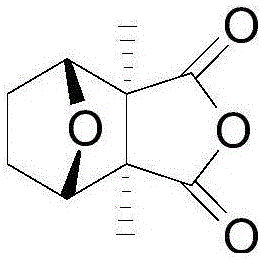
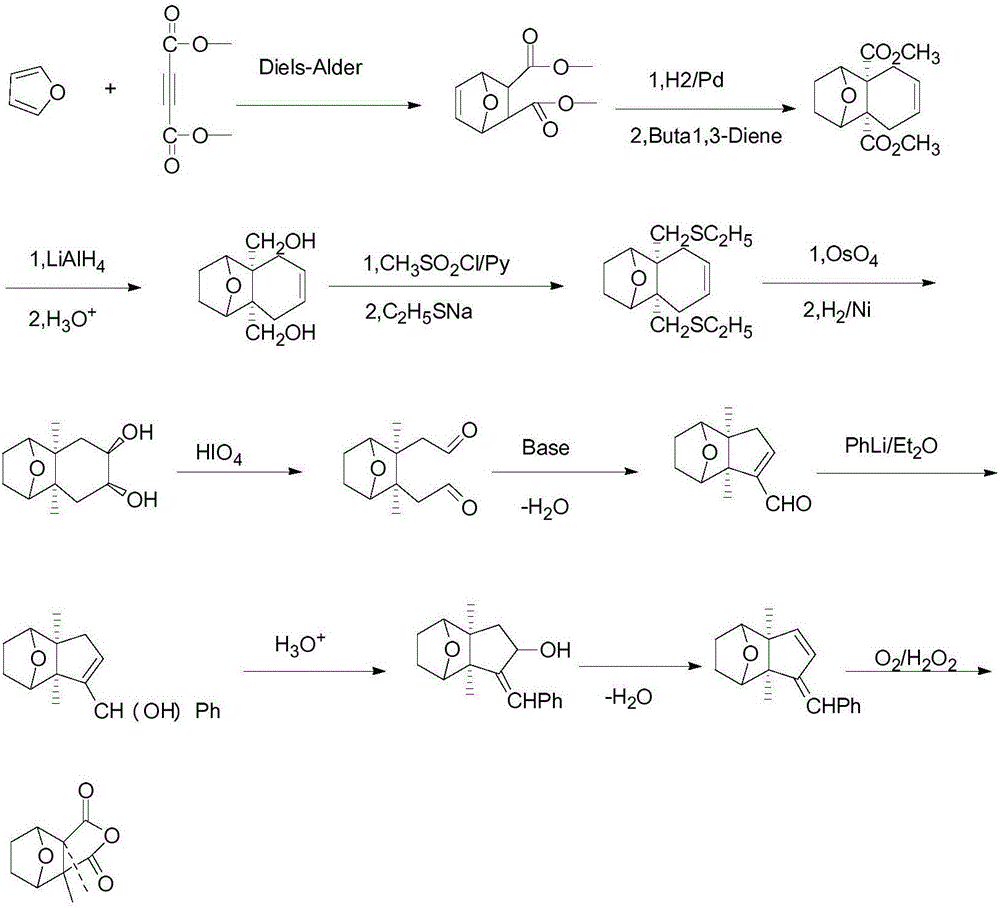
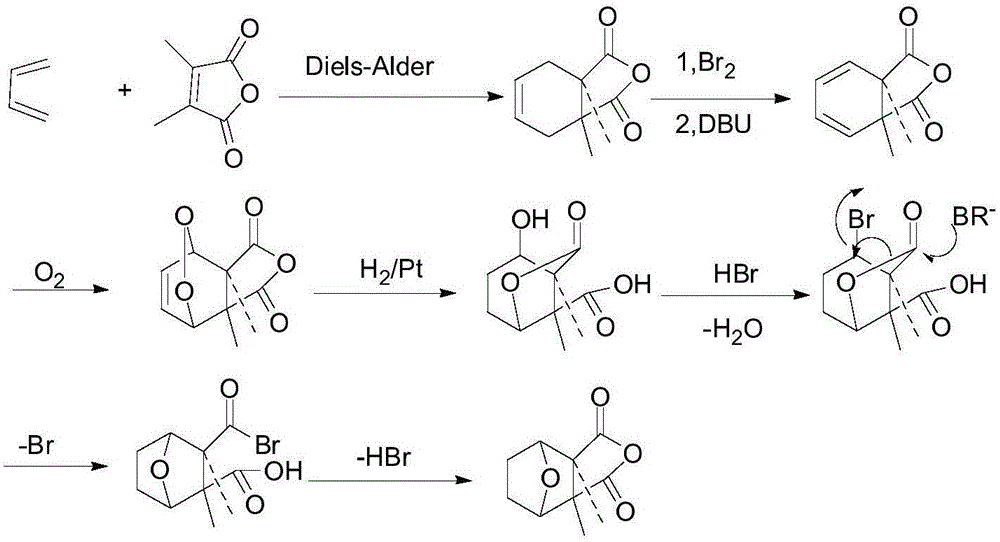
Novel green and environment-friendly synthetic process for cantharidin
A green, environmentally friendly, synthetic technology, applied in organic chemistry and other directions, can solve problems such as low conversion rate, and achieve the effects of high conversion rate, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 3-cyano-3-hydroxy-4-formic acid methyl ester-2,5-tetrahydrothiophene
[0029] Add 315g of NaCN aqueous solution (mass percentage concentration: 23.2%) and 620mL of purified water into a 5L reaction kettle under ice-bath conditions, quickly add sodium bisulfite aqueous solution to adjust the pH of the reaction solution to 7-8, slowly add it dropwise within 40min 132g / 75mL of 3-oxo-4-carboxymethylthiophene / methanol solution, warmed to room temperature and reacted for 16h after dropping. It was extracted three times with dichloromethane, and the dichloromethane was evaporated under vacuum to obtain 197 g of yellow oily substance 3-cyano-3-hydroxy-4-methyl carboxylate-2,5-tetrahydrothiophene, with a yield of 80%.
[0030] (2) Preparation of 3-cyano-4-formic acid methyl ester-2,5-dihydrothiophene
[0031] Add 40g of 3-cyano-3-hydroxy-4-methyl-4-carboxylate-2,5-tetrahydrothiophene, 35.2g of benzene, and 60g of pyridine into a 1L reactor, control the intern...
Embodiment 2
[0040] (1) Preparation of 3-cyano-3-hydroxy-4-formic acid methyl ester-2,5-tetrahydrothiophene
