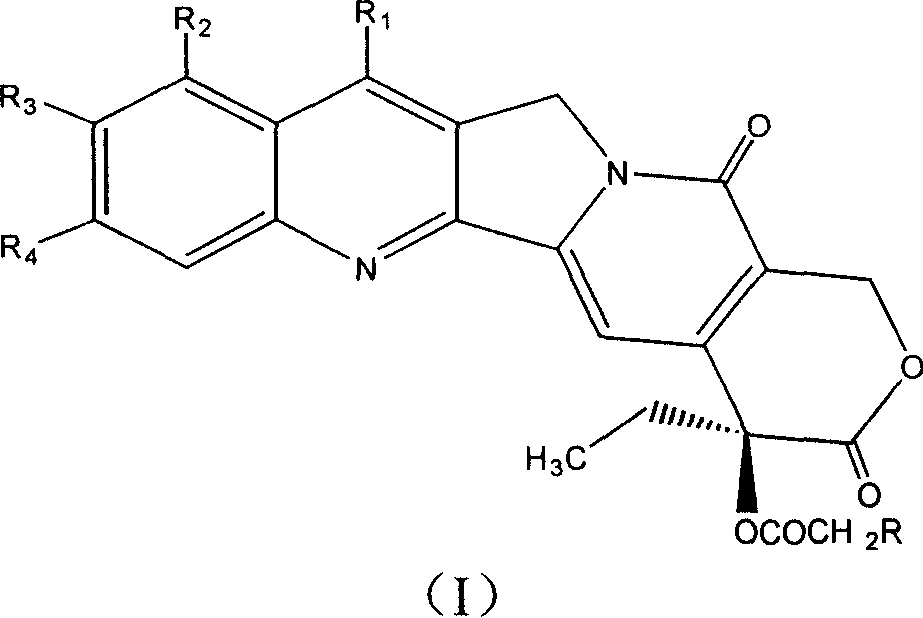
20-bit esterified camptothecine derivate, its preparation method and drug composite and use
A compound and low-level technology, which can be used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve the problems of high toxicity and low anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
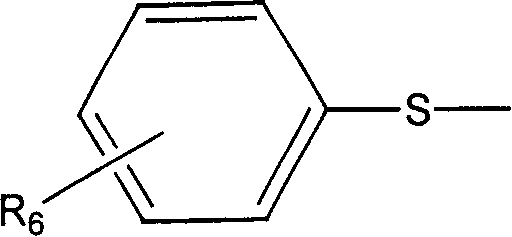
[0110] Camptothecin-20-O-2-(pyridine-4-)thioacetic acid ester
[0111] Add 30 mg (0.0861 mmol) camptothecin, 30 mg (0.172 mmol) 2-(pyridine-4-) thioglycolic acid, 66 mg (0.344 mmol) 1-[(3- Dimethylamino)propyl]-3-ethylcarboximide hydrochloride (EDCI), 10 mg (0.0819 mmol) 4-dimethylaminopyridine (DMAP) and 5 mL of anhydrous dichloromethane, and the reaction mixture was stirred at room temperature After 10 hours, the reaction solution was diluted with 50 mL of chloroform, then successively washed with 50 mL of water, 50 mL of saturated NaHCO 3 solution and 50mL saturated brine, adding anhydrous MgSO 4 Dry, filter to remove MgSO 4 Finally, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: chloroform-methanol 100:1) to obtain 26 mg of white solid, yield: 60%, mp: 207-210°C, 1 H NMR (400MHZ, CDCl 3 ): δ8.41(s, 1H, Ar-H), 8.24(m, 3H, Ar-H), 7.97(d, J=8.0Hz, 1H, Ar-H), 7.88(t, J=7.2Hz , 1H, Ar-H), 7.7...
Embodiment 2
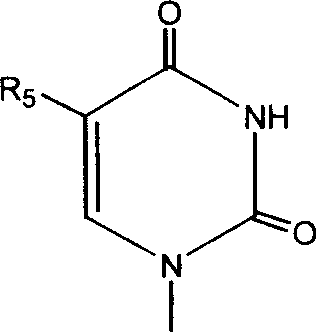
[0113] Camptothecin-20-O-2-(thymine-1-)acetate
[0114]
[0115] Add 40 mg (0.106 mmol) camptothecin, 60 mg (0.326 mmol) 2-(thymine-1-) acetic acid, 80 mg (0.424 mmol) 1-[(3-di Methylamino)propyl]-3-ethylcarbimide hydrochloride (EDCI), 10 mg (0.0819 mmol) 4-dimethylaminopyridine (DMAP) and 5 mL of anhydrous dichloromethane, and the reaction mixture was stirred at room temperature for 24 Hours, the reaction solution was diluted with 50mL chloroform, then successively with 50mL water, 50mL saturated NaHCO 3 solution and 50mL saturated brine, adding anhydrous MgSO 4 Dry, filter to remove MgSO 4 Finally, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: chloroform-methanol 100:1) to obtain 14 mg of a yellow solid, yield: 24%, mp: 206-208°C, 1 H NMR (400MH Z , CDCl 3 ): δ8.50(s, 1H, Ar-H), 8.44(d, J=8.0Hz, 1H, Ar-H), 8.00(d, J=8.0Hz, 1H, Ar-H), 7.91(t , J=7.6Hz, 1H, Ar-H), 7.74(t, J=7.6Hz, 1H, A...
Embodiment 3
[0117] Camptothecin-20-O-2-[(4-methylpyrimidine)-2-]thioglycolate
[0118]
[0119] Add 40mg (0.115mmol) camptothecin, 52mg (0.282mmol) 2-[(4-methylpyrimidine)-2-]thioglycolic acid, 90mg (0.469mmol) 1-[(3-Dimethylamino)propyl]-3-ethylcarboximide hydrochloride (EDCI), 10 mg (0.0819 mmol) 4-dimethylaminopyridine (DMAP), and 5 mL of anhydrous dichloromethane , the reaction mixture was stirred at room temperature for 12 hours, the reaction solution was diluted with 50 mL of chloroform, then successively washed with 50 mL of water, 50 mL of saturated NaHCO 3 solution and 50mL saturated brine, adding anhydrous MgSO 4 Dry, filter to remove MgSO 4 Finally, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: chloroform-methanol 100:1) to obtain 50 mg of a yellow solid, yield: 85%, mp: 231-233°C, 1 H NMR (400MH Z , CDCl 3 ): δ8.40(s, 1H, Ar-H), 8.21(d, J=8.0Hz, 1H, Ar-H), 7.97(d, J=8.0Hz, 1H, Ar-H), 7.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com