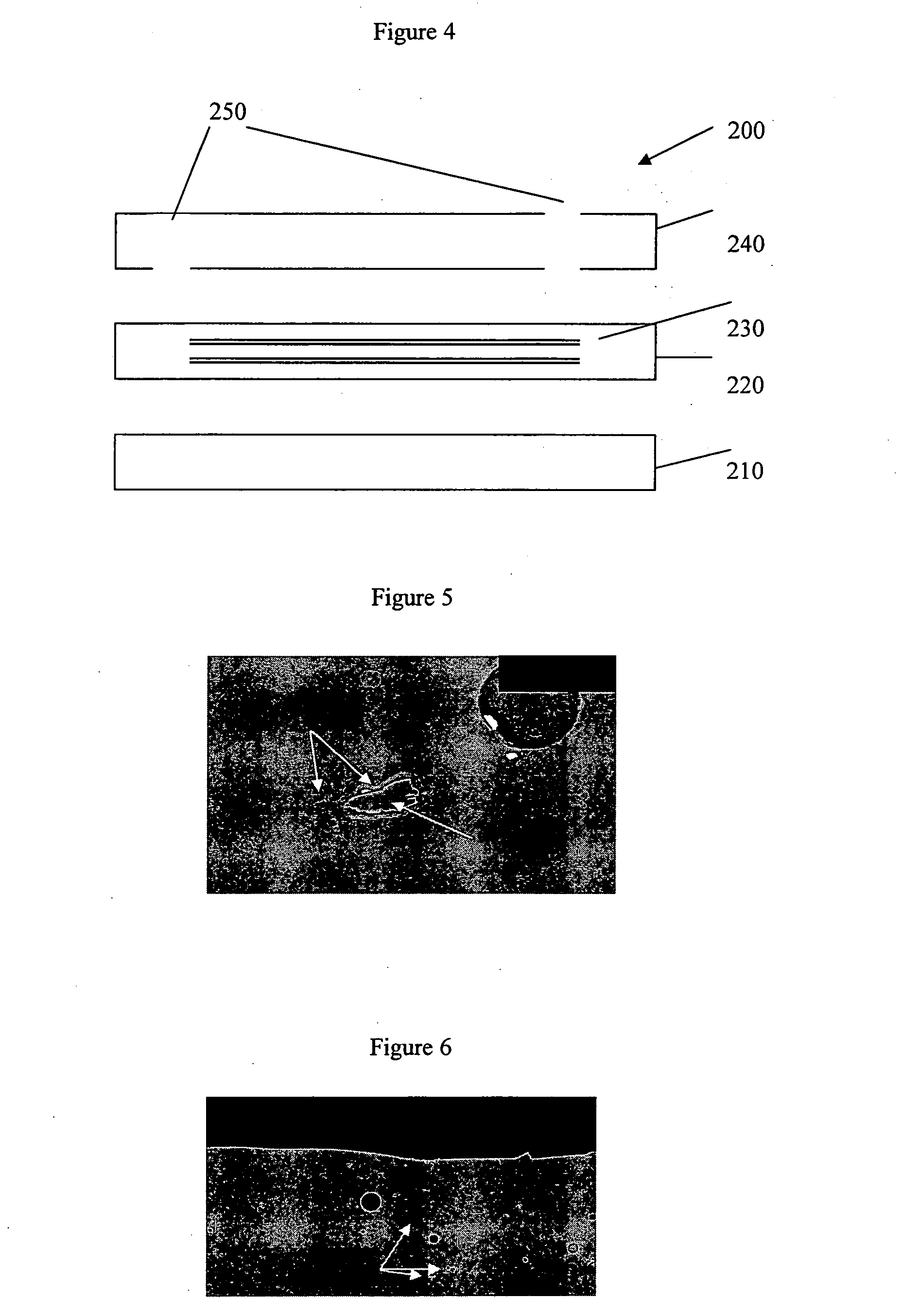
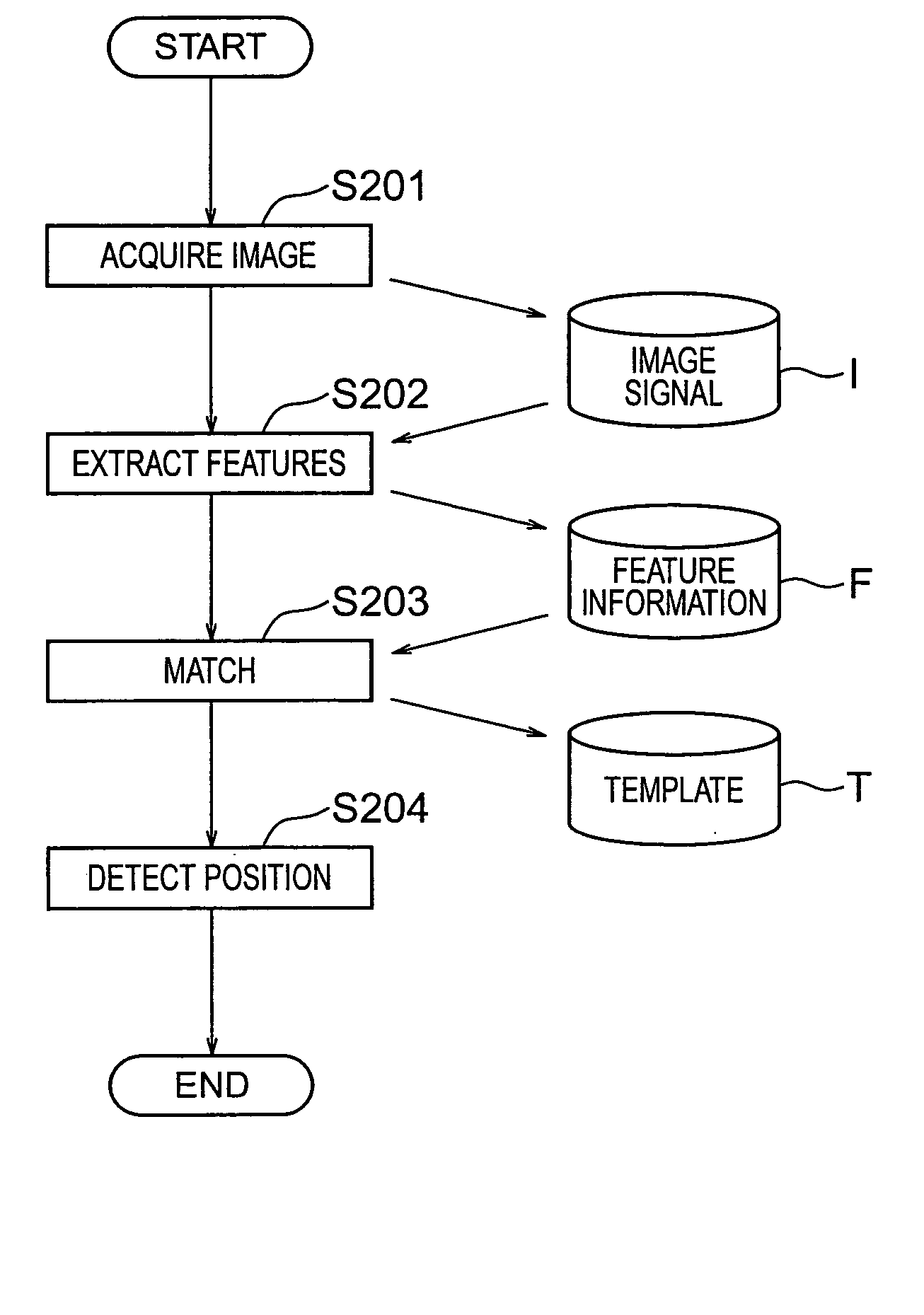
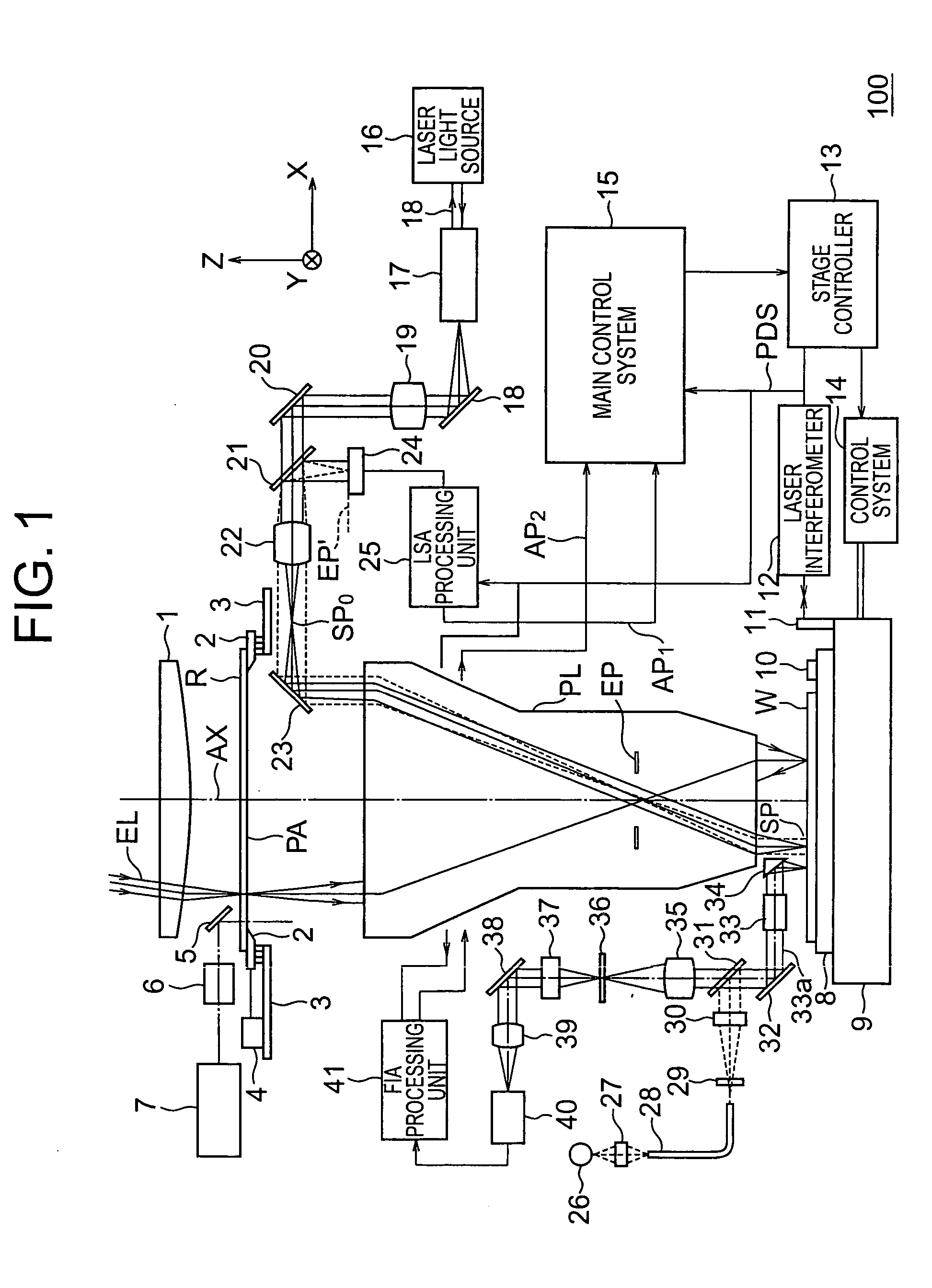
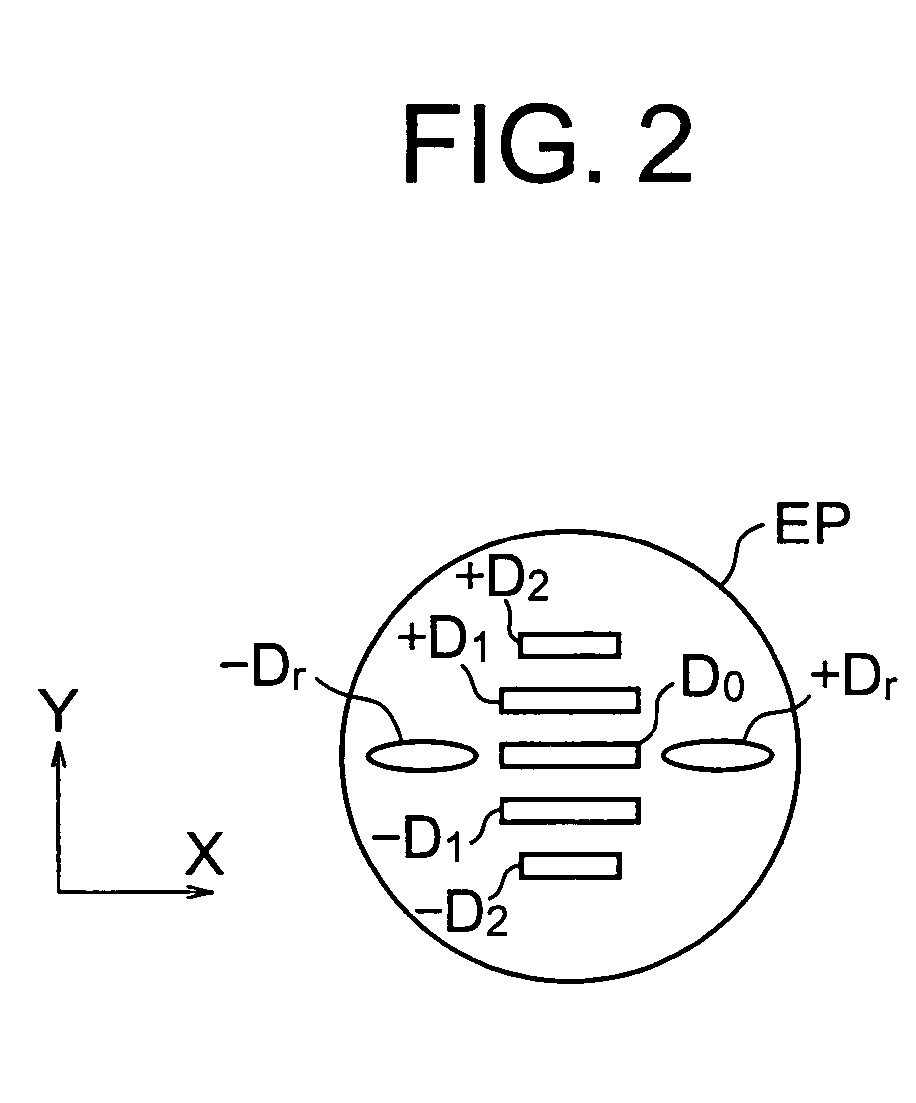
InactiveUS20060126916A1Promote generationSuitable for detectionSemiconductor/solid-state device manufacturingCharacter and pattern recognitionTemplate matchingImage deformation
A method enabling easy generation of a template handling pattern deformation due to optical conditions or process conditions and suitably detecting pattern position is disclosed. A first method extracts a feature component, that is, symmetry, not affected by optical conditions etc. from the pattern image information and uses it as a template. At the time of pattern detection, image information is projected into a symmetry feature space and matching performed in the feature space to detect a pattern. Suitable template matching can be performed with being affected by pattern deformation. A second method generates a model of a pattern from input pattern data, calculates a plurality of pattern images (virtual models) obtained by imaging of the model for each imaging condition using an image deformation simulator, and determines a template considering their average or correlation.
