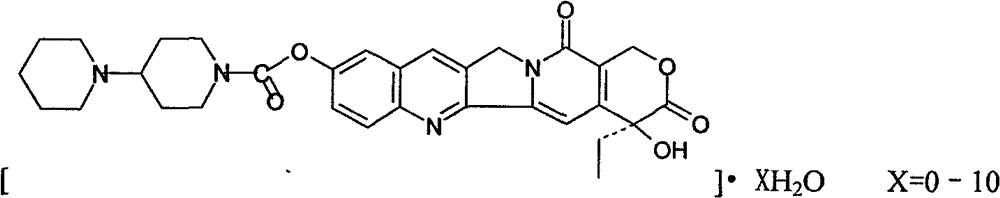
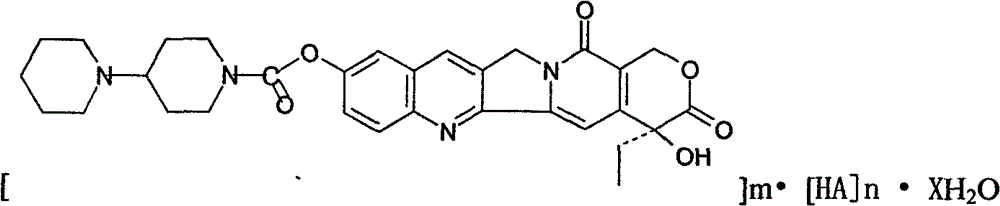
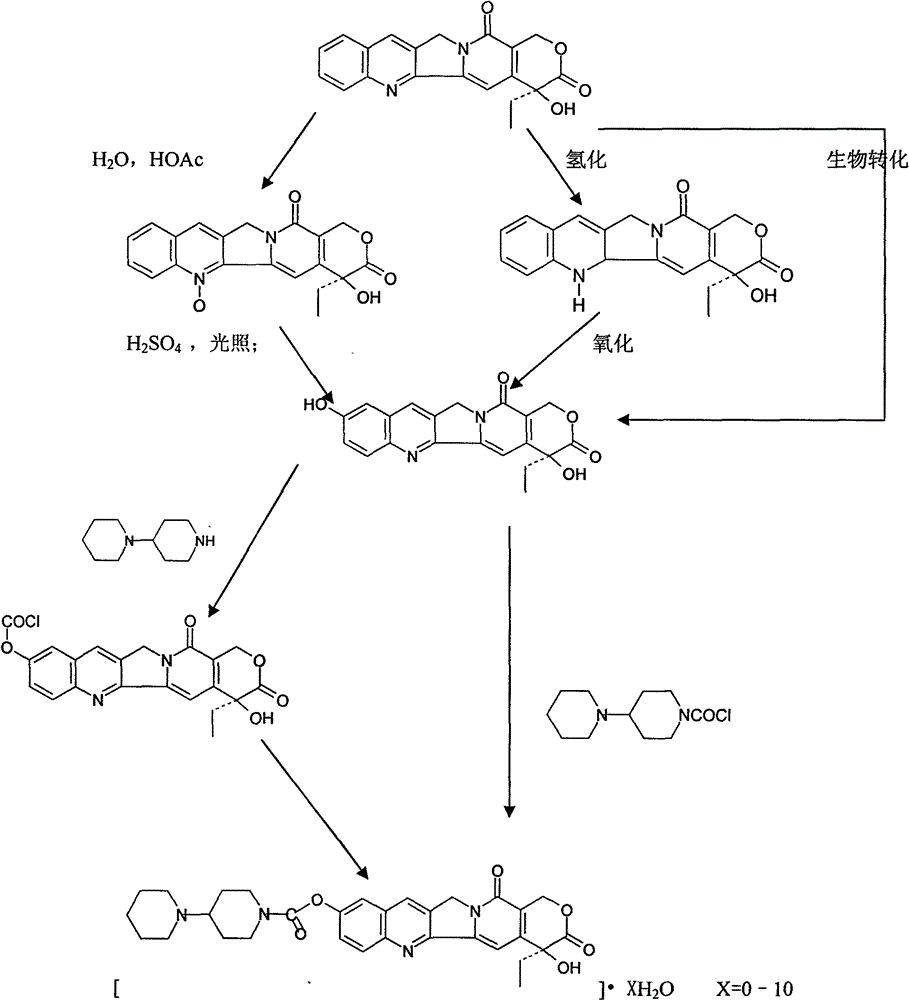
Preparation and clinic application of hydroxycamptothecin derivative and preparation thereof
A technology of hydroxycamptothecin and its derivatives, which is applied in the preparation and clinical application of a class of hydroxycamptothecin derivatives and their preparations, and can solve the problems of high anti-tumor effect, poor water solubility and ester solubility, and poor ring-opening effect And other problems, to achieve the effect of high curative effect, stable quality, good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0063] Synthesis of bipiperine hydrochloride (from hydroxycamptothecin)
[0064] (1) Synthesis of 4-piperidinylpiperidinecarbonyl chloride
[0065] Add 2.82g (9.5mmol) of triphosgene and 20ml of dichloromethane into a 100ml reaction flask equipped with a drying tube, cool to -10°C, add dropwise 1.6g (9.5mmol) of 4-piperidinylpiperidine, dichloromethane The mixture composed of 20ml and 63.9ml (28.5mmol) of triethylamine was naturally warmed to room temperature after the addition, filtered, and the filtrate was concentrated to half, and filtered again. The filter cake was discarded, and the filtrate was concentrated to dryness to obtain 2.02 g of the product, yield: 81%.
[0066] (2) Synthesis of Bipiperidin Hydrochloride
[0067] Take 1.4g (2.6mmol) of 10-hydroxycamptothecin and 50ml of pyridine, dissolve them with an electromagnetic stirrer, dissolve 1.16g (5.04mmol) of 4-piperidinylpiperidinecarbonyl chloride in 30ml of dichloromethane, add dropwise to react solution, stir...
example 2
[0069] Synthesis of bipiperine hydrochloride (from hydroxycamptothecin)
[0070] (1) Synthesis of 10-chloroformyloxycamptothecin:
[0071] Suspend 500 mg (1.27 mmol) of 7-ethyl-10-hydroxycamptothecin in dry dioxane (400 ml), add 2 ml of triethylamine, and heat to dissolve it. While stirring at room temperature while temporarily adding phosgene, the phosgene dimer (trichloromethyl chloroformate) (400 ml) was decomposed in the presence of activated carbon catalyst. After 0.5 hours, when the disappearance of the raw materials was confirmed, the insoluble matter was filtered off, and the solvent was evaporated under reduced pressure to obtain 565 mg (97.0%) of white powder of 10-chloroformyloxycamptothecin.
[0072] (2) Synthesis of Bipiperidin Hydrochloride
[0073] Suspend 300mg (0.66mmol) of 10-chloroformyloxycamptothecin in dry dioxane (50ml), add 330mg (1.96mmol) of 4-piperidinylpiperidine, and stir without heating or heating. Raw material disappears. Then the solvent was...
example 3
[0075] Preparation of biphenhydrin hydrochloride for injection
[0076] (1) Prescription
[0077]
[0078]
[0079] (2) Operation method
[0080] Take bipiperidine hydrochloride (equivalent to 500mg of hydroxycamptothecin) and dissolve it in water, add 0.1mol / L hydrochloric acid solution to adjust the pH value to 2.5-6.0, add 10g of mannitol, decolorize with activated carbon, remove pyrogen, and filter through microporous Membrane filtration, dilute to 200ml, divide into 2ml / bottles, freeze-dry to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com