[0041] Add 420g NaCN aqueous solution (mass 23.2%) and 800mL purified water into a 5L reaction kettle under ice-bath conditions, quickly add sodium bisulfite aqueous solution to adjust the pH of the reaction solution to 7-8, slowly add 244g / 100mL of 3 -Oxo-4-methyl carboxylate-thiophene / methanol solution, react for 16 hours after dropping. Dichloromethane was extracted three times, and dichloromethane was evaporated under vacuum to obtain 250 g of yellow oily substance 3-cyano-3-hydroxy-4-methyl-4-carboxylate-2,5-tetrahydrothiophene with a yield of 76%.
[0042] (2) Preparation of 3-cyano-4-formic acid methyl ester-2,5-dihydrothiophene
[0043] Control the internal temperature at 4-6°C, put 80g of 3-cyano-3-hydroxy-4-carboxylic acid methyl ester-2,5-tetrahydrothiophene, 88g of benzene, and 140g of pyridine into a 2L reaction kettle, and slowly add 250g of POCl 3 , after the reac...
Embodiment 3
[0051] (1) Preparation of 3-cyano-3-hydroxy-4-formic acid methyl ester-2,5-tetrahydrothiophene
[0052] Add 500g NaCN aqueous solution (mass 23.2%) and 1000mL purified water into a 10L reaction kettle under ice-bath conditions, quickly add sodium bisulfite aqueous solution to adjust the pH of the reaction solution to 7-8, slowly add 336g / 120mL of 3 -Oxo-4-methyl carboxylate-thiophene / methanol solution, react for 16 hours after dropping. Dichloromethane was extracted three times, and the dichloromethane was evaporated under vacuum to obtain 295 g of yellow oily substance 3-cyano-3-hydroxy-4-methyl-4-carboxylate-2,5-tetrahydrothiophene with a yield of 75%.
[0053] (2) Preparation of 3-cyano-4-formic acid methyl ester-2,5-dihydrothiophene
[0054] Control the internal temperature at 8-10°C, add 160g 3-cyano-3-hydroxy-4-carboxylic acid methyl ester-2,5-tetrahydrothiophene, 176g benzene, 280g pyridine into the 2L reaction kettle, and slowly drop 500g POCl 3 , after the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com