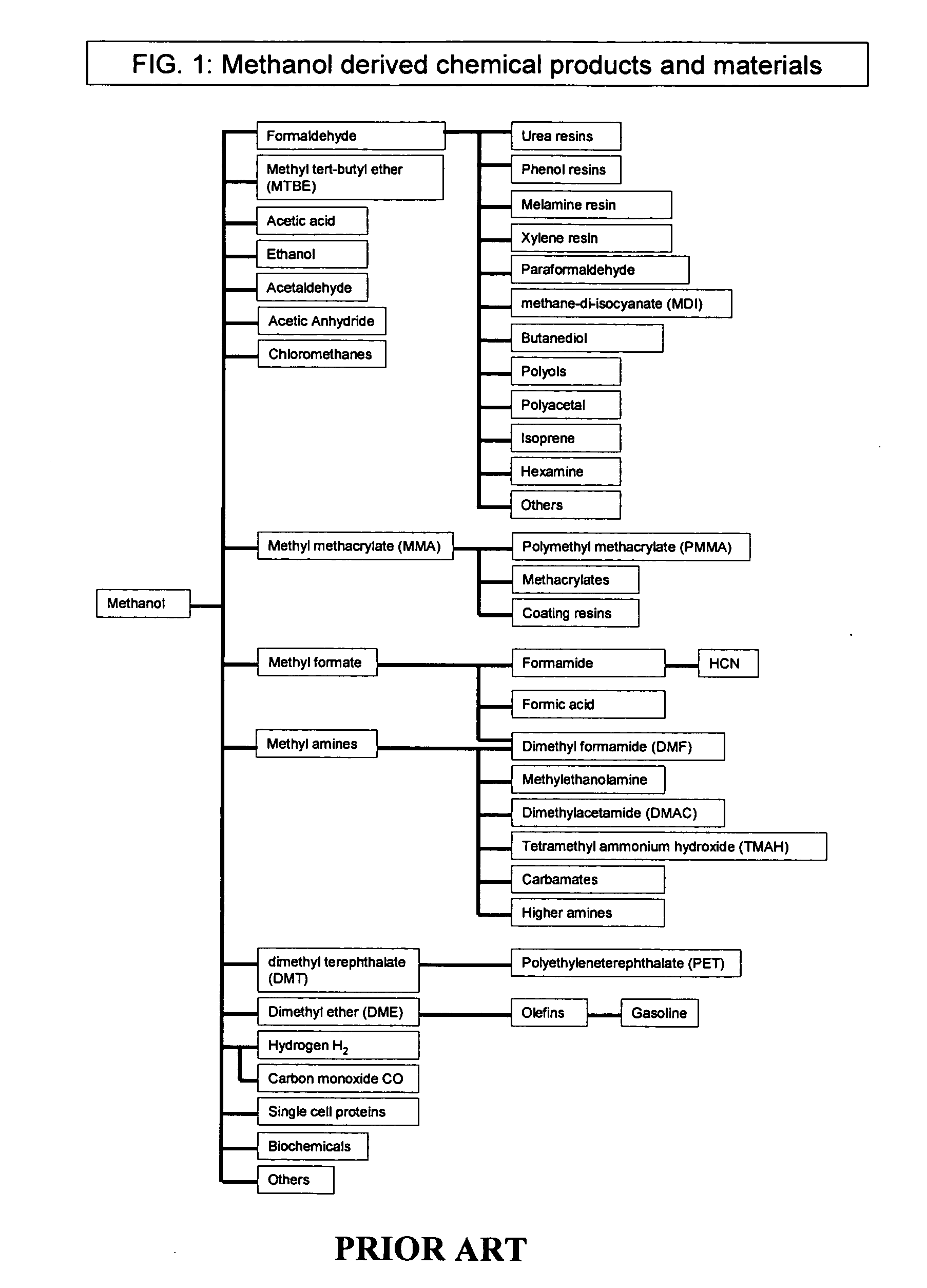
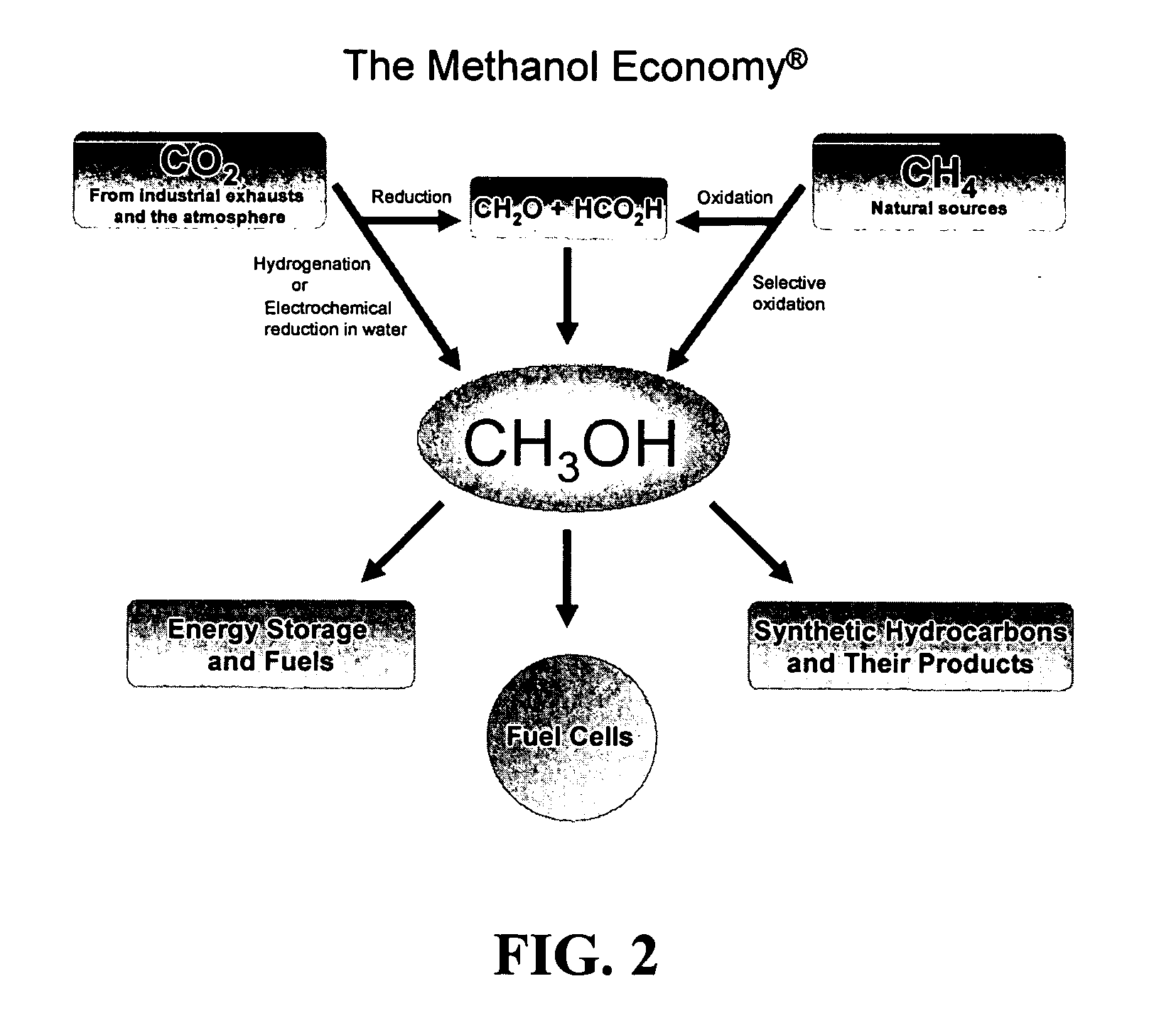