Owner:NIKON CORP
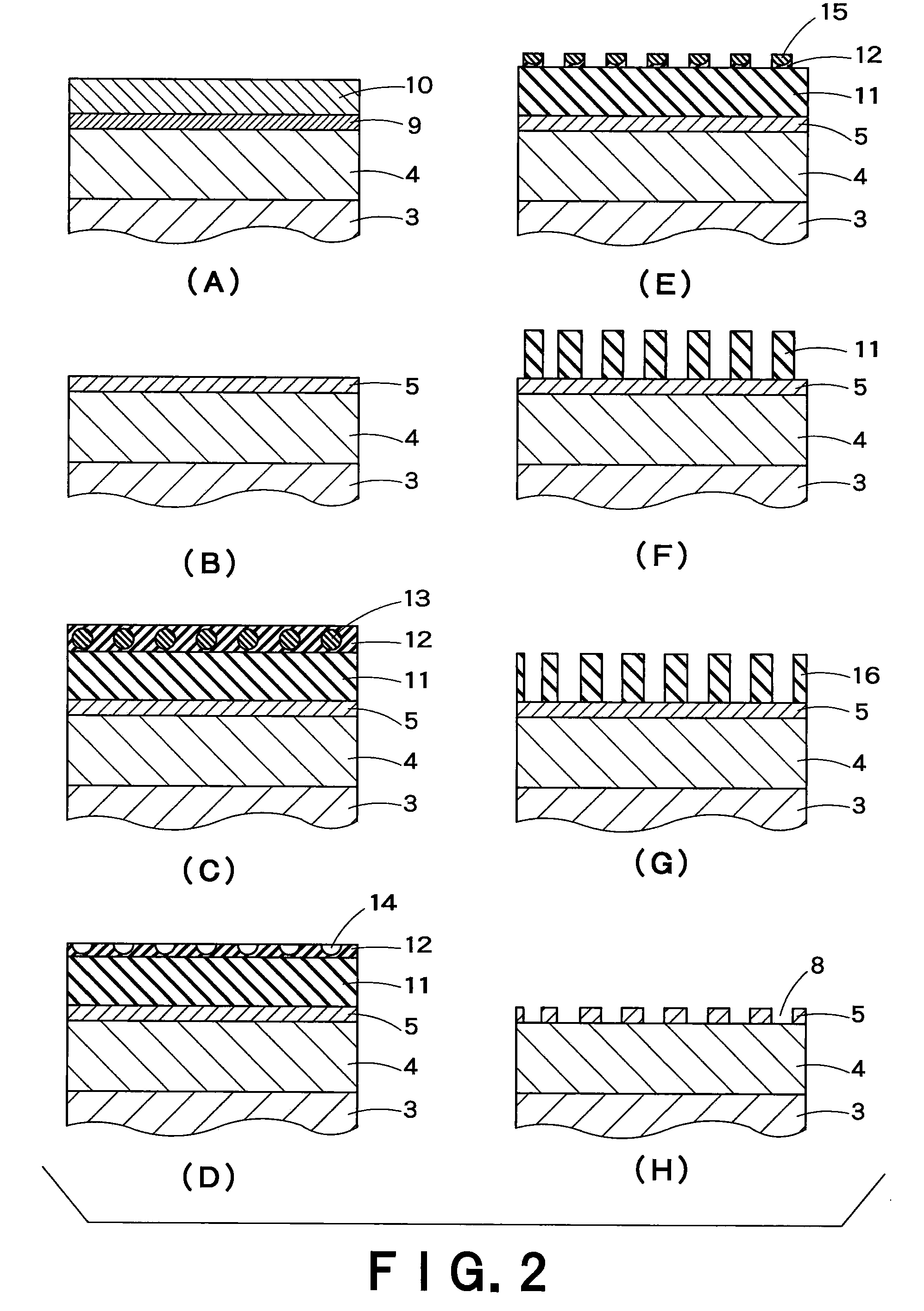
Multilayer wiring substrate and method of manufacturing the same
InactiveUS20120153463A1Avoid layeringDistribute pressureSemiconductor/solid-state device detailsPrinted circuit aspectsRough surfaceInsulation layer
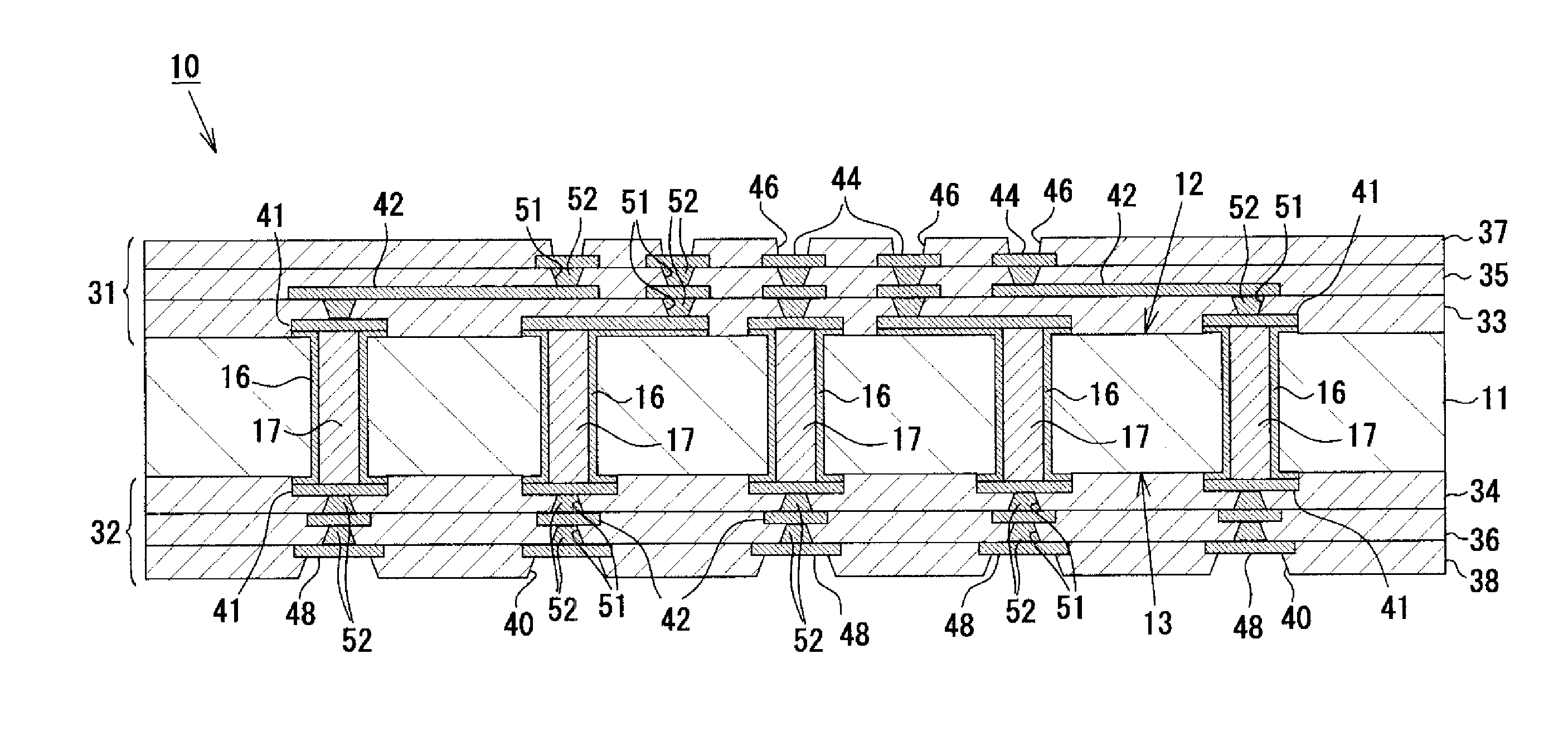
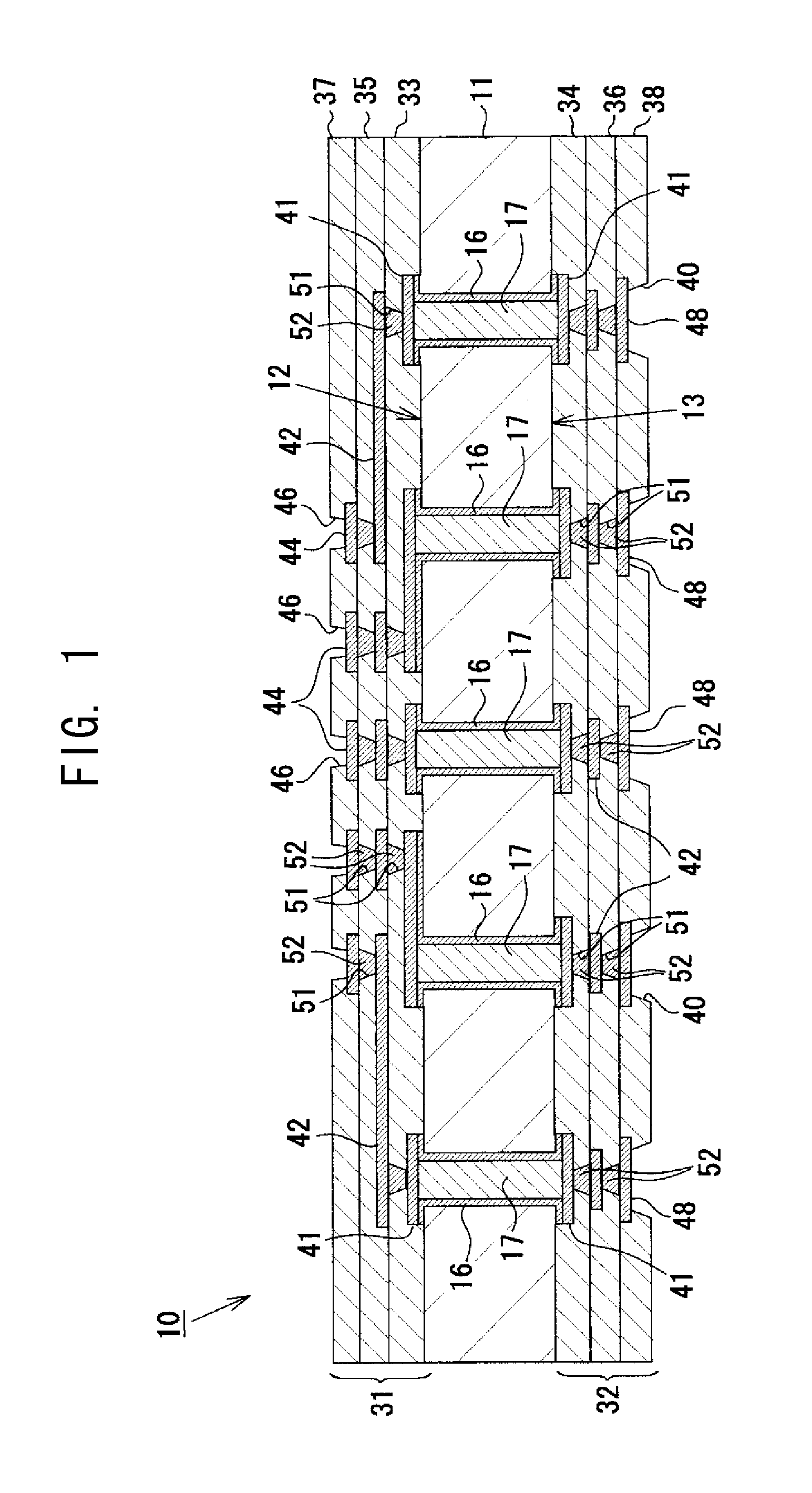
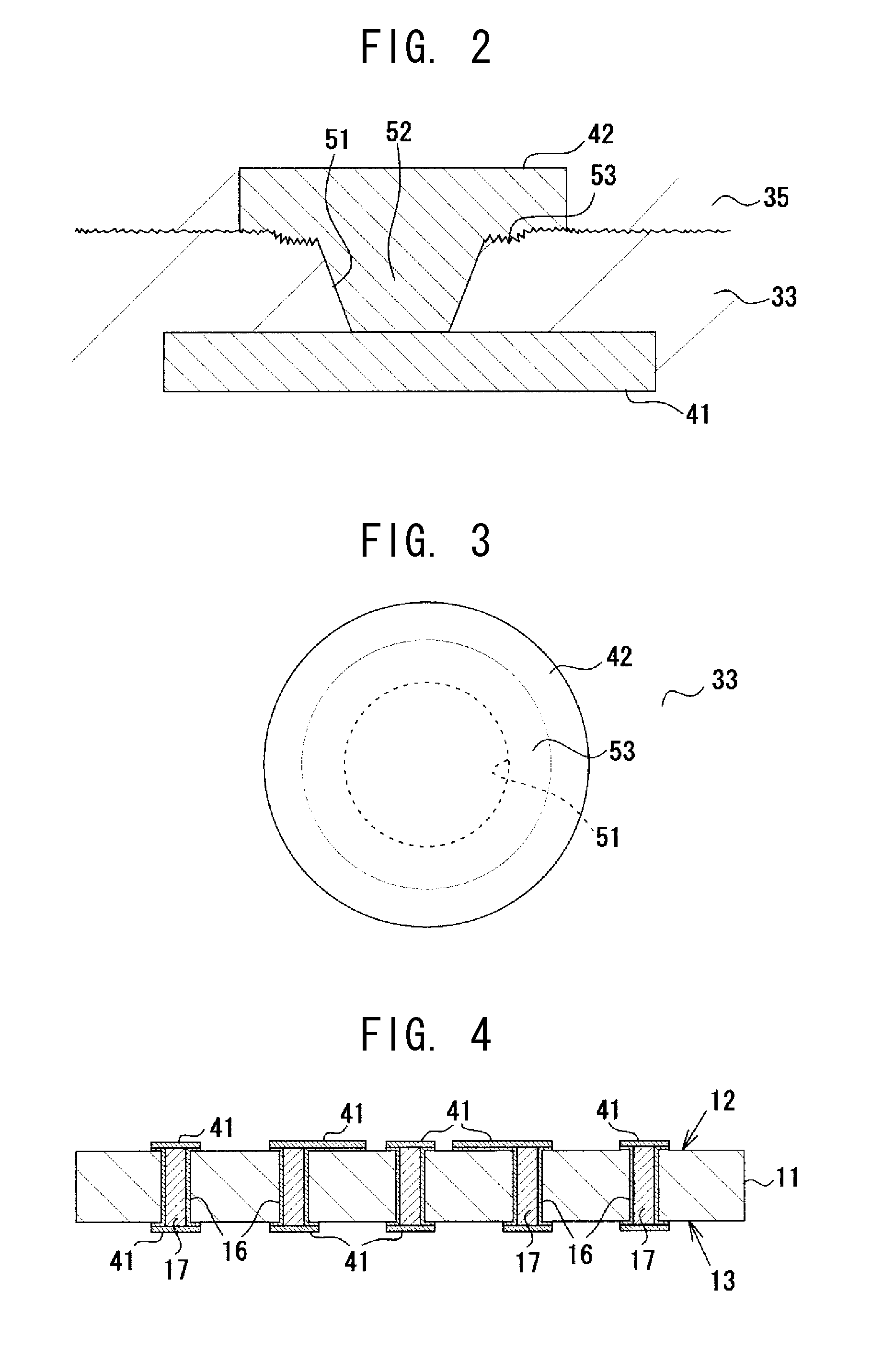
To provide a multilayer wiring substrate in which the connection reliability of via conductors is enhanced, via holes are formed in a resin interlayer insulation layer which isolates a lower conductor layer from an upper conductor layer, and via conductors are formed in the via holes for connecting the lower conductor layer and the upper conductor layer. The surface of the resin interlayer insulation layer is a rough surface, and the via holes open at the rough surface of the resin interlayer insulation layer. Stepped portions are formed in opening verge regions around the via holes such that the stepped portions are recessed from peripheral regions around the opening verge regions. The stepped portions are higher in surface roughness than the peripheral regions.
