Preparation of norcantharidin targeted liposome and application of freeze-drying preparation of norcantharidin targeted liposome
A technology of methylcantharidin lipid and norcantharidin, which is applied in the preparation of norcantharidin liver-targeted liposomes and its application in freeze-dried preparations, and can solve the problems of large tablet dosage, adverse reactions, and clinical application limitations and other problems, to achieve the effect of simple operation and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The phospholipid material lecithin 60mg, cholesterol 8mg, and stearyl glycyrrhetinate 6mg were dissolved in chloroform, dissolved by ultrasound, and then placed in an eggplant-shaped bottle and rotary steamed at 37°C to form a film. Dissolve 6mg of norcantharidin in 50ml phosphate buffer (pH 7.4), then add it to a thin eggplant-shaped bottle and hydrate for 1 hour. After hydration, the suspension probe will be sonicated (150W, 3min) to obtain fat. The plastid solution has a measured particle size of about 200nm.
Embodiment 2
[0028] The phospholipid material lecithin 60mg, cholesterol 8mg, and stearyl glycyrrhetinate 6mg were dissolved in 150ml ether, and then placed in an eggplant-shaped bottle, and rotary steamed at room temperature to form a film. Add ether to dissolve the membrane, dissolve 6mg of norcantharidin in 50ml phosphate buffer (pH 7.4), add lipid-containing ether, phacoemulsify the probe in an ice water bath, and then rotate the emulsion on a rotary evaporator After 20 minutes, the liposome suspension was obtained, and the probe was sonicated (150W, 3 minutes) to size the liposome solution, and the measured particle size was about 150 nm.
Embodiment 3
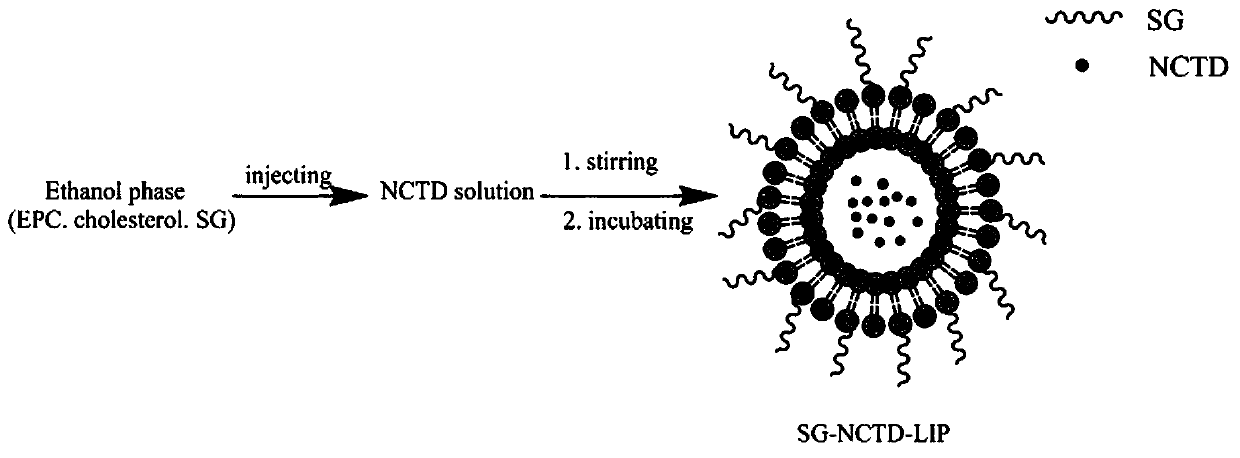
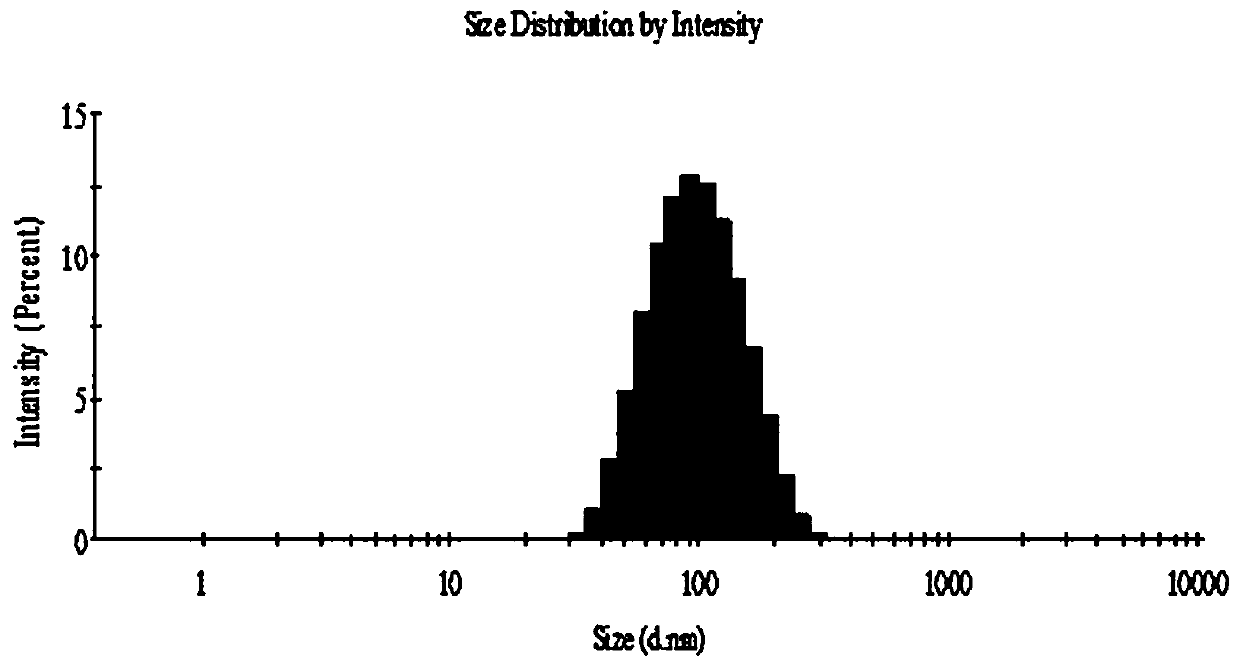
[0030] Ethanol injection method (such as figure 1 Shown): Dissolve 6 mg of norcantharidin in phosphate buffer (pH 7.4), place it on a magnetic stirrer and stir at a water bath temperature of 50°C; phospholipid material lecithin 60 mg, cholesterol 8 mg, and stearyl glycyrrhetinate 6mg is dissolved in 5ml ethanol, the lipid-containing ethanol solution is slowly injected into the medicated PBS solution with a syringe, incubated for 0.5h, and then rotary steamed to remove residual ethanol to obtain liposome suspension, and the probe is ultrasonically adjusted The liposome solution is obtained from the granule, and the measured particle size is about 90nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com