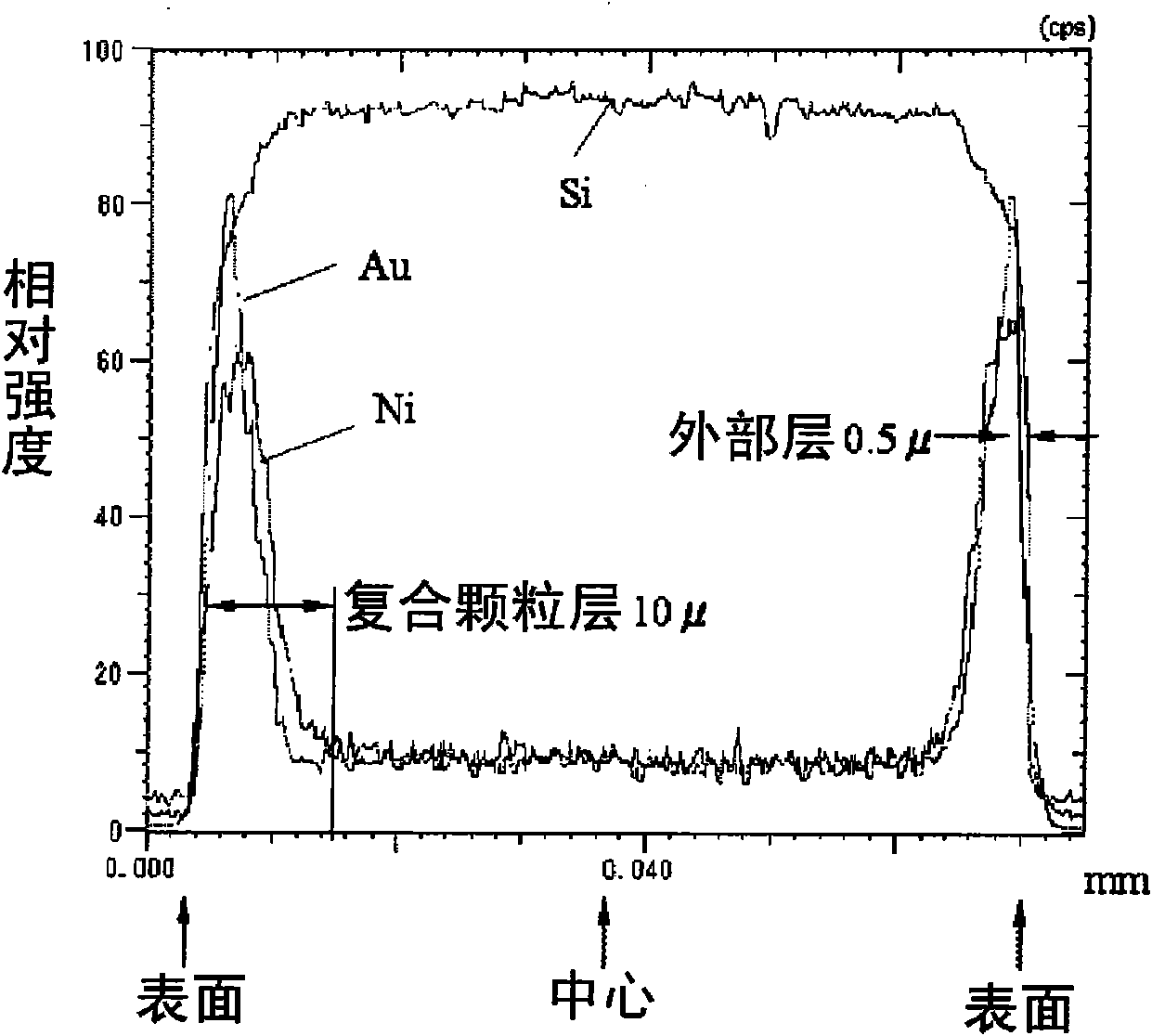
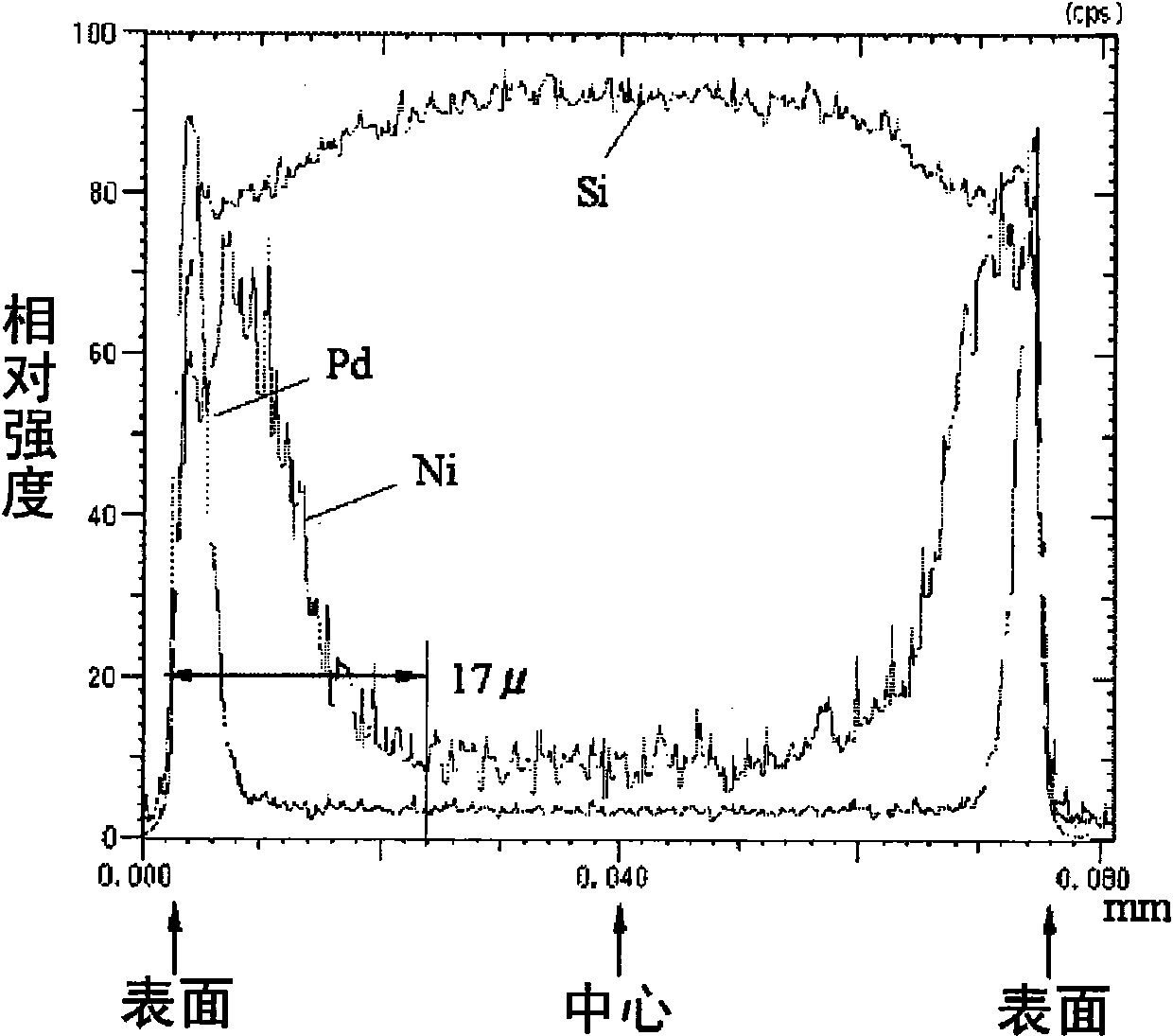
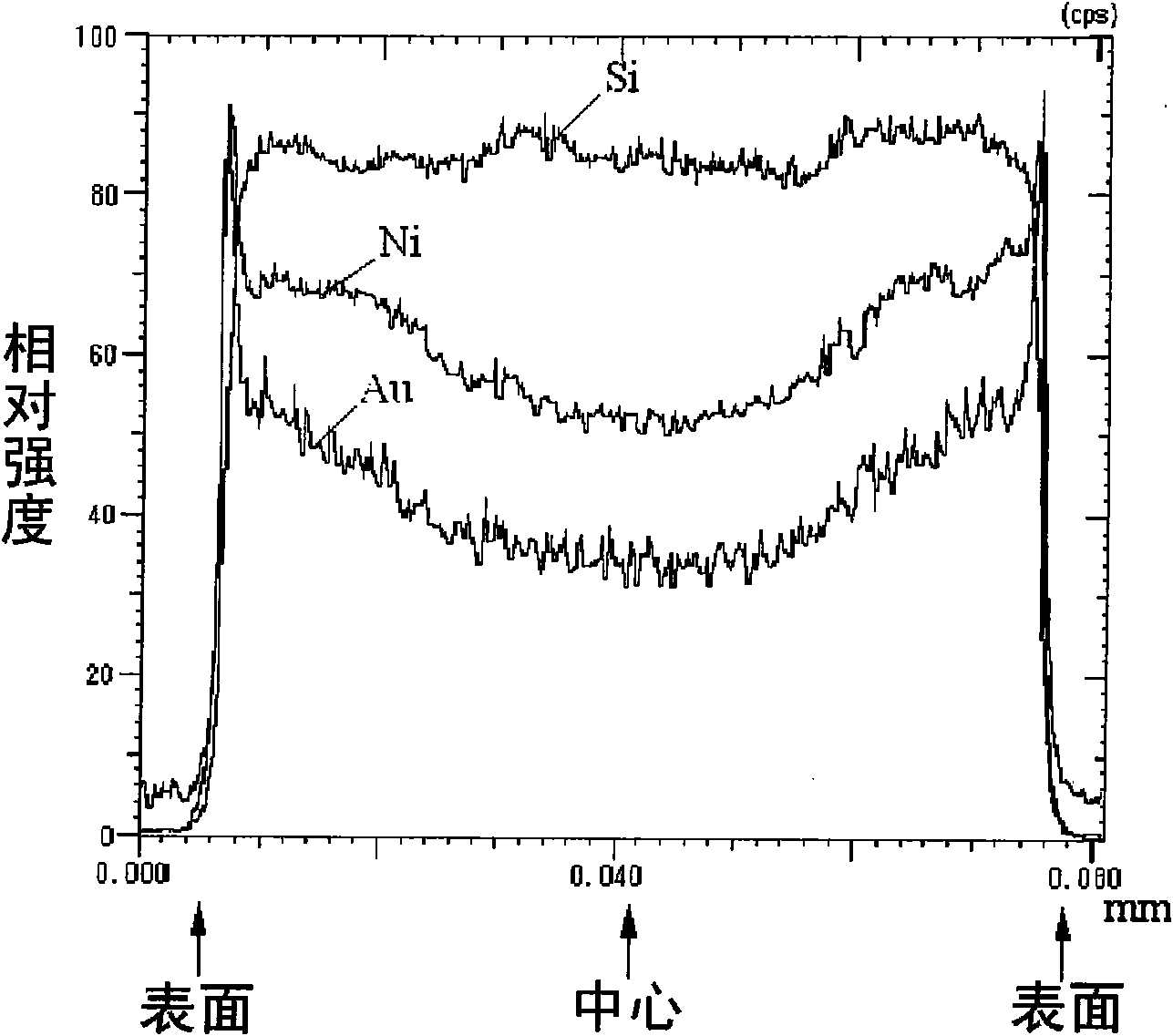
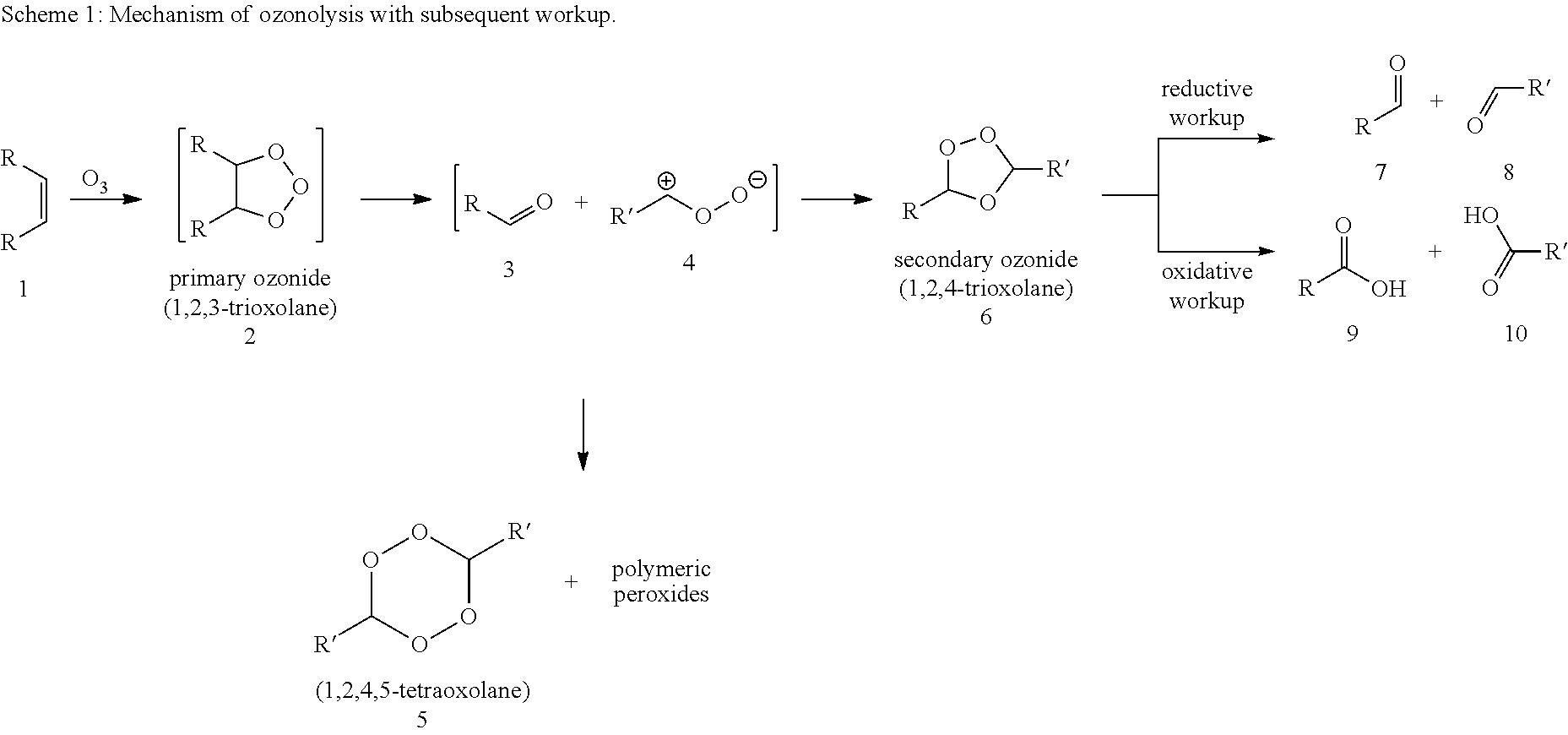
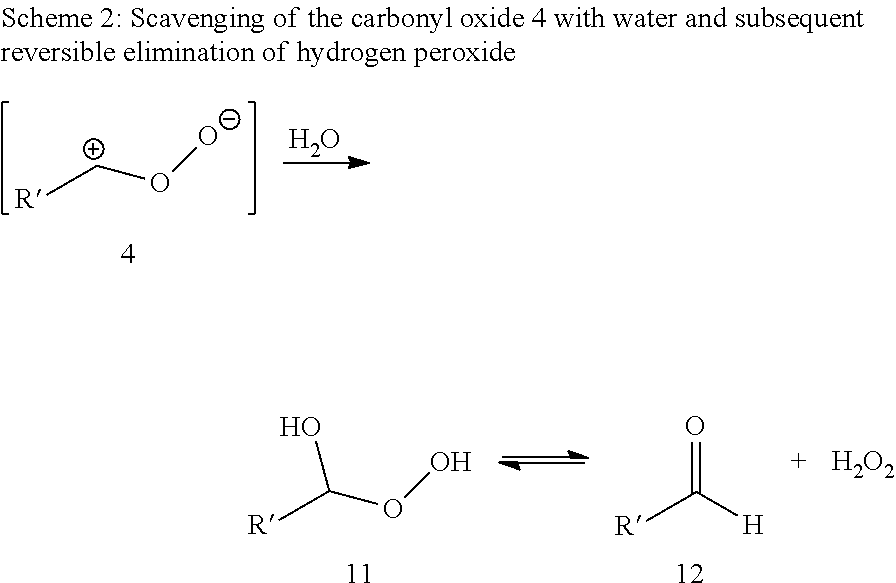
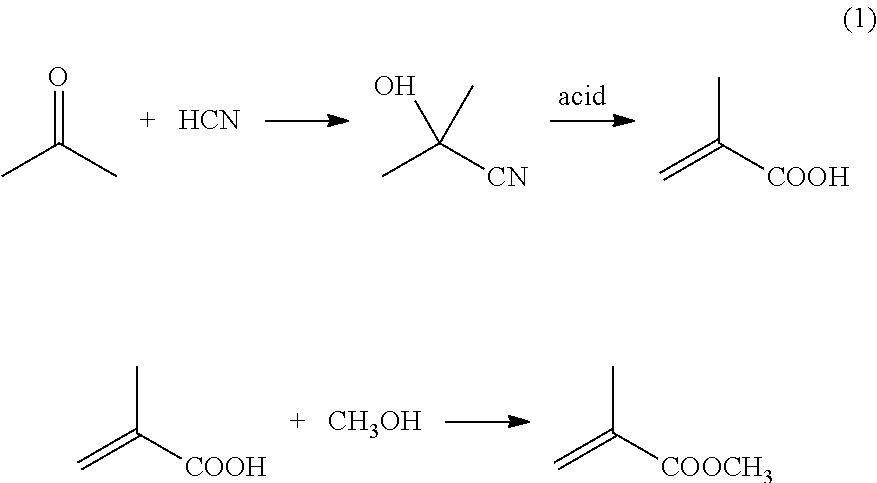
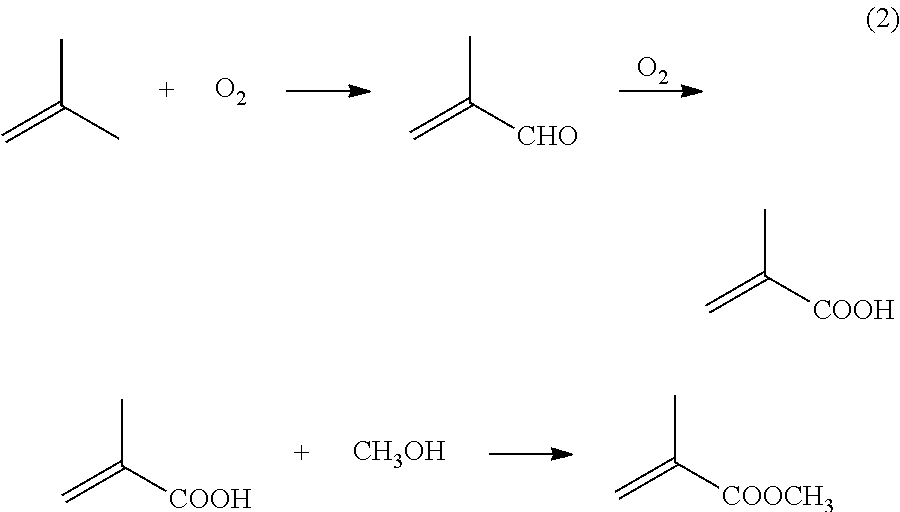
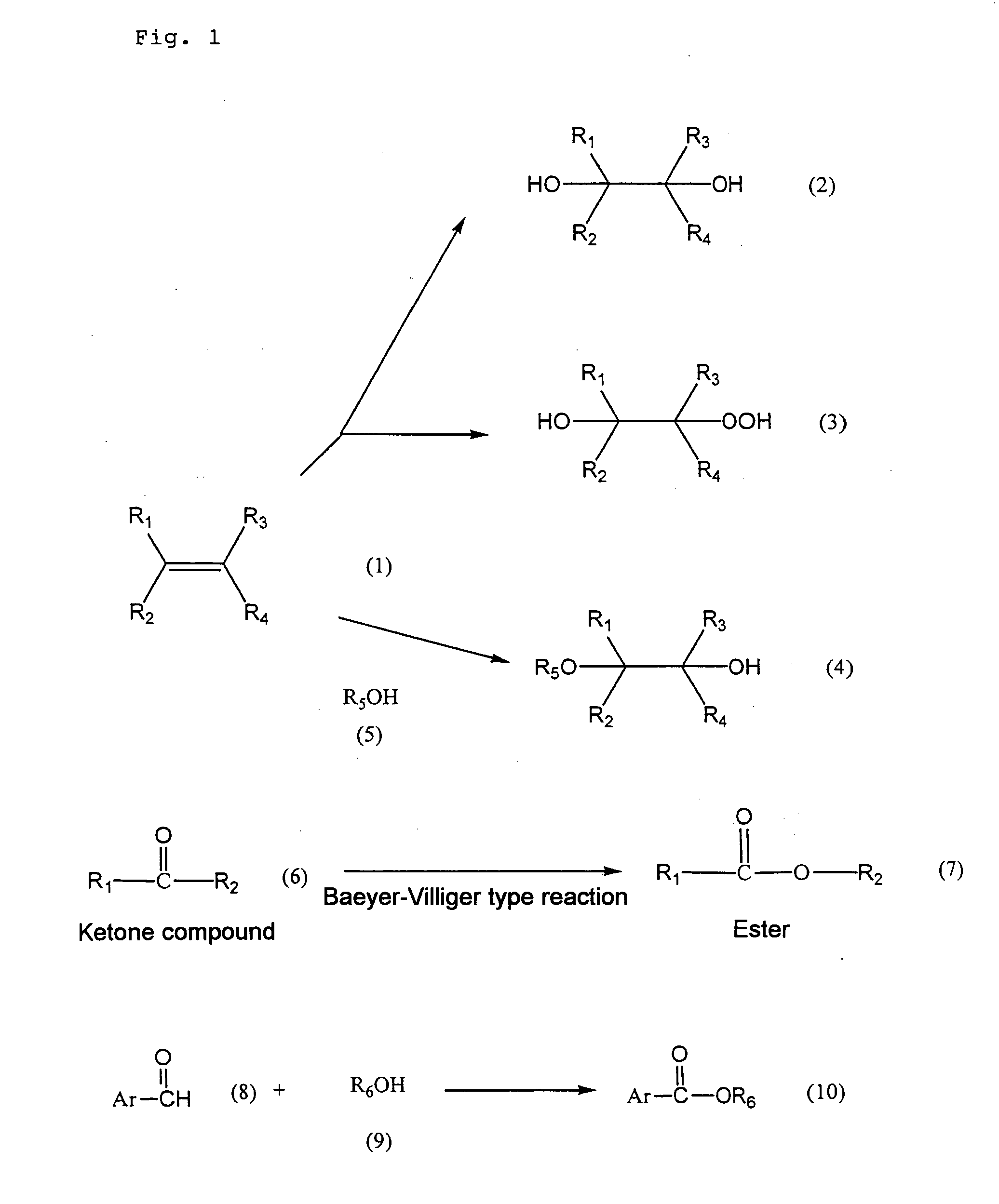
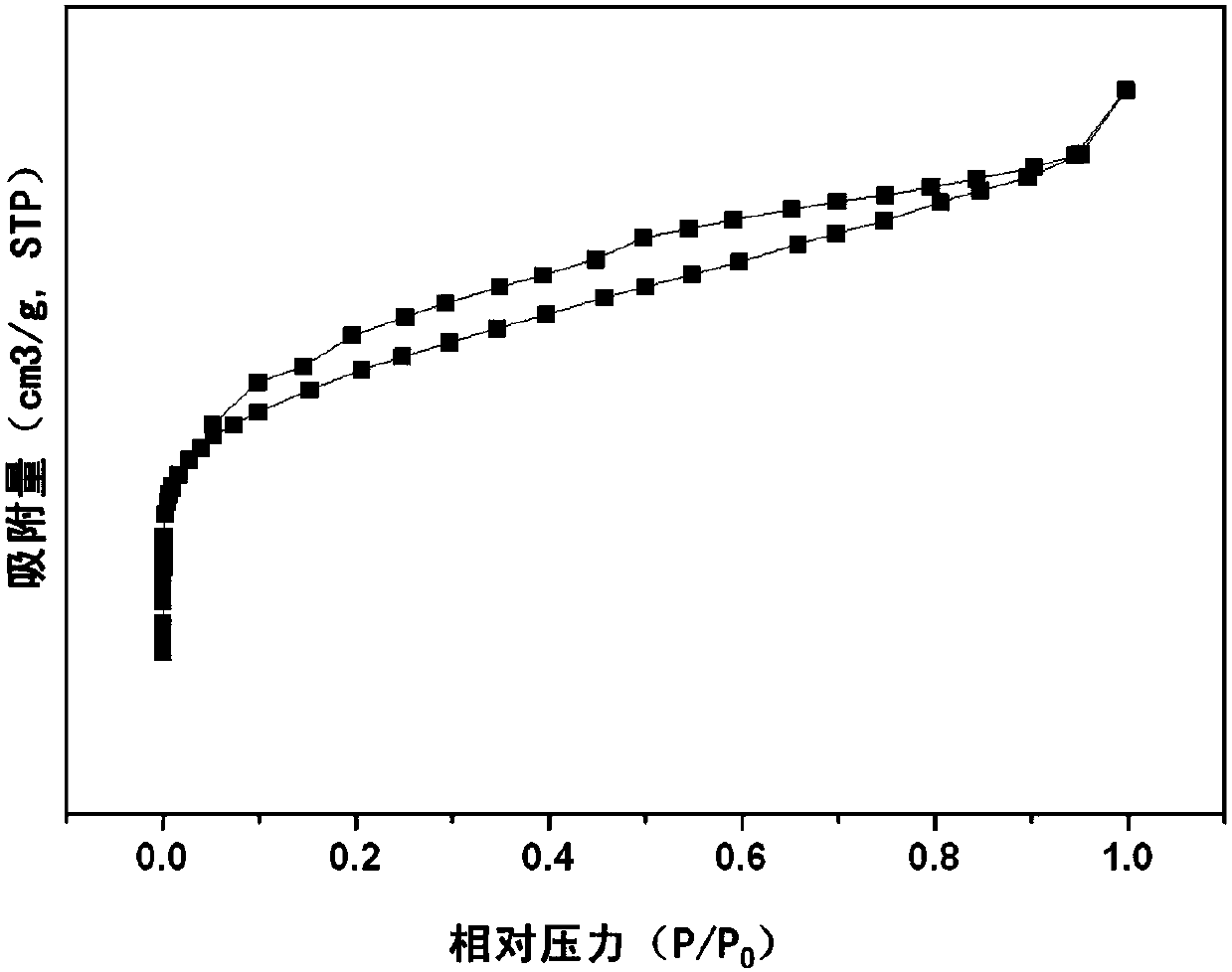
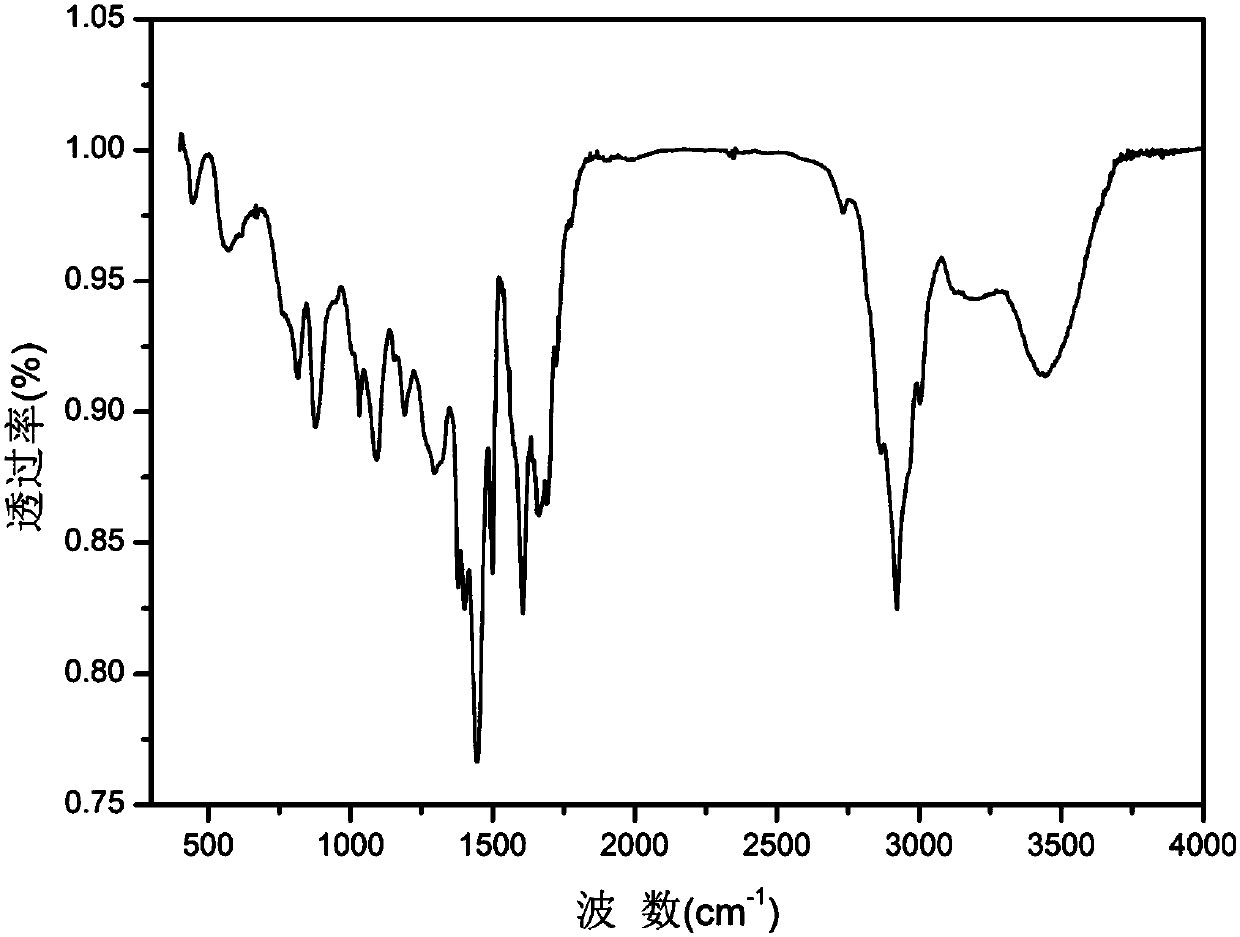
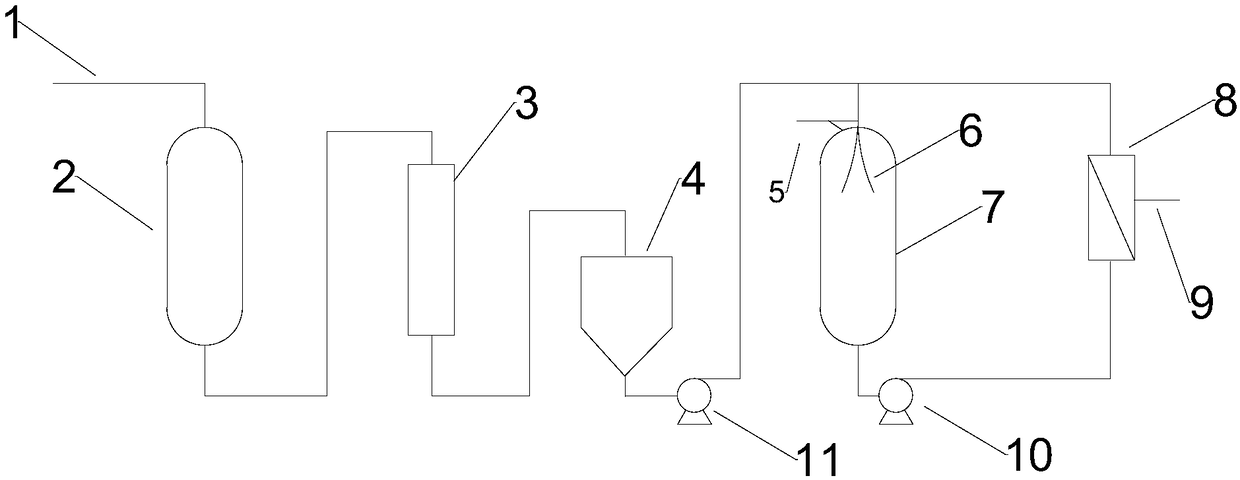
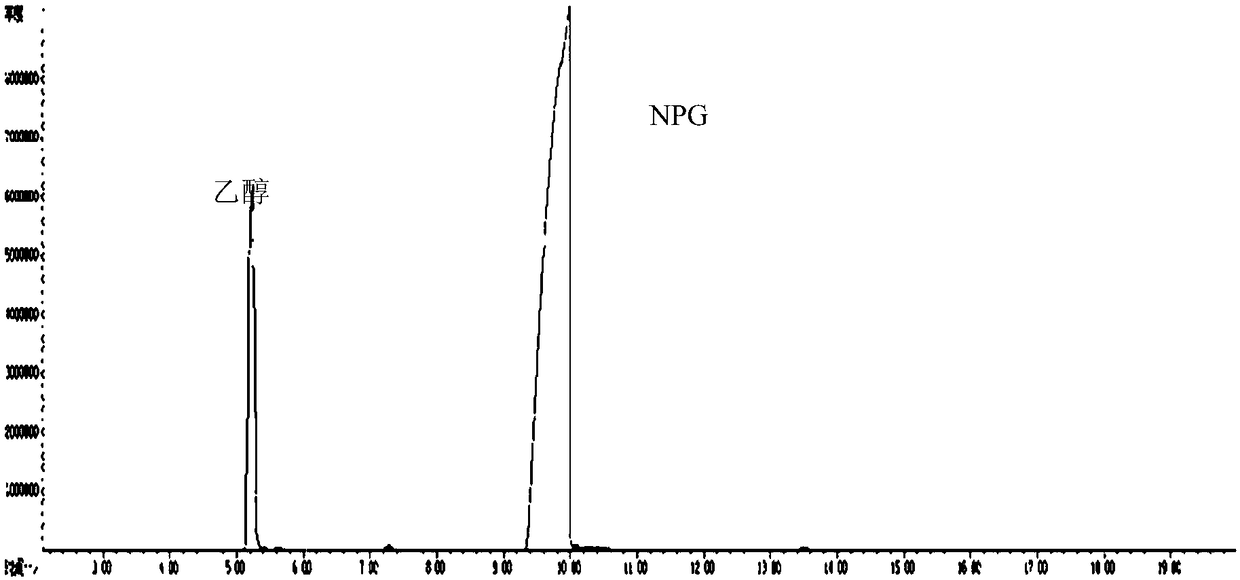
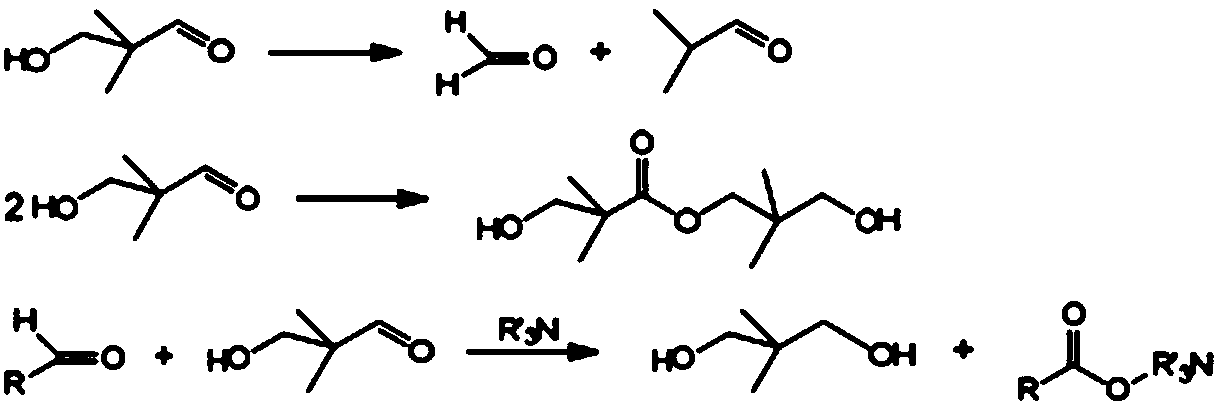
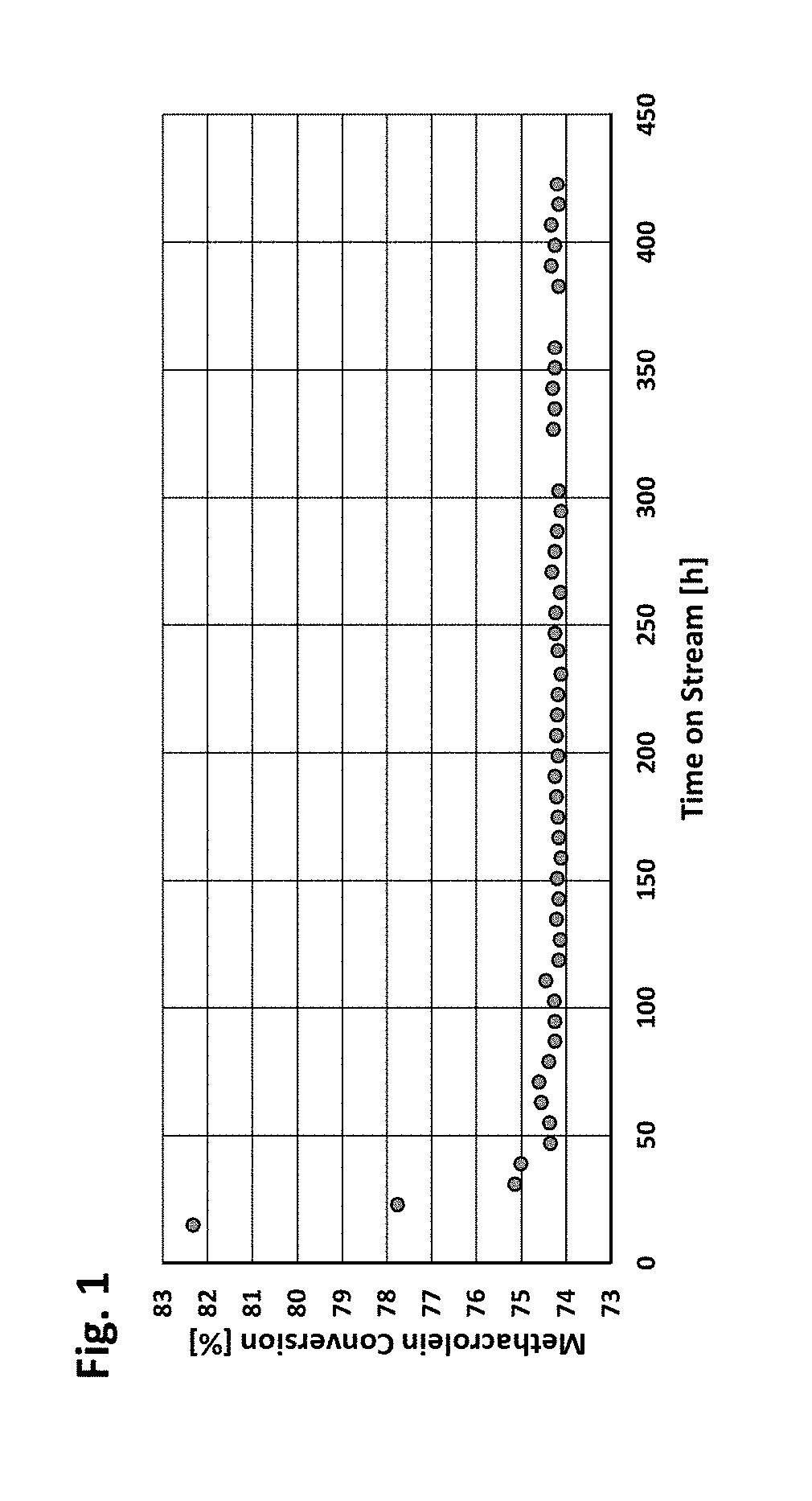
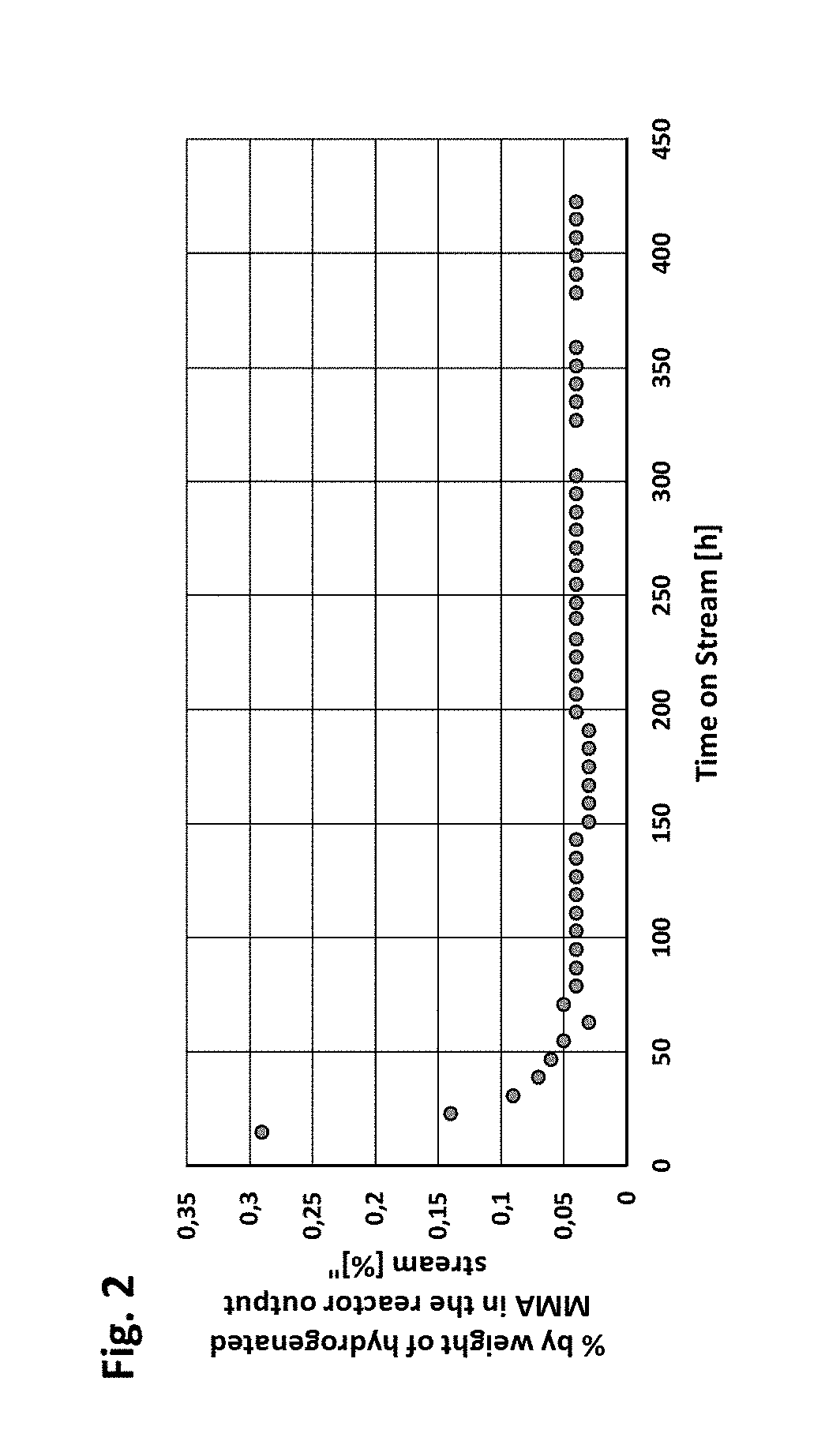
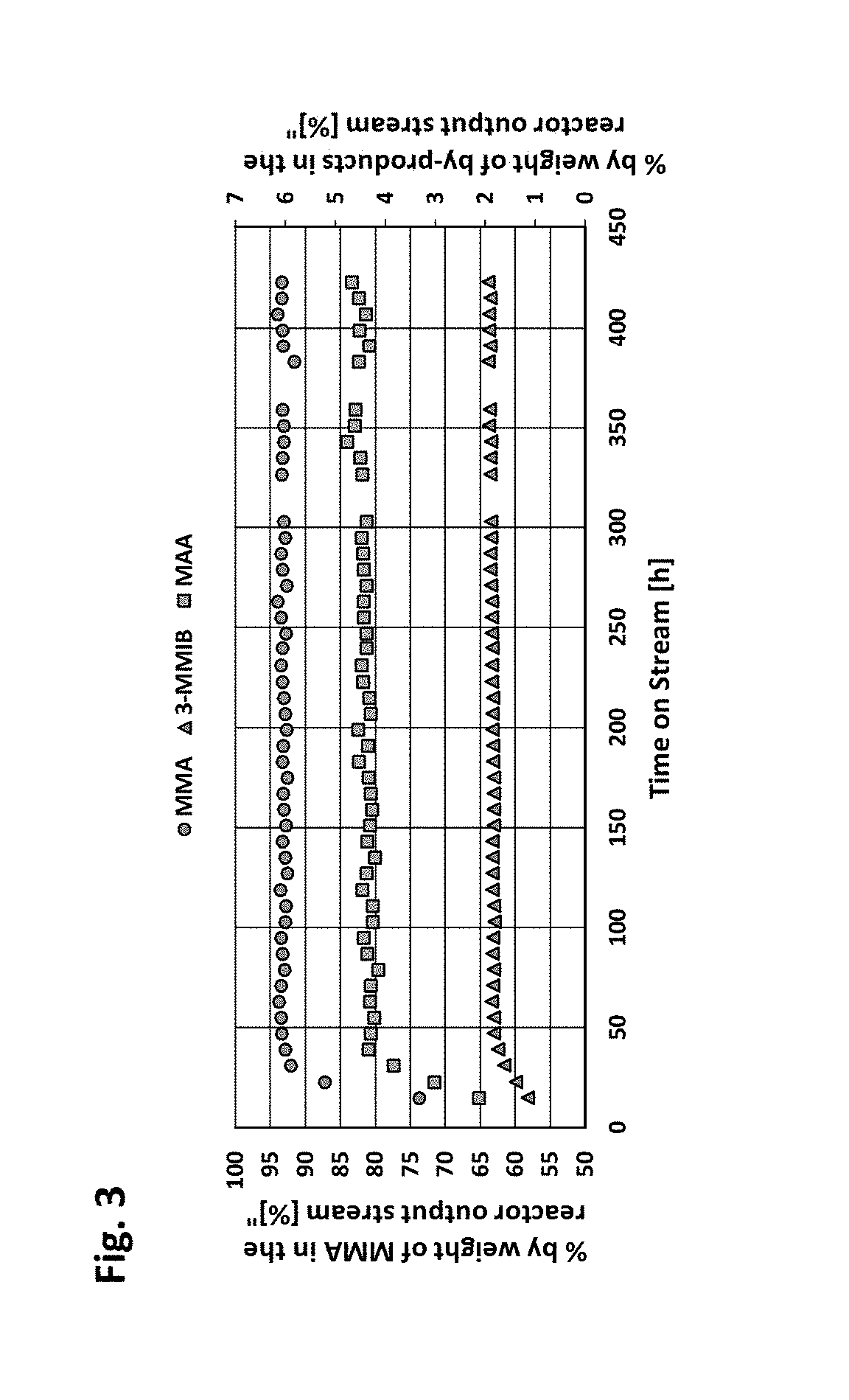
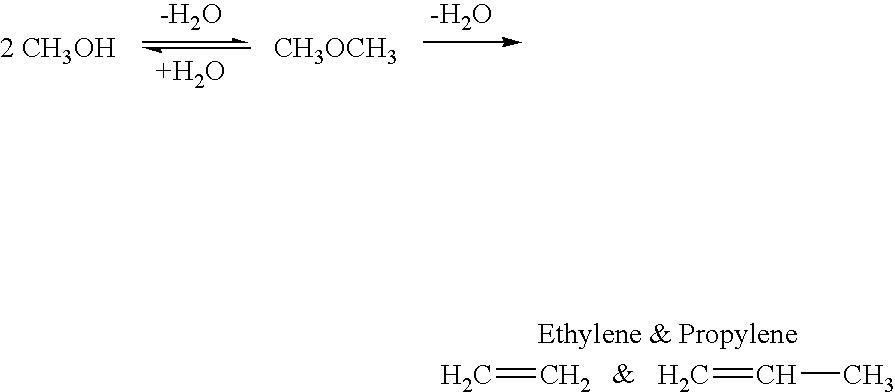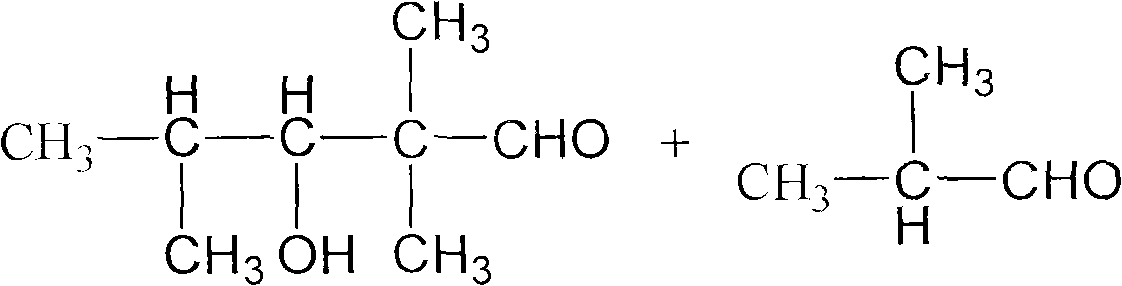