Owner:NGK SPARK PLUG CO LTD
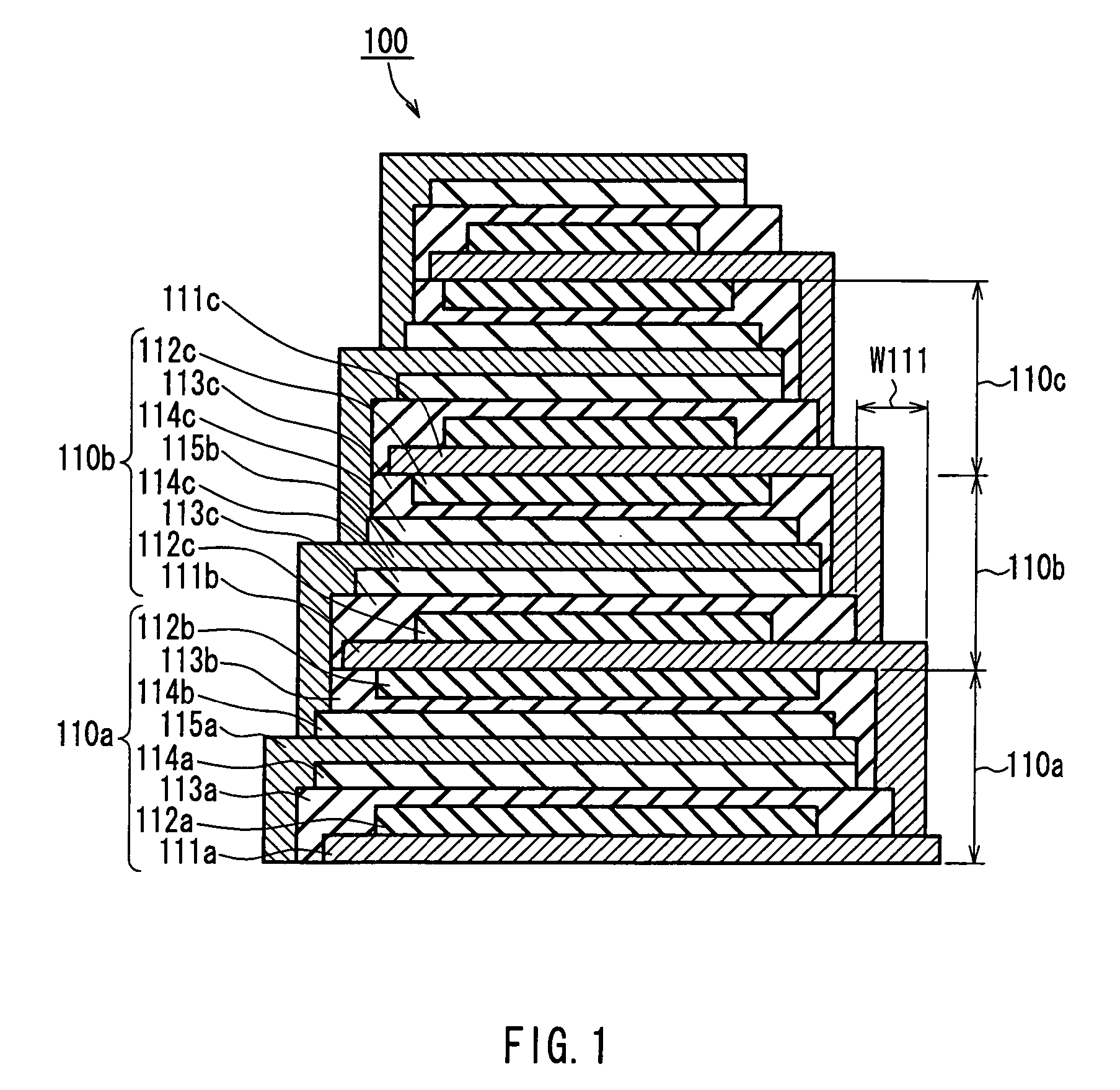
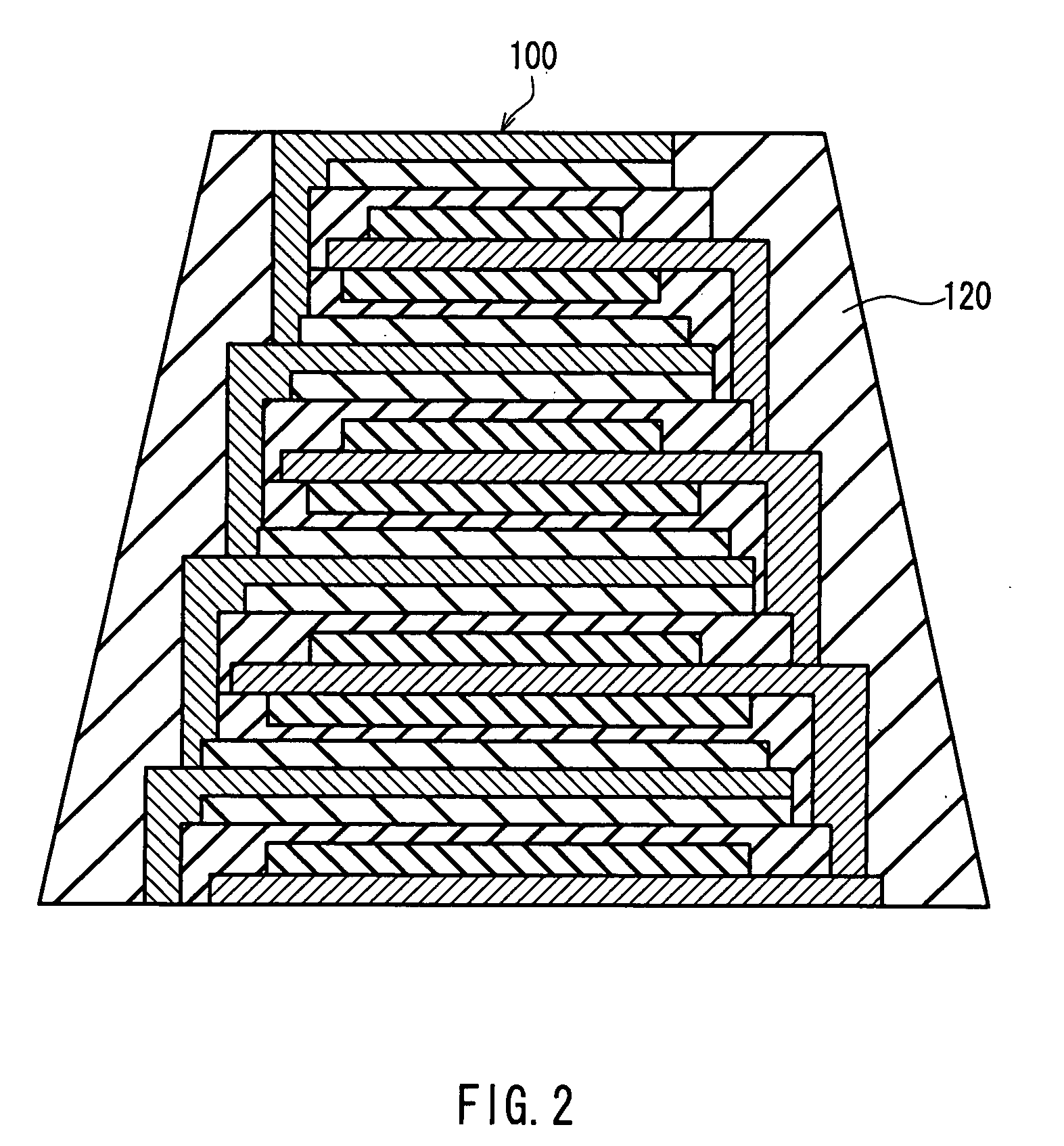
Thin-film laminated body, thin-film cell, capacitor, and method and equipment for manufacturing thin-film laminated body
InactiveUS20050141170A1Improve connection reliabilityQuality improvementCell electrodesFinal product manufactureEngineeringAdhesion strength
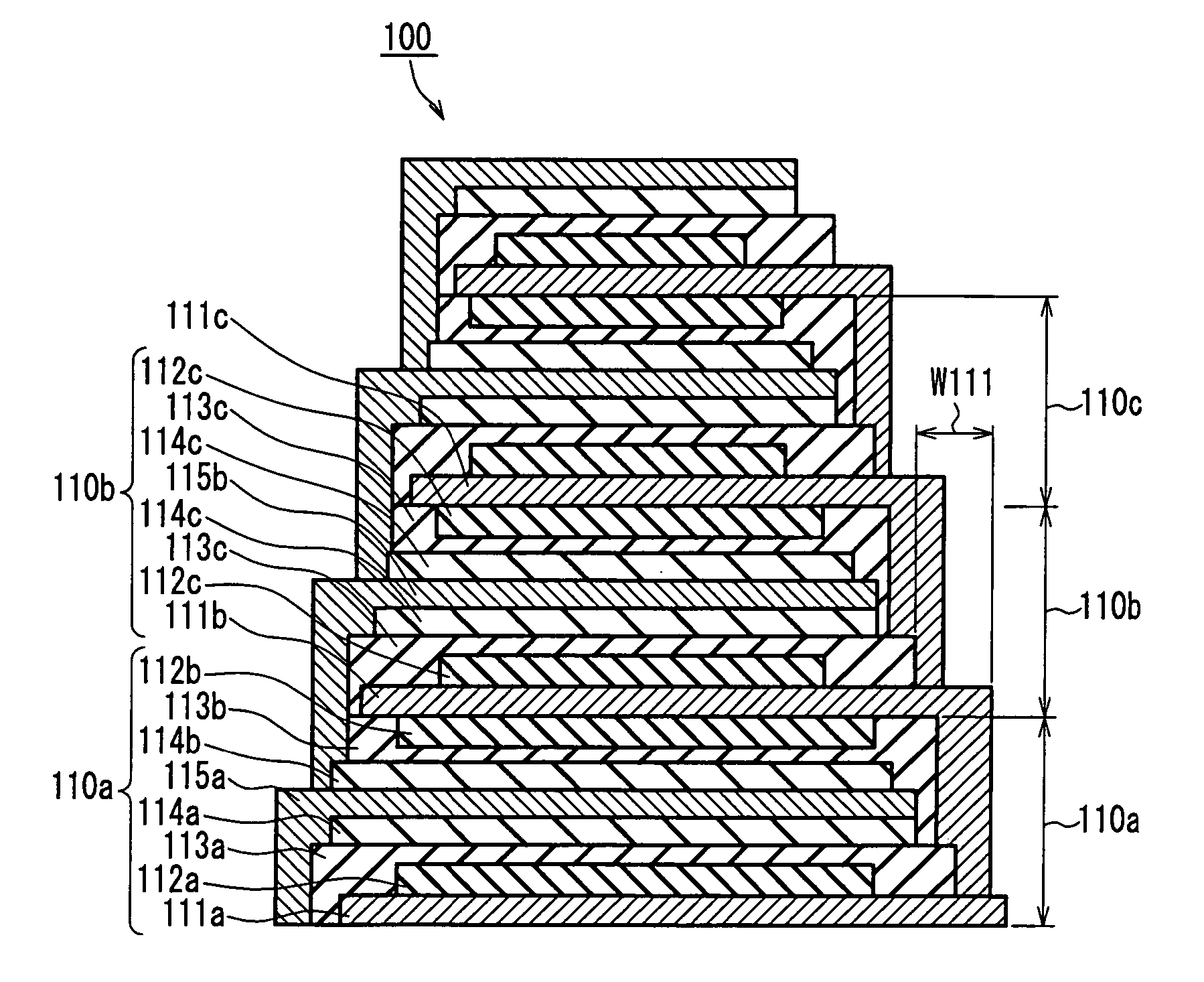
A thin film layered product is composed of at least two deposition units, each of which includes at least a first thin film layer and a second thin film layer. At least one of the first thin film layer and the second thin film layer in each of the at least two deposition units is laminated so as to have an area decreased in a direction from a lower layer toward an upper layer. Thus, the reliability of connection between layers can be improved, and when a protective layer is formed on side faces, the formability and adhesion strength can be improved.
Owner:PANASONIC CORP
Laser annealing apparatus and annealing method of semiconductor thin film using the same
InactiveUS20050170572A1Prolong lifeCost of apparatus can be reducedLaser detailsElectrode and associated part arrangementsOptical axisLight beam
A laser beam temporally modulated in amplitude by a modulator and shaped into a long and narrow shape by a beam shaper is rotated around the optical axis of an image rotator inserted between the beam shaper and a substrate. Thus, the longitudinal direction of the laser beam having the long and narrow shape is rotated around the optical axis on the substrate. In order to perform annealing in a plurality of directions on the substrate, the laser beam shaped into the long and narrow shape is rotated on the substrate while a stage mounted with the substrate is moved only in two directions, that is, X- and Y-directions. In such a manner, the substrate can be scanned at a high speed with a continuous wave laser beam modulated temporally in amplitude and shaped into a long and narrow shape, without rotating the substrate. Thus, a semiconductor film can be annealed.
Owner:PANASONIC LIQUID CRYSTAL DISPLAY CO LTD +1
Pore forming material for porous body, manufacturing method of pore forming material for porous body, manufacturing method of porous body, porous body, and honeycomb structural body
InactiveUS20060154021A1High porosityAvoid crackingSolid waste managementExhaust apparatusInorganic particleInorganic particles
The pore forming material for a porous body of the present invention comprises organic polymer particles and inorganic particles. As the embodiment thereof, there may be mentioned one having a structure in which the inorganic particles are contained in the organic polymer particles, and an aggregate body of the organic polymer particles and the inorganic particles. The inorganic particles may be inorganic micro-balloons, and the organic polymer particles may be organic micro-balloons.
Owner:IBIDEN CO LTD
Plastic tolerance compensating assembly
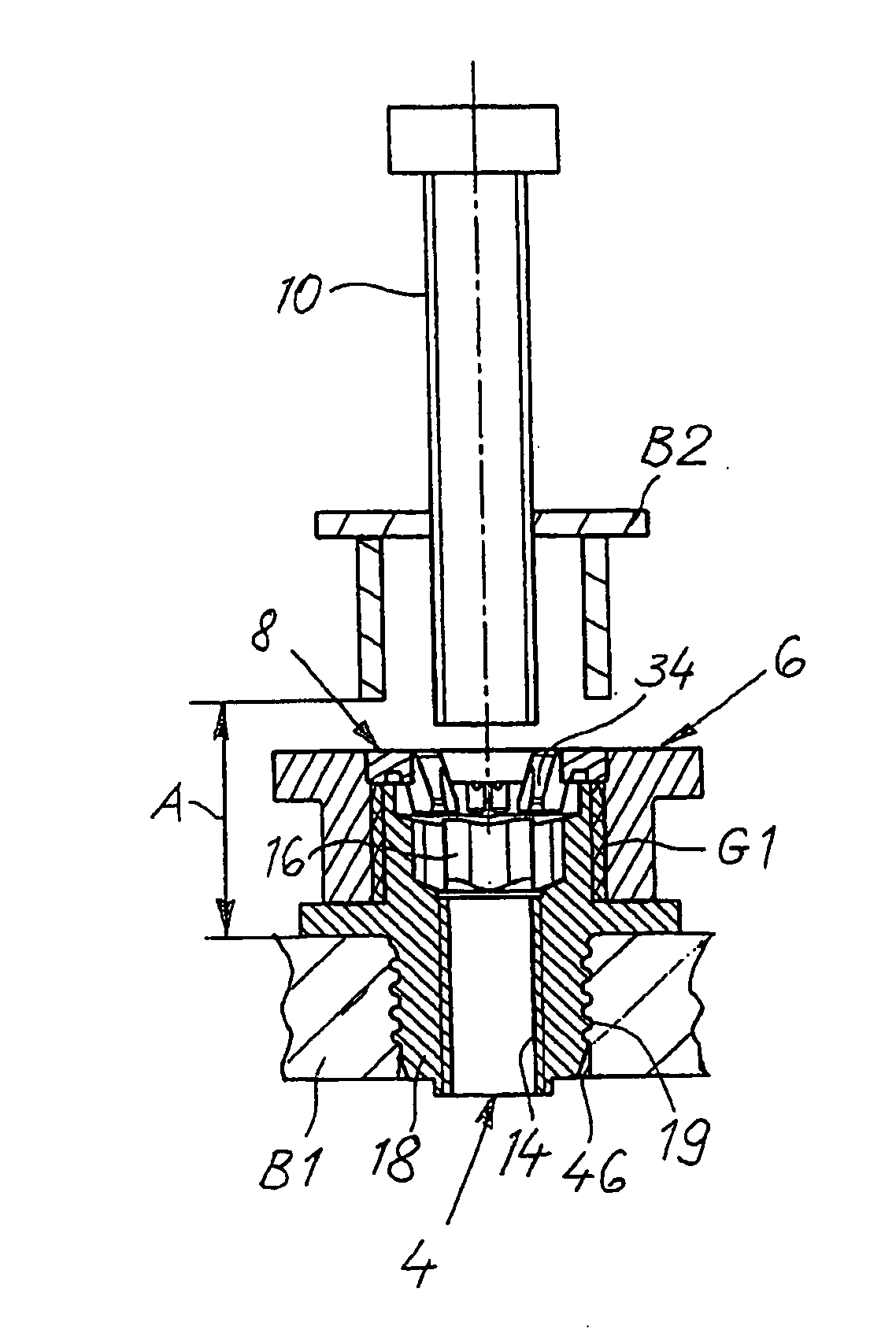
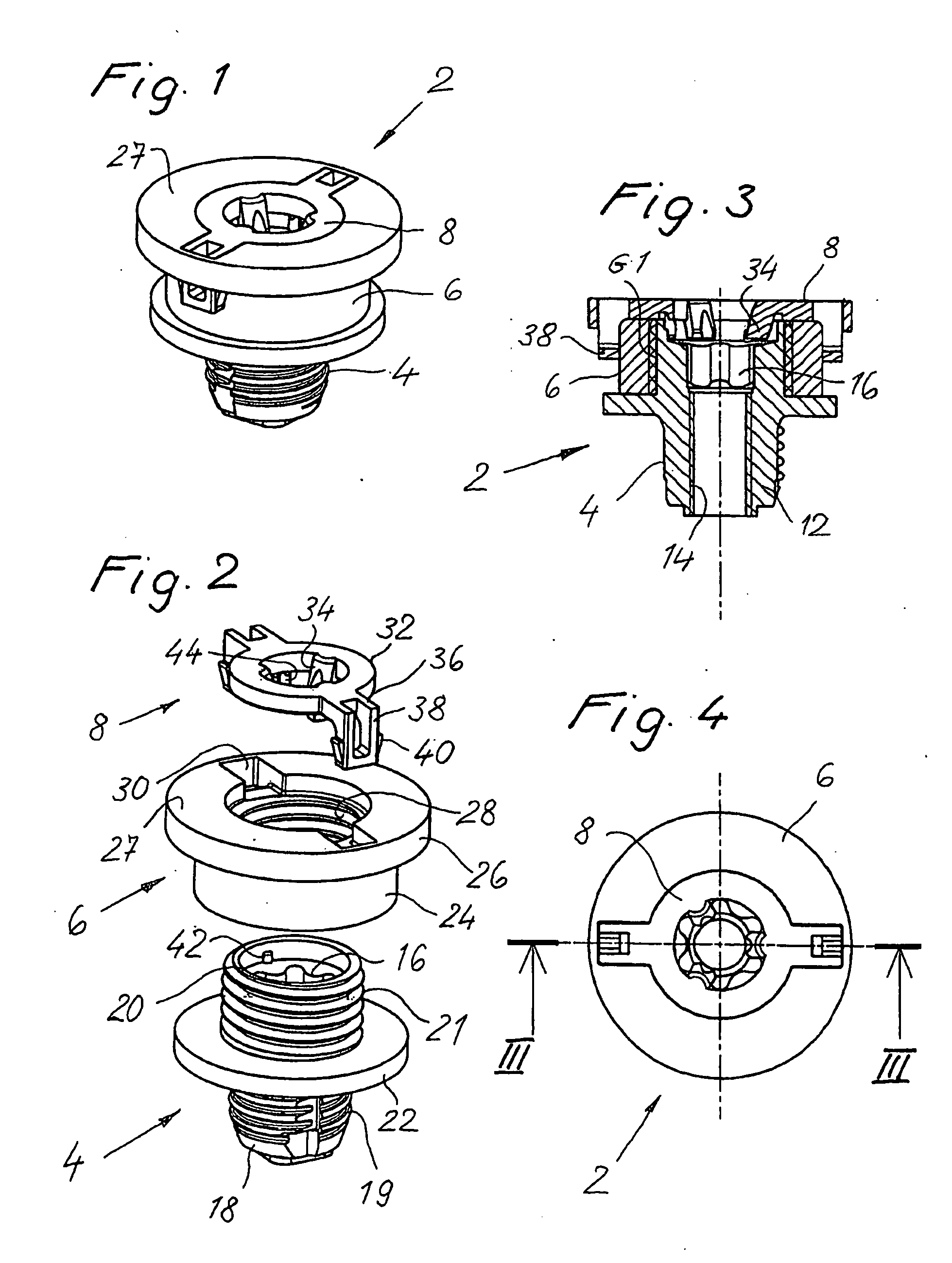
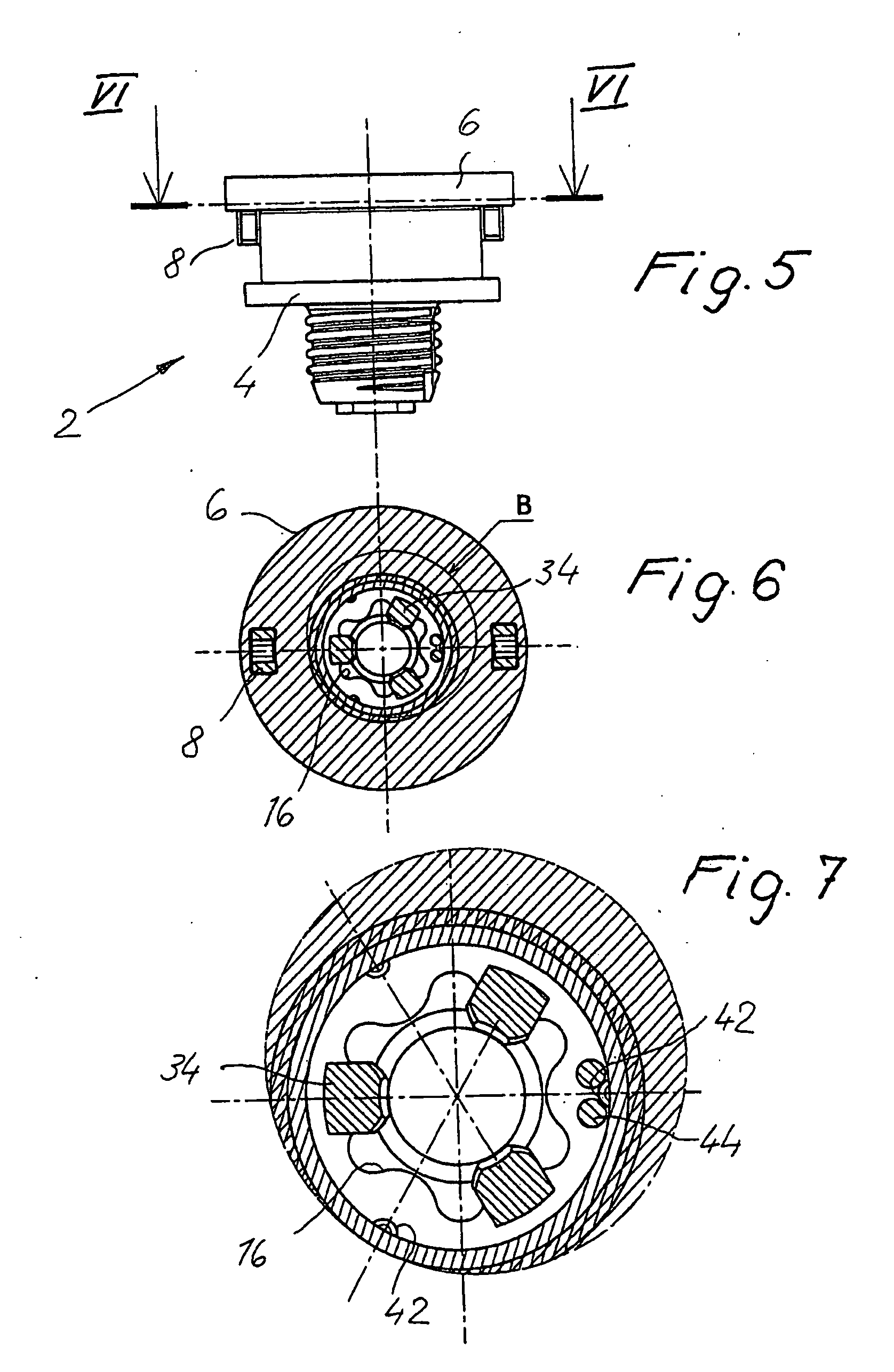
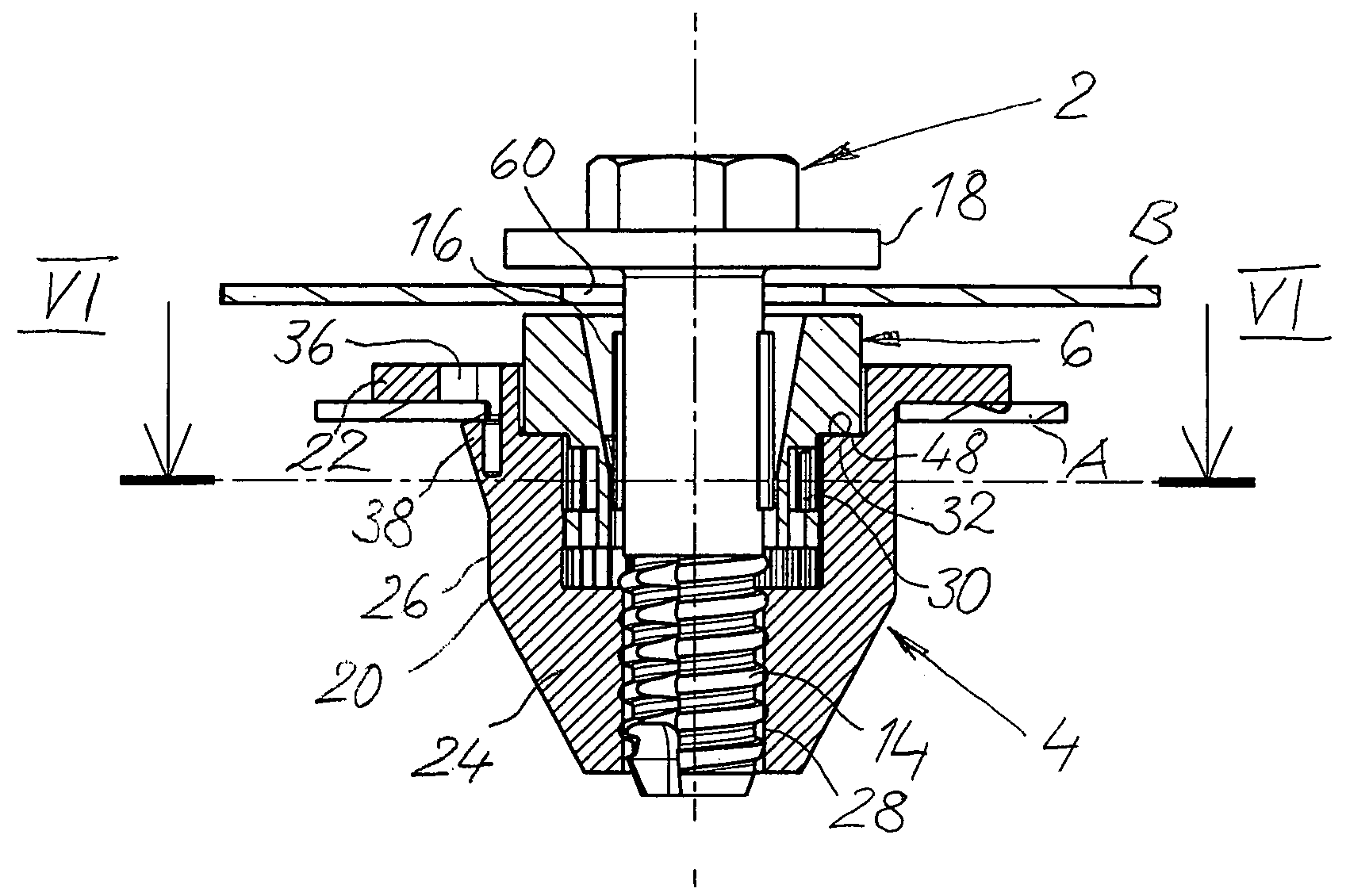
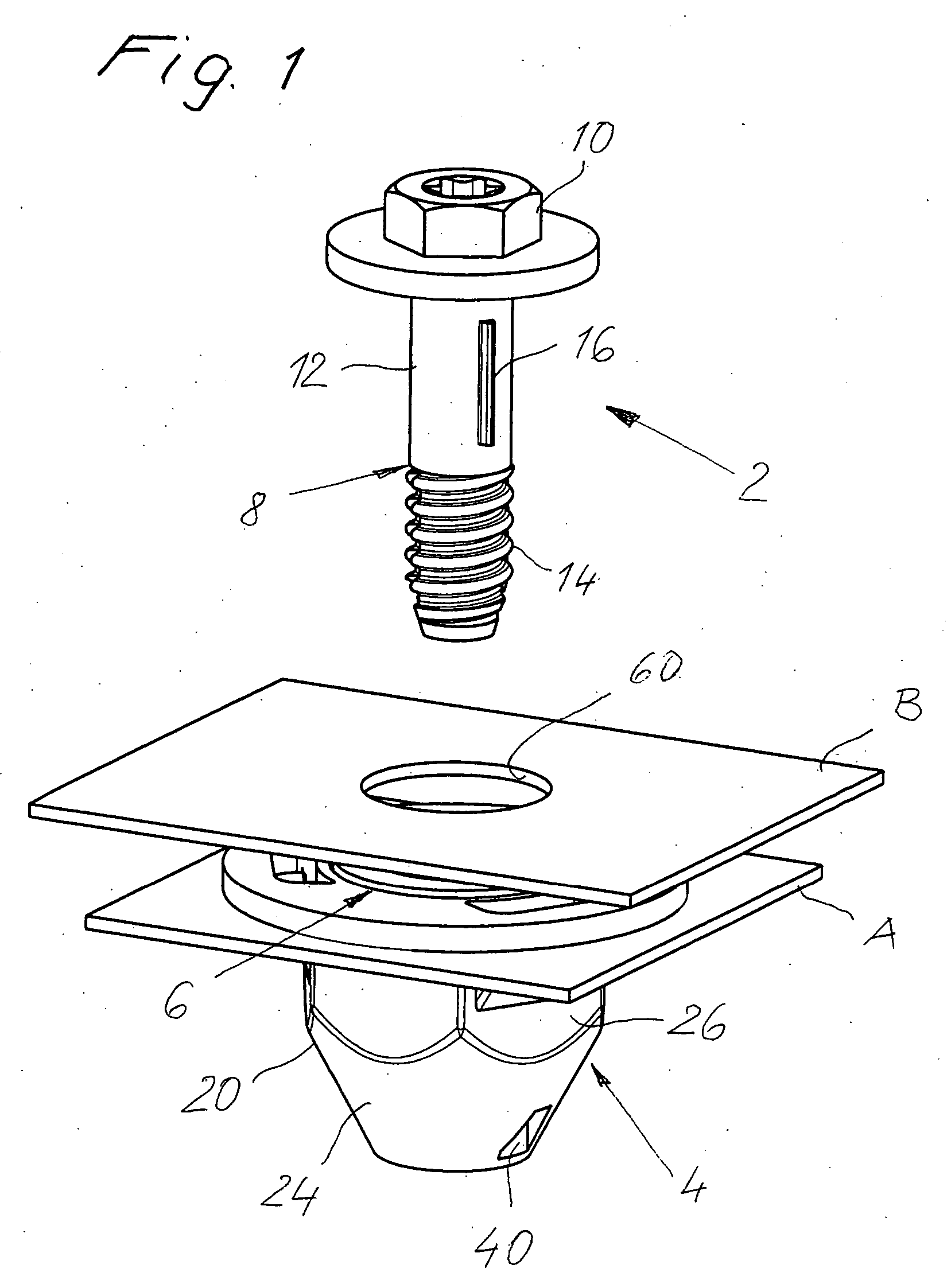
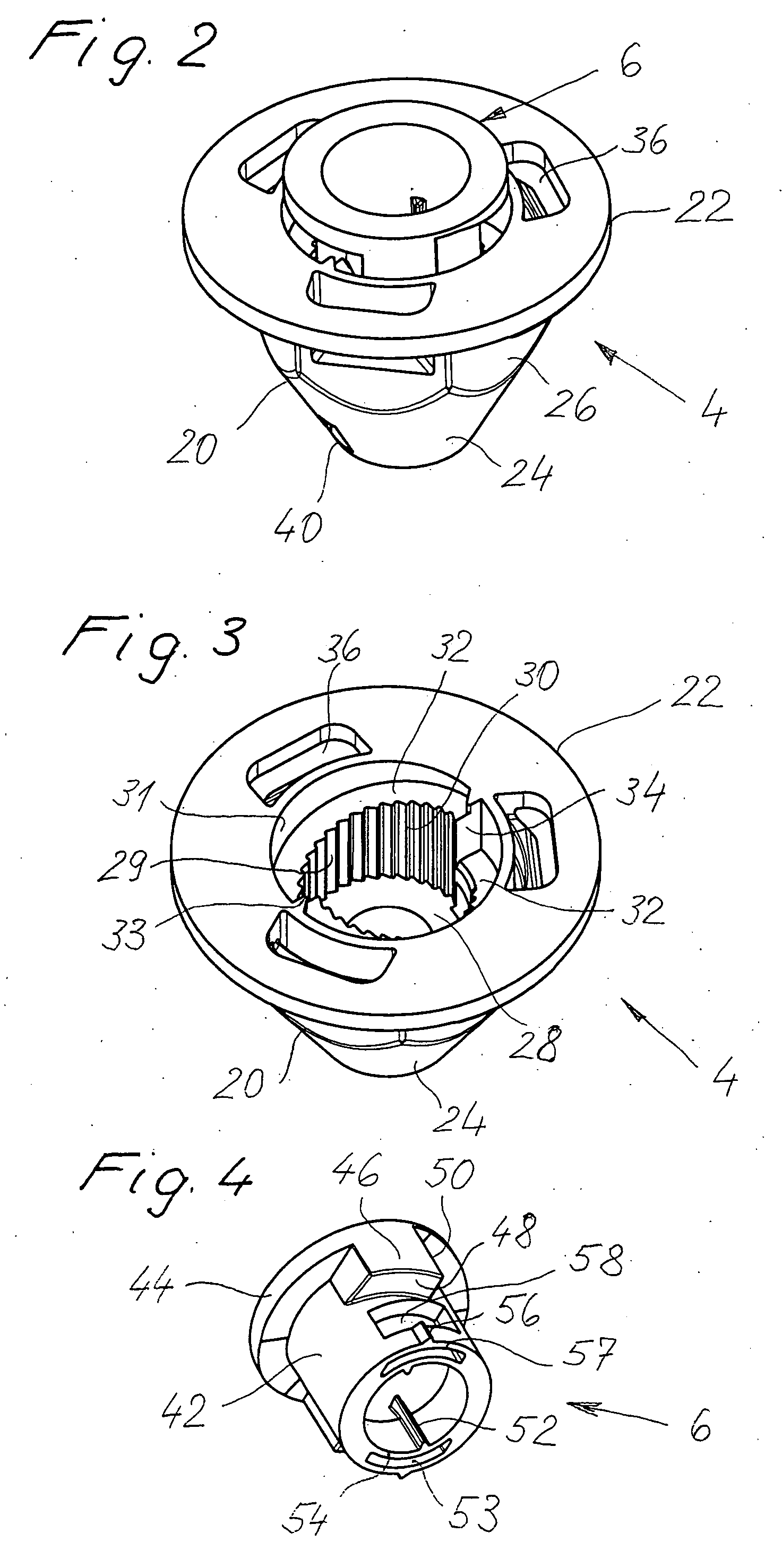
InactiveUS20060280579A1Economically manufacturedSuitable for manufactureWashersSheet joiningMechanical engineeringTorsional Forces
A tolerance compensating assembly of automatically compensating tolerances in the spacing between two structural members comprises a mounting bolt 10, a base element 4, an adjustment sleeve 6 and a driver 8. The base element 4 and the adjustment sleeve 6 form a first thread pairing G1 of a predetermined spiral direction for adjusting the adjustment sleeve 6 relative to the base element 4. The base element 4 and the mounting bolt 10 form a second thread pairing G2 in the opposite spiral direction for clamping the two structural members B1, B2. The driver 8 is a separate structural member and disengageably connected to the adjustment sleeve 6 and has a plurality of flexibly resilient clamping portions 34 spaced along its periphery, which provide for frictional contact with the thread of the mounting bolt 10 above a predetermined torsional force.
Owner:BOLLHOFF VERBINDUNGSTECHNIK GMBH
Semiconductor light-emitting element and process for production thereof
InactiveUS20090242925A1Difficult to manufactureSuitable for manufactureSemiconductor/solid-state device manufacturingSemiconductor devicesOhmOhmic contact
The present invention provides a semiconductor light-emitting element comprising an electrode part excellent in ohmic contact and capable of emitting light from the whole surface. An electrode layer placed on the light-extraction side comprises a metal part and plural openings. The metal part is so continuous that any pair of point-positions in the part is continuously connected without breaks, and the metal part in 95% or more of the whole area continues linearly without breaks by the openings in a straight distance of not more than ⅓ of the wavelength of light emitted from an active layer. The average opening diameter is of 10 nm to ⅓ of the wavelength of emitted light. The electrode layer has a thickness of 10 nm to 200 nm, and is in good ohmic contact with a semiconductor layer.
Owner:KK TOSHIBA
Dielectric-free metal-only dipole-coupled broadband radiating array aperture with wide field of view
InactiveUS20150162665A1Suitable for manufactureReflection coefficientPolarised antenna unit combinationsAntenna feed intermediatesPhysicsBroadband
Dielectric-free, metal-only, dipole-coupled broadband radiating array aperture with wide field of view.
Owner:CUBIC CORPORATION
Solid polyurethane powder coating compositions containing uretdione groups that are hardenable at low temperatures
InactiveUS20050003206A1Suitable for manufactureHigh glossOrganic chemistrySynthetic resin layered productsPolymer sciencePtru catalyst
A highly reactive polyurethane powder coating composition, which contains uretdione groups that are hardenable at low temperatures, contains A) at least one uretdione-containing powder coating hardener based on aliphatic, (cyclo)aliphatic or cycloaliphatic polyisocyanates and hydroxyl-containing compounds, the hardener having a melting point of from 40 to 130° C., a free NCO content of less than 5% by weight, and a uretdione content of 6-18% by weight; B) at least one hydroxyl-containing polymer having a melting point of from 40 to 130° C., and an OH number of between 20 and 200 mg KOH / g; C) 0.001-3% by weight of at least one tetraalkyl ammonium carboxylate catalyst, based on a total amount of components A) and B). Components A) and B) are present in a ratio so that for each hydroxyl group of component B) there is from 0.3 to 1 uretdione group of component A).
Owner:EVONIK DEGUSSA GMBH
Mask blank manufacturing method and sputtering target for manufacturing the same
ActiveUS20050170263A1Prevent and reduce generationStably manufactureCellsVacuum evaporation coatingMetalElution
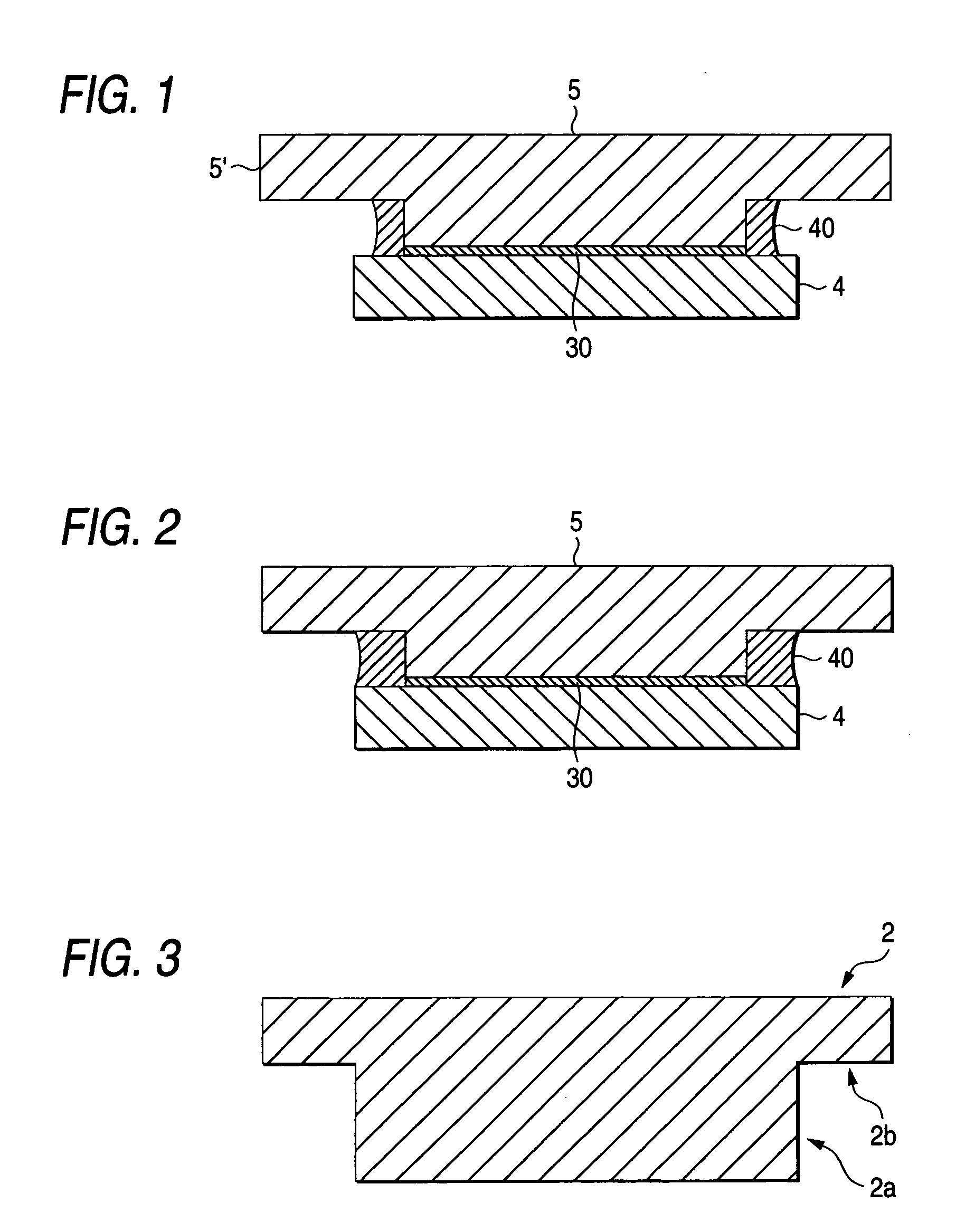
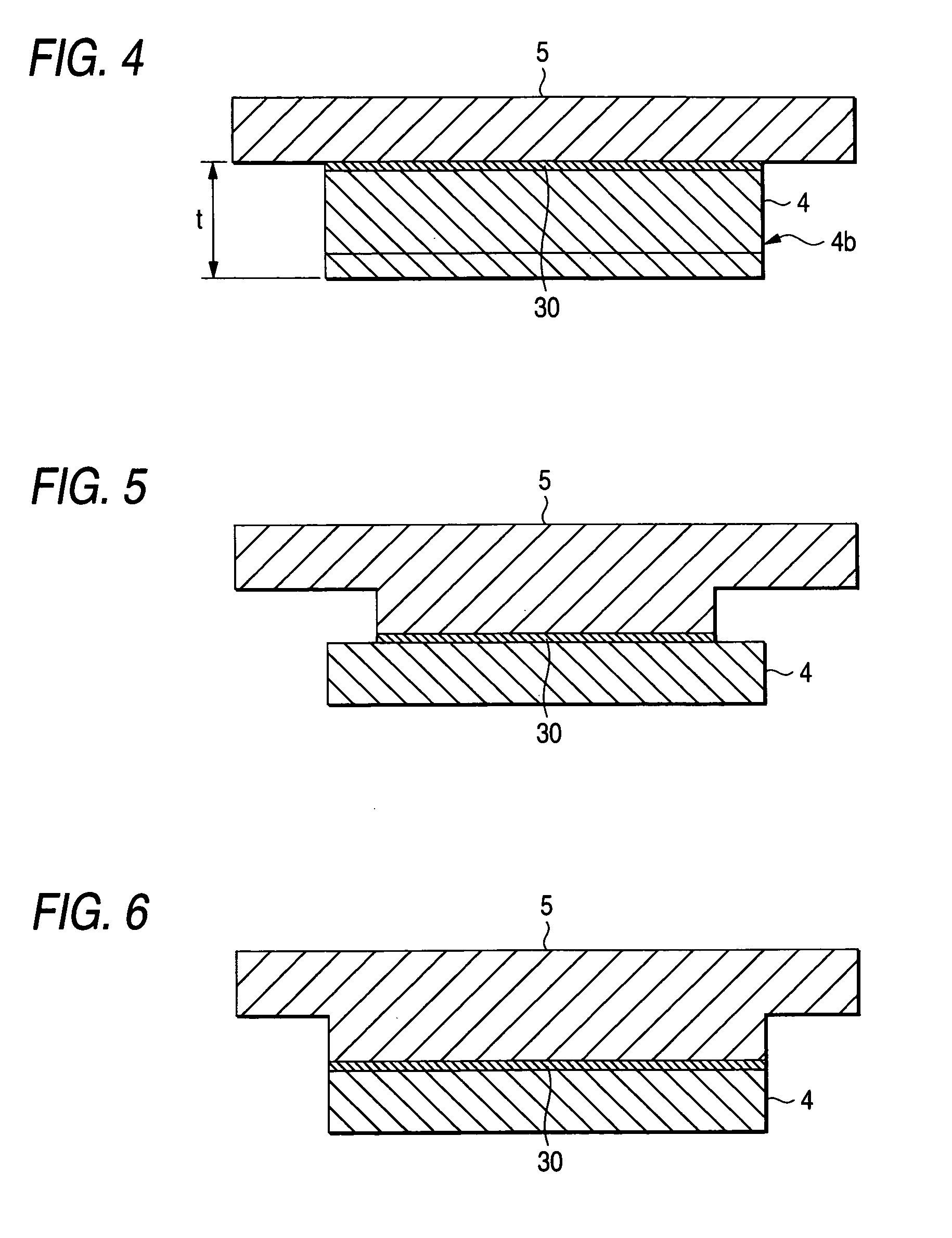
A sputtering target for manufacturing a mask blank having a backing plate 5 where a portion for bonding a target member 4 is protruded like the convex with respect to a base portion 5′, and the target member 4 being formed to have a larger surface area than the area of the bonding portion of the backing plate 5 with extending from the bonding portion over a whole periphery with a bonding agent 30 interposed in-between, and further a metal 40 is deposited to a concave portion formed by a combination of the two structures in such a manner that the elution of the bonding agent 30 can be sealed.
Owner:HOYA CORP
Plastic tolerance compensating device
InactiveUS20070009342A1Simple processEconomically manufacturedWashersSheet joiningEngineeringAbutment
The tolerance compensating assembly of the present invention comprises a mounting bolt 2, a receiving part 4, and an adjustment sleeve 6. The receiving part 4 can be fixed to a first of two structural members A, B and can be screwed with the mounting bolt 2 to clamp the structural members A, B. The adjustment sleeve 6 is supported on the receiving part 4 by ramped sliding surfaces 32, 48 and has at least one driver projection 52 which co-operates with a driver projection 16 of the mounting bolt 2 such that the adjustment sleeve 6 is also driven by the mounting bolt 2 upon the mounting bolt 2 being screwed into the receiving part 4 and is moved into abutment with the second structural member B to compensate tolerance as a function of a corresponding relative movement of the ramped sliding surfaces 32, 48.
Owner:BOLLHOFF VERBINDUNGSTECHNIK GMBH
Nanocrystal and photovoltaic device comprising the same
InactiveUS20070151597A1Improve light absorption efficiencyHigh light absorption efficiencyMaterial nanotechnologyFinal product manufactureInfraredHigh energy
A nanocrystal with high light absorption efficiency and a broad absorption spectrum, and a photovoltaic device comprising the nanocrystal are disclosed. The nanocrystal of the present invention comprises a core, a first shell grown and formed on the surface of the core, and a second shell grown and formed on the surface of the core or the surface of the first shell. Besides, the core, the first shell, and the second shell are a low energy gap material, a middle energy gap material, and a high energy gap material, respectively. Therefore, the nanocrystal has a great absorption in the ultraviolet range, the visible light range, and the infrared range; and the solar spectrum can be converted effectively to improve the light conversion efficiency thereof.
Owner:IND TECH RES INST
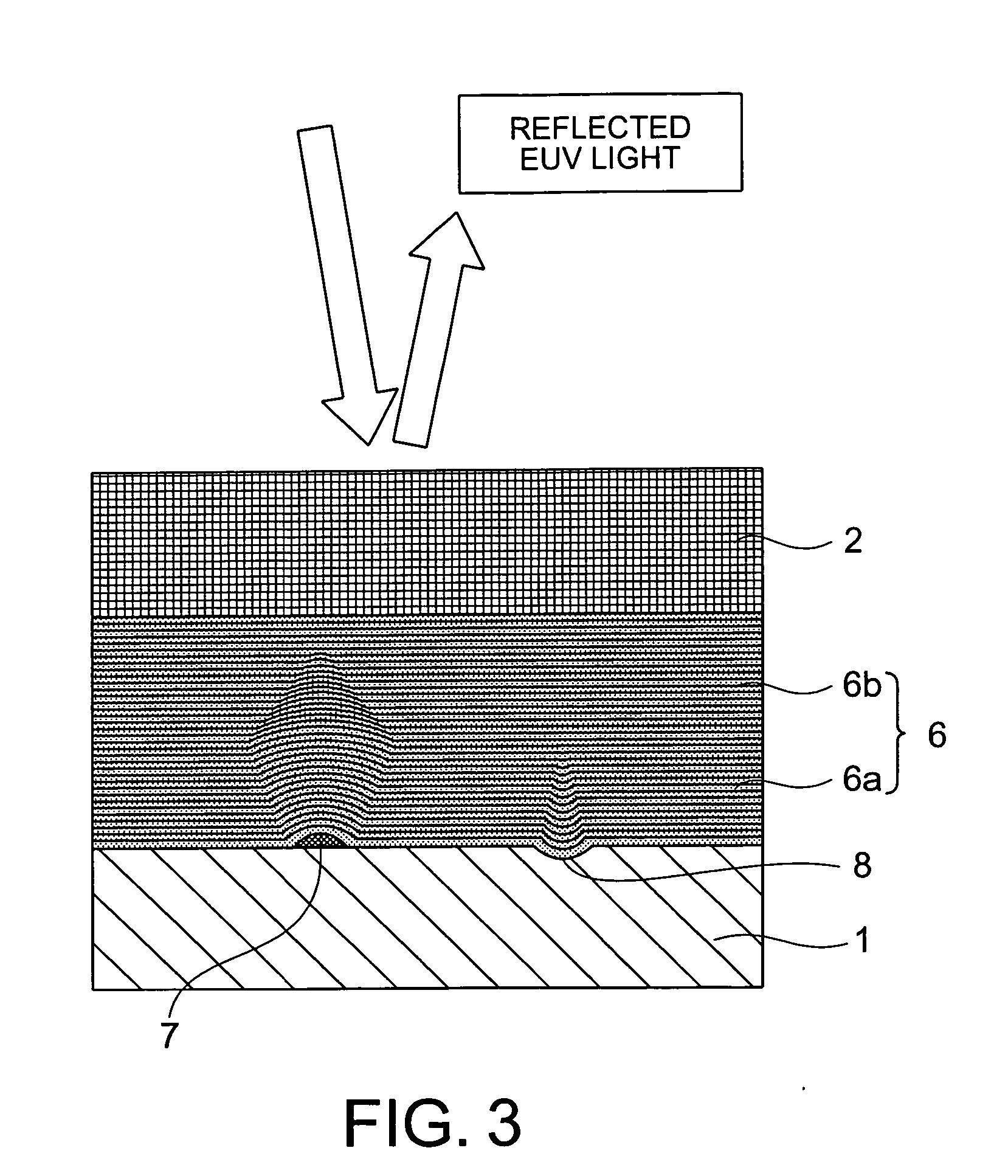
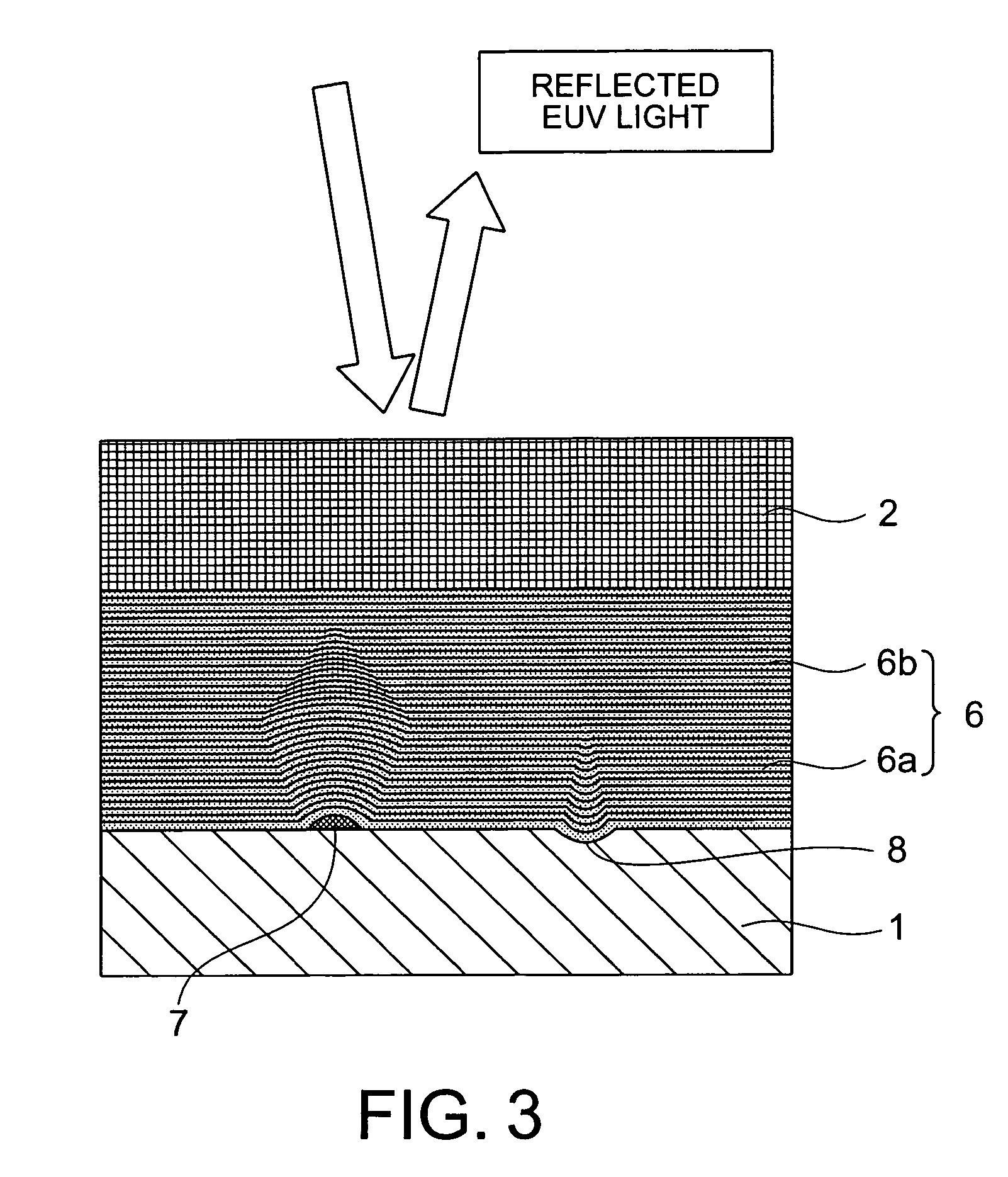
Multilayer reflective film coated substrate, manufacturing method thereof, reflective mask blank, and reflective mask
InactiveUS20070091420A1Improve surface smoothnessFew defectMirrorsNanoinformaticsOptoelectronicsPhysics
A multilayer reflective film coated substrate includes a multilayer under film comprised of Mo / Si alternately-layered films and a multilayer reflective film comprised of Mo / Si alternately-layered films for reflecting exposure light. The multilayer under film and the multilayer reflective film are formed on a substrate in this order. Given that a cycle length of the multilayer under film is d bottom (unit: nm) and a cycle length of the multilayer reflective film is d top (unit: nm), a relationship of a formula (1) is satisfied when d bottom>d top, the formula (1) given by(n+0.15)×d top≦d bottom≦(n+0.9)×d topwhere n is a natural number equal to or greater than 1.
Owner:HOYA CORP
Semiconductor light-emitting element and process for production thereof
InactiveUS8101964B2Improve efficiencyReduced scattering effectSemiconductor/solid-state device manufacturingSemiconductor devicesOhmic contactLength wave
The present invention provides a semiconductor light-emitting element comprising an electrode part excellent in ohmic contact and capable of emitting light from the whole surface. An electrode layer placed on the light-extraction side comprises a metal part and plural openings. The metal part is so continuous that any pair of point-positions in the part is continuously connected without breaks, and the metal part in 95% or more of the whole area continues linearly without breaks by the openings in a straight distance of not more than ⅓ of the wavelength of light emitted from an active layer. The average opening diameter is of 10 nm to ⅓ of the wavelength of emitted light. The electrode layer has a thickness of 10 nm to 200 nm, and is in good ohmic contact with a semiconductor layer.
Owner:KK TOSHIBA
Process of manufacturing a curved member having a high-grade design surface and member manufactured by the process
ActiveUS20120225241A1Low costHigh standardLayered productsDomestic articlesCompression moldingGlass transition
It is an object of the present invention to provide a process capable of manufacturing various types of curved members having a high-grade design surface which are used as glazing members for means of transport such as automobiles at a low cost.The present invention is the process of manufacturing a curved member having a high-grade design surface, comprises the steps of:(1) preparing a sheet having a high-grade design surface by injection compression molding a resin material containing a thermoplastic resin;(2) preheating the sheet at a temperature of (Tg+5)° C. to (Tg+70)° C. (Tg(° C.) is the glass transition temperature of the resin material) to soften it; and(3) applying pressure to the softened sheet to curve the high-grade design surface.
Owner:TEIJIN KASEK KK
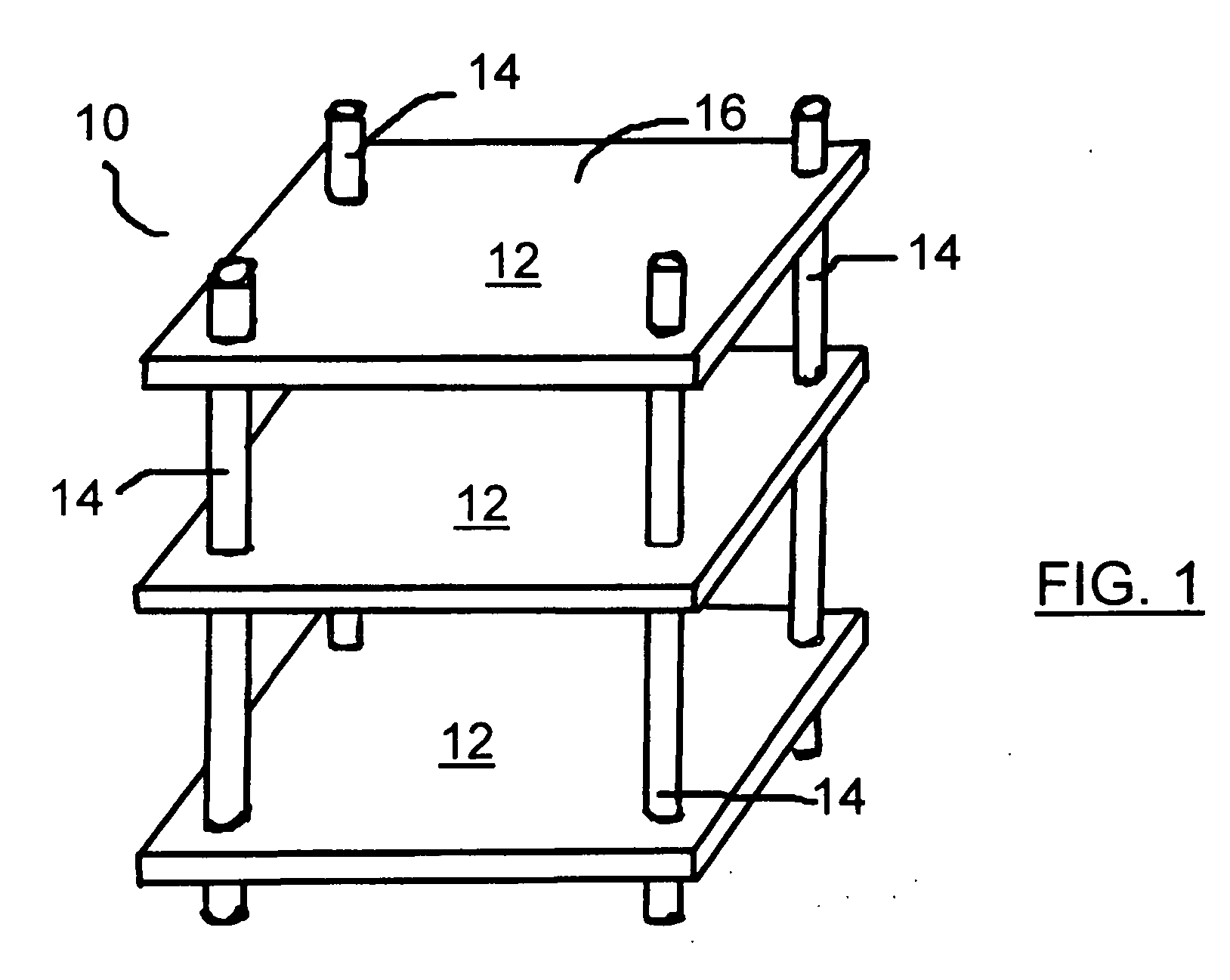
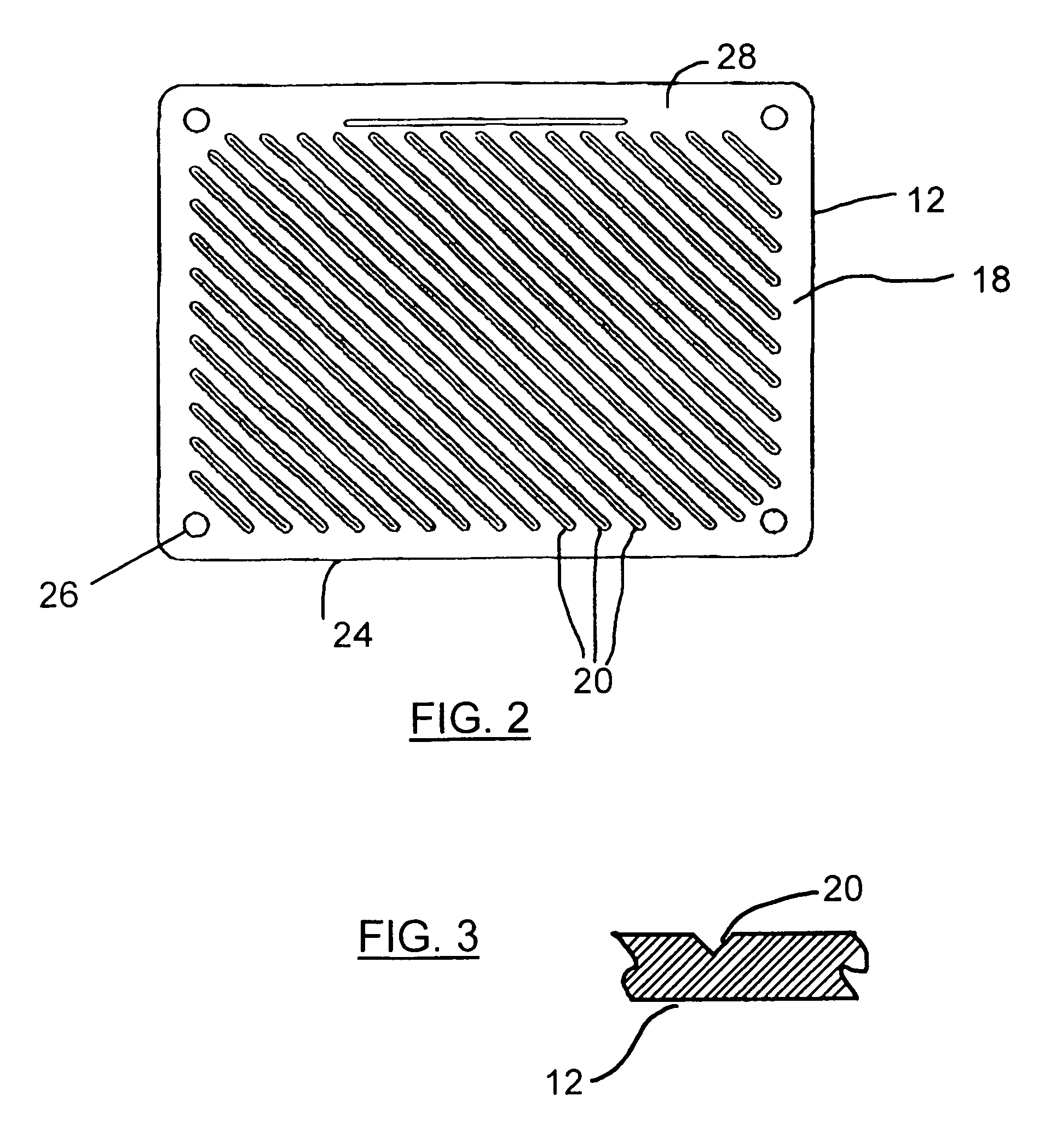
Audio equipment storage device
InactiveUS20070278170A1Reduce and ameliorate and eliminate deleterious effectSuitable for manufactureFolding cabinetsSectional furnitureFiberCarbon fibers
Modifications to the structural elements of racks / shelves for component shelves, and in particular, audio components are provided in order to improve the sound produced by the system by minimizing the effects of vibration on the equipment positioned on the shelves. In particular, adding a series of grooves to the lower surface of each shelf was found to be of assistance in attenuating the vibration measured on the shelf surface. Additionally, using vertical supports manufactured of a tube of a composite fibre material, such as a carbon fibre / graphite reinforced plastic (GRP) or a solid metallatic support, such as a support made of an aluminum rod, was also found to attenuate the vibrations measured on the shelf surface. As such, a method and device for ameliorating the effects of vibration on vibration sensitive equipment is provided.
Owner:WIEBE JOHN
Multilayer reflective film coated substrate, manufacturing method thereof, reflective mask blank, and reflective mask
InactiveUS20070091421A1Improve surface smoothnessExcellent in pattern transferabilityMirrorsNanoinformaticsOptoelectronicsPhysics
A multilayer reflective film coated substrate includes a multilayer under film comprised of Mo / Si alternately-layered films, an intermediate layer in the form of a Si film, and a multilayer reflective film comprised of Mo / Si alternately-layered films for reflecting exposure light. The multilayer under film, the intermediate layer, and the multilayer reflective film are formed on a substrate in this order. Given that a cycle length of the multilayer under film is d bottom (unit:nm), a thickness of the intermediate layer is d Si (unit:nm), and a cycle length of the multilayer reflective film is d top (unit:nm), relationships of a formula (1) and a formula (2) are satisfied, the formula (1) given by n×d top−0.05≦d bottom≦n×d top+0.05where n is a natural number equal to or greater than 1, and the formula (2) given by m×d top−1.2≦d Si≦−m×d top+1.2where m is an integer equal to or greater than 0.
Owner:HOYA CORP
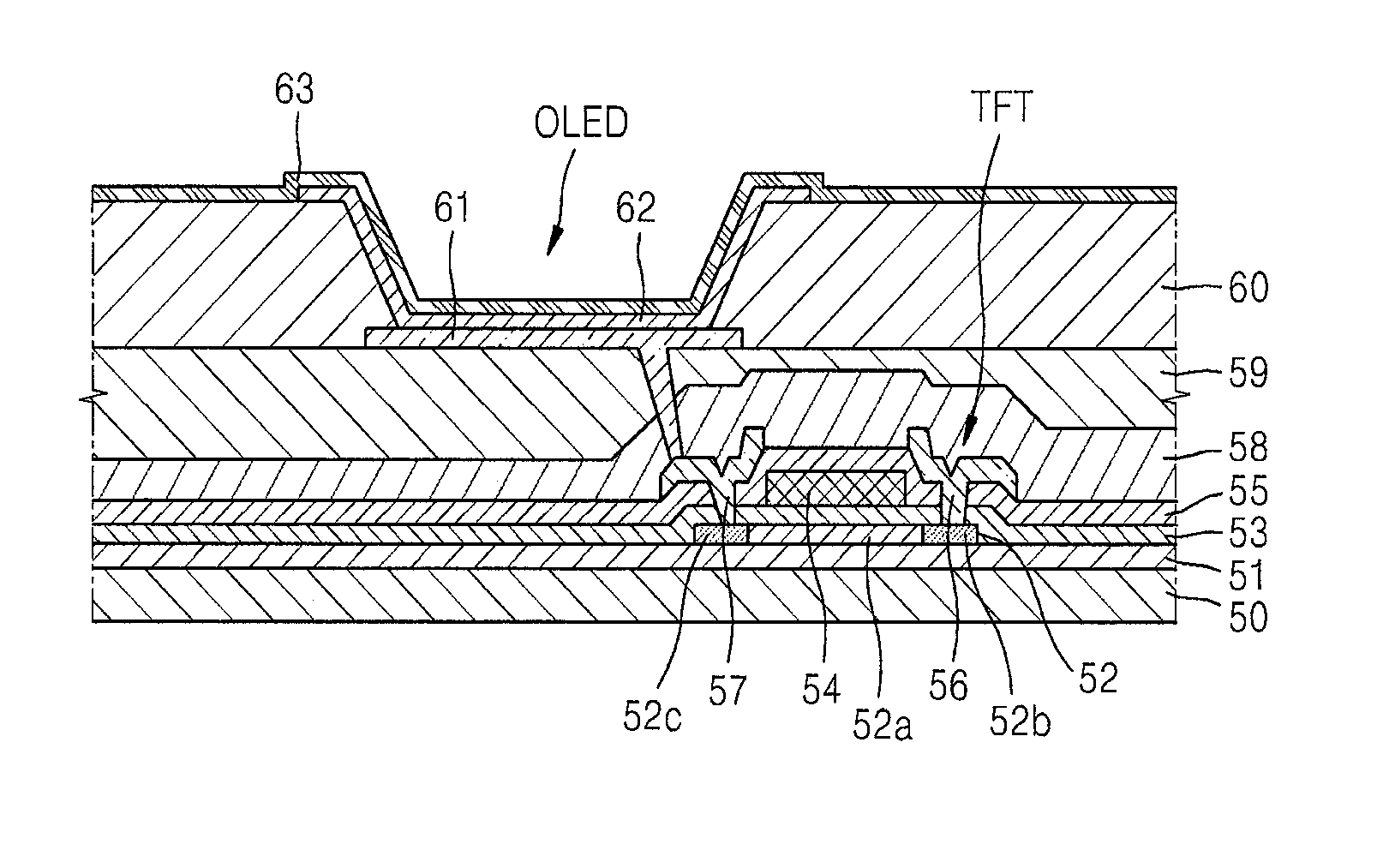
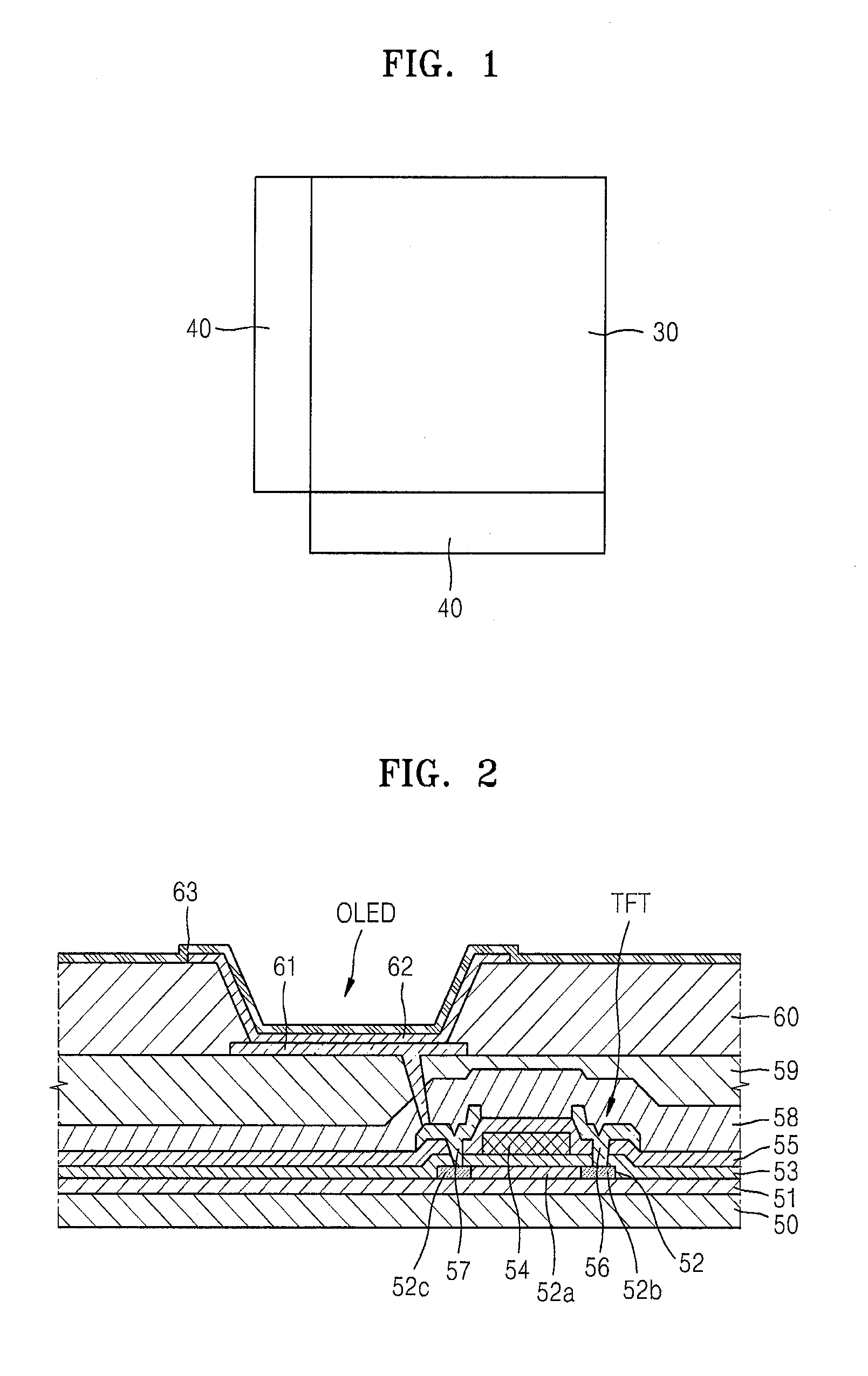
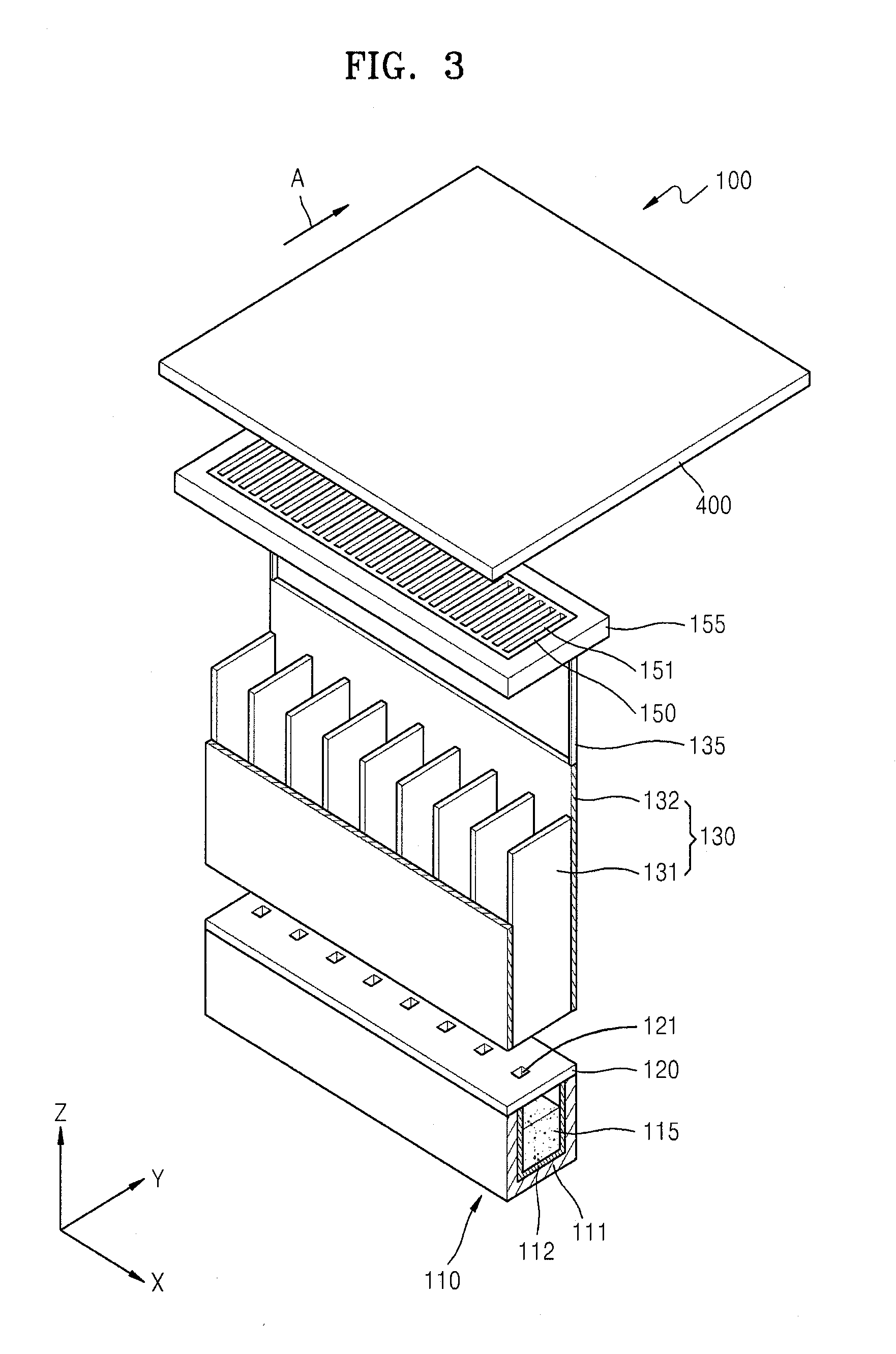
Thin film deposition apparatus, method of manufacturing organic light-emitting display device by using the thin film deposition apparatus, and organic light-emitting display device manufactured by using the method
ActiveUS20120068199A1Suitable for manufactureLiquid surface applicatorsSpraying apparatusDisplay deviceEngineering
A thin film deposition apparatus that is suitable for manufacturing large-sized display devices on a mass scale and that can be used for high-definition patterning, a method of manufacturing an organic light-emitting display device by using the thin film deposition apparatus, and an organic light-emitting display device manufactured by using the method. The thin film deposition apparatus includes: a deposition source that discharges a deposition material; a deposition source nozzle unit disposed at a side of the deposition source and including a plurality of deposition source nozzles arranged in a first direction; a patterning slit sheet disposed opposite to the deposition source nozzle unit and including a plurality of patterning slits arranged in the first direction; a first barrier plate assembly including a plurality of first barrier plates that are disposed between the deposition source nozzle unit and the patterning slit sheet in the first direction, and that partition a space between the deposition source nozzle unit and the patterning slit sheet into a plurality of sub-deposition spaces; and a second barrier plate disposed at one side of the patterning slit sheet, wherein an inner part of the second barrier plate is partitioned into a plurality of spaces by a plurality of barrier ribs.
Owner:SAMSUNG DISPLAY CO LTD
Polymer compositions suitable for intraocular lenses and related methods
ActiveUS20090088839A1Reduces hydride/vinyl ratioPrevent curingTissue regenerationAdhesivesCross-linkPolymer science
A polymeric material with a molecular response time that makes it suitable for use near fragile body tissues. The polymeric material is useful for both low modulus and high modulus applications thereby simplifying the multi-part polymeric article manufacturing process and creating better integrated multi-part polymeric articles. Cross-linked polymers with different moduli may be obtained utilizing the same or similar starting materials but modifying the amount of catalyst, the amount of cross-linking agent, and / or the amount of methyl vinyl cyclics.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Meloxicam compositions
InactiveUS20050038018A1Increase doseImprove efficacyCosmetic preparationsBiocideJoint arthralgiaStimulant
A pharmaceutical composition comprising: (a) meloxicam or a pharmaceutically acceptable salt thereof; and (b) one or more additional pharmaceutically active compounds selected from antacids, sedatives, and central nervous system stimulants, and the use of such composition of an inflammatory disease, symptoms of an inflammatory disease, including various symptoms thereof, and / or headache, toothache, ache after tooth extraction, sore throat, otalgia, arthralgia, neuralgia, lumbago, myalgia, muscle stiffness of shoulder, pain of contusion, pain of fracture, pain of sprain, menstrual pain, traumatic pain, chill, exothermic reaction and / or cold and various symptoms of cold such as sore throat, chill, pyrexia, headache, arthralgia and muscle pain.
Owner:BOEHRINGER INGELHEIM INT GMBH
Natural microtubule encapsulated phase-change materials and preparation thereof
InactiveUS20100071882A1Cheap and easily available natural microfibersLarge specific surface areaHeat storage plantsMetal-working apparatusMicrotubulePolymer science
Microtubule encapsulated microcapsules of a phase-change material and preparation thereof are provided. The microcapsules of a phase-change material consist of a phase-change material, truncated microtubules, and a polymer. The truncated microtubules are formed by truncating hollow tubular natural fibers into fiber segments with a length of 0.1 mm-5 cm. The diameter of the hollow tubular natural fiber is 0.1-1000 μm. The phase-change material is encapsulated in the truncated microtubules and the truncated microtubules are covered with the polymer. The microtubules have high energy storage density due to high hollowness, and can transfer energy stably due to the closed structure, transfer heat rapidly due to the very fine micro-tubular structures, and may be used for a long term in view of the heat and chemical stability.
Owner:ETERNAL MATERIALS CO LTD
Multi-Component Medicine Evaluation Method
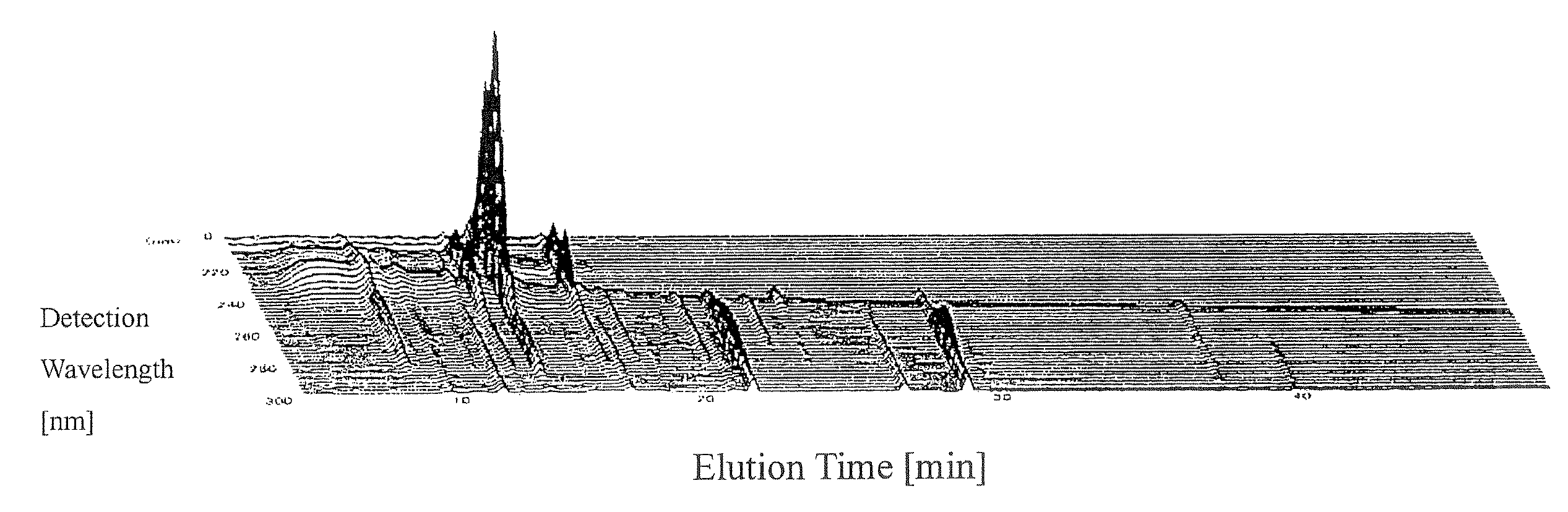
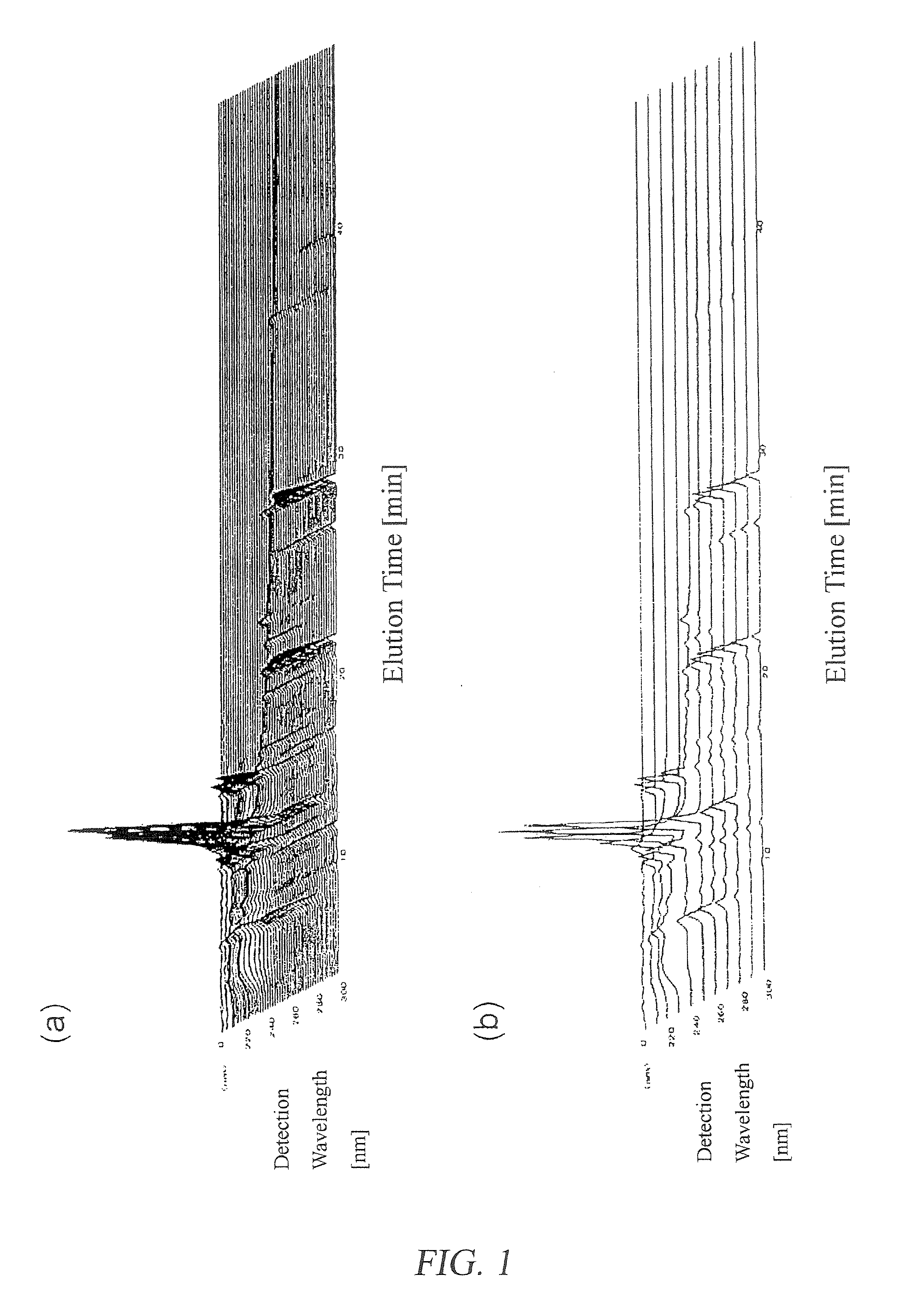
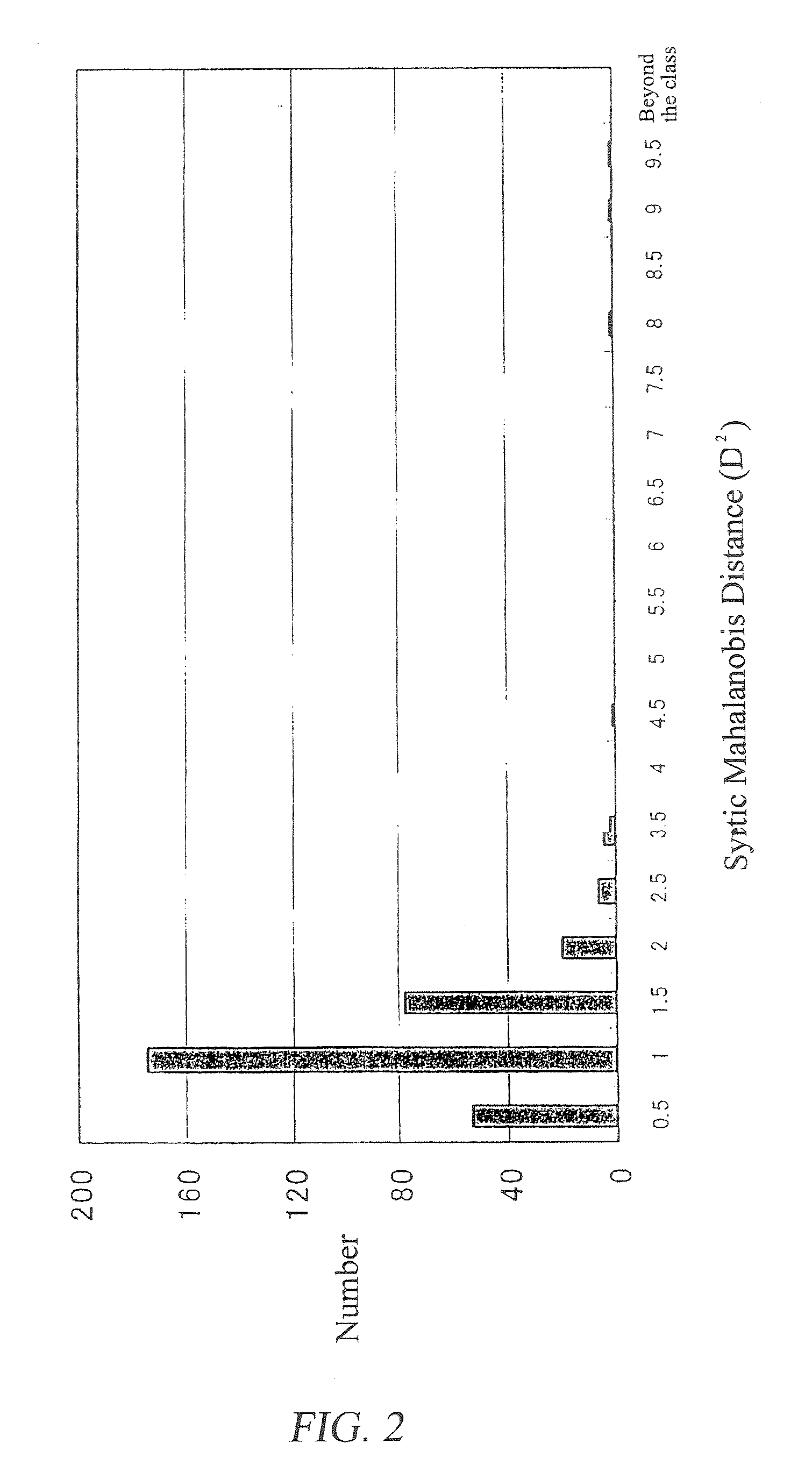
ActiveUS20080140375A1Efficiently employedWide wavelength rangeComponent separationAnalogue computers for chemical processesFingerprintData point
A method for evaluation of a multi-component medicine comprising judging the degree of difference of the multi-component medicine to be evaluated from a group of multi-component medicines selected as a reference group by using a Mahalanobis distance obtained by a process comprising the following steps (1) to (8),(1) a step of obtaining fingerprint data of three-dimensional high performance liquid chromatography of the multi-component medicine to be evaluated,(2) a step of combining the fingerprint data obtained in (1) with fingerprint data of three-dimensional high performance liquid chromatography of other multi-component medicines of the same kind forming a reference group,(3) a step of allocating variable axes in the MT method to the number of multi-component medicine and either the elution time or detection wavelength of the fingerprint data of (2) above and regarding a signal strength as a characteristic amount in the MT method,(4) a step of obtaining a unit space from the characteristic amount of (3) using the MT method,(5) a step of obtaining the Mahalanobis distance of all multi-component medicines for each detection wavelength or elution time using the MT method from the unit space obtained in (4),(6) a step of allocating variable axes in the MT method to the number of the multi-component medicine and either the elution time or detection wavelength to which the variable axes are not allocated in the step (3) and regarding the Mahalanobis distance obtained in step (5) as a characteristic amount in the MT method,(7) a step of obtaining a second unit space from the characteristic amount of (6) using the MT method, and(8) a step of obtaining the Mahalanobis distance of the multi-component medicine to be evaluated using the MT method from the second unit space obtained in (7).According to the present invention, because the waveform processing of HPLC peaks is unnecessary, dispersion among data are small, thereby bringing highly reliable results; because the amount of information (the number of data points) is not limited to the number of peaks of a specific component, the amount of information can be freely increased or decreased; in addition, because it is not necessary to judge by combining the value of the content of two or more components, but can be judged using a single numerical value, the degree of difference of the multi-component medicine to be evaluated from a reference group can be simply judged.
Owner:TSUMURA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com