Water soluble camptothecin derivative and medicinal composition containing same
A technology of camptothecin and derivatives, applied in the field of medicine based on camptothecin, can solve the problem of not being used as a CPT prodrug and the like, and achieve the effects of improving stability and reducing drug toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
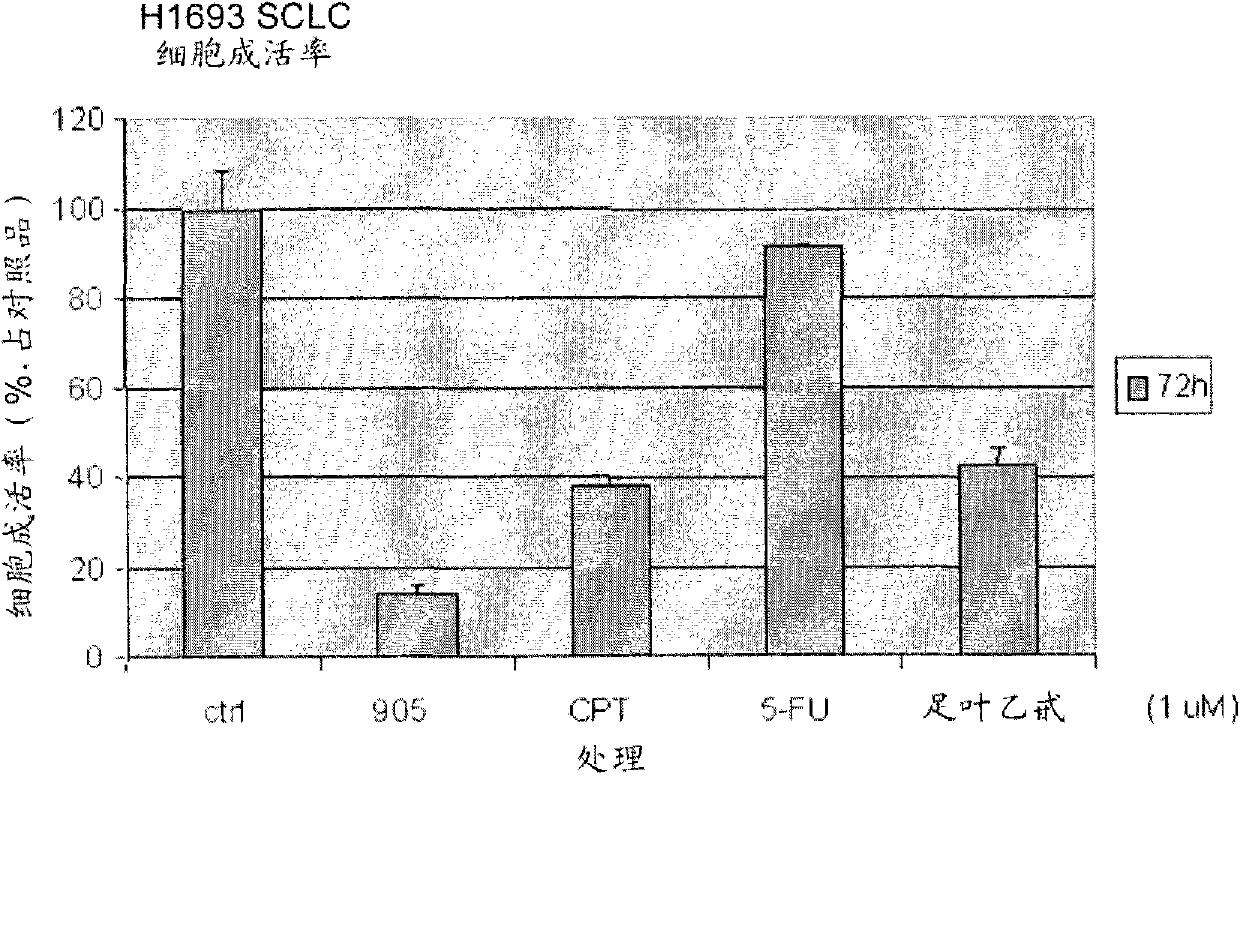
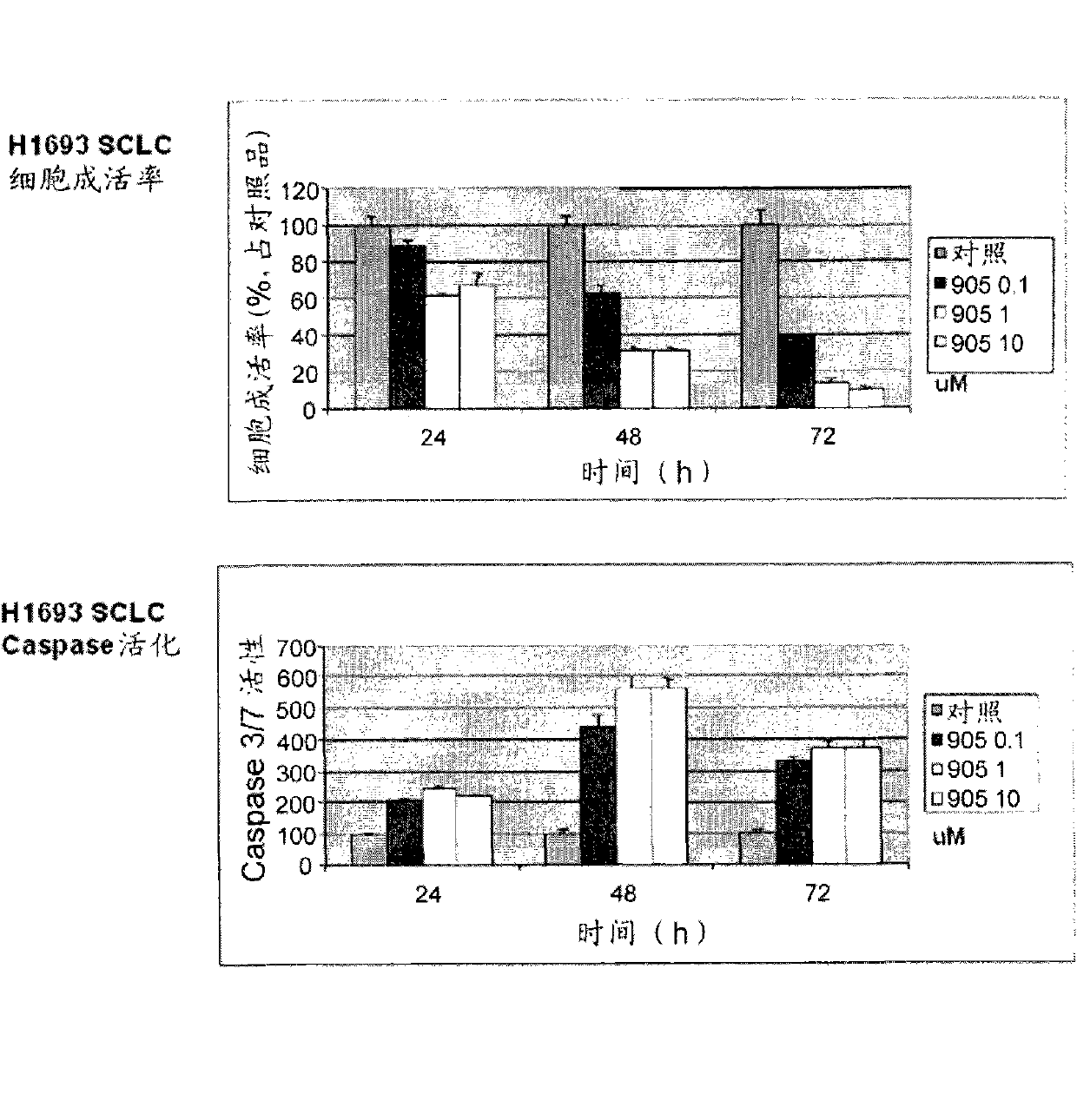
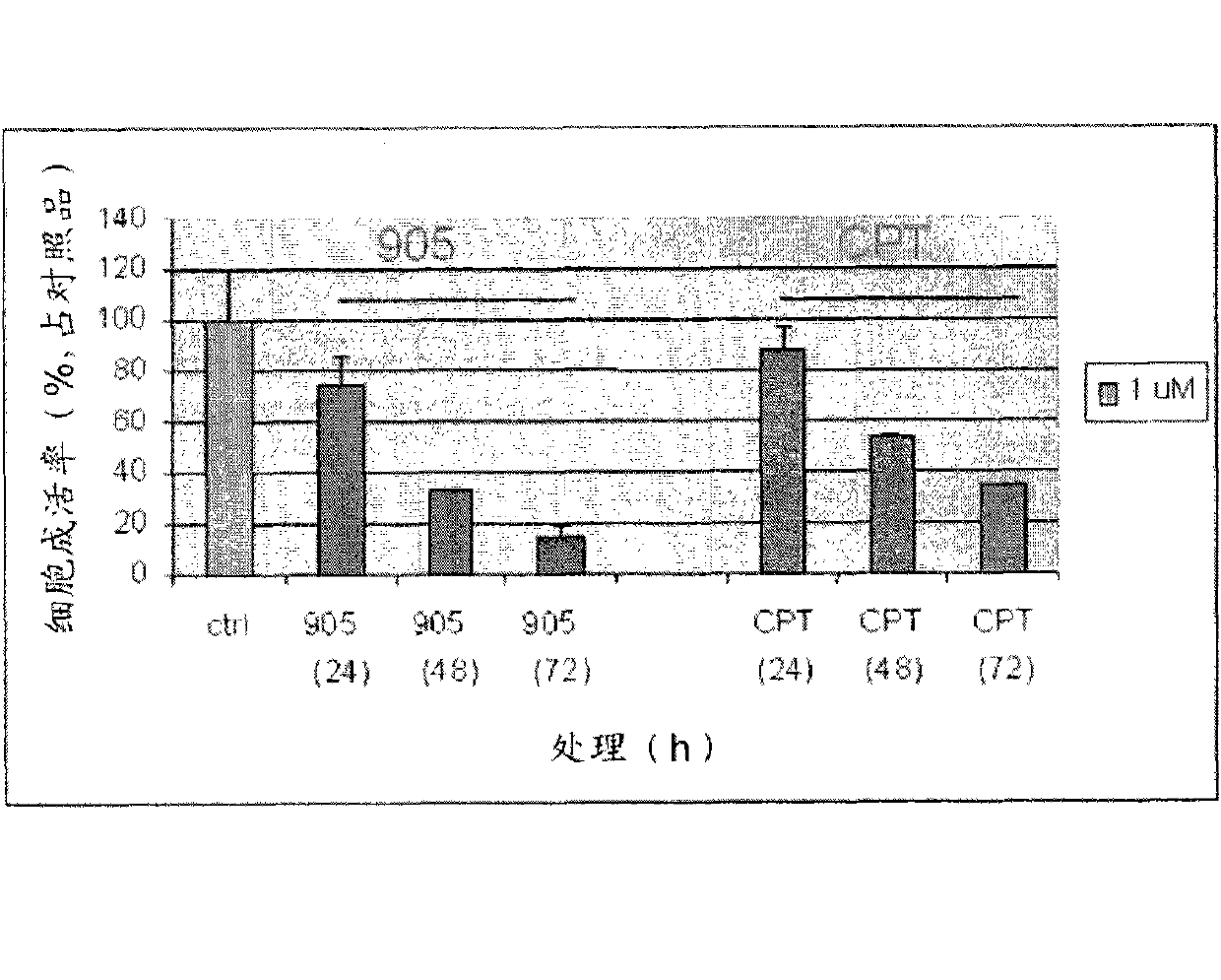
[0135] Example 1: Synthesis and anticancer evaluation of Dix-905
[0136] Compounds of formula I of the present invention include compounds Dix-905 having the structure
[0137]
[0138] (a) Synthesis of Dix-905
[0139] Under nitrogen protection, CPT (174 mg, 1 equivalent) was dissolved in anhydrous solvent (10 ml DMF), stirred and cooled to 0-4 ° C, and 4-dimethylaminopyridine (6.7 mg, 0.11 equivalent), di Isopropylethylamine (258mg, 2eq) and 2-cyanoethyl N,N-diisopropylphosphoramidite chloride (236mg, 2eq). The mixture was stirred for 1 hour, then cooled to room temperature. After confirming complete consumption of CPT (about 4 hours), a small amount of water (1 ml) was added to stop the reaction. The reaction solvent was removed by evaporation and the residue was resuspended in dichloromethane (10ml), extracted with ice-cold 10% sodium carbonate solution, brine and dried over sodium sulfate. Dichloromethane was evaporated to dryness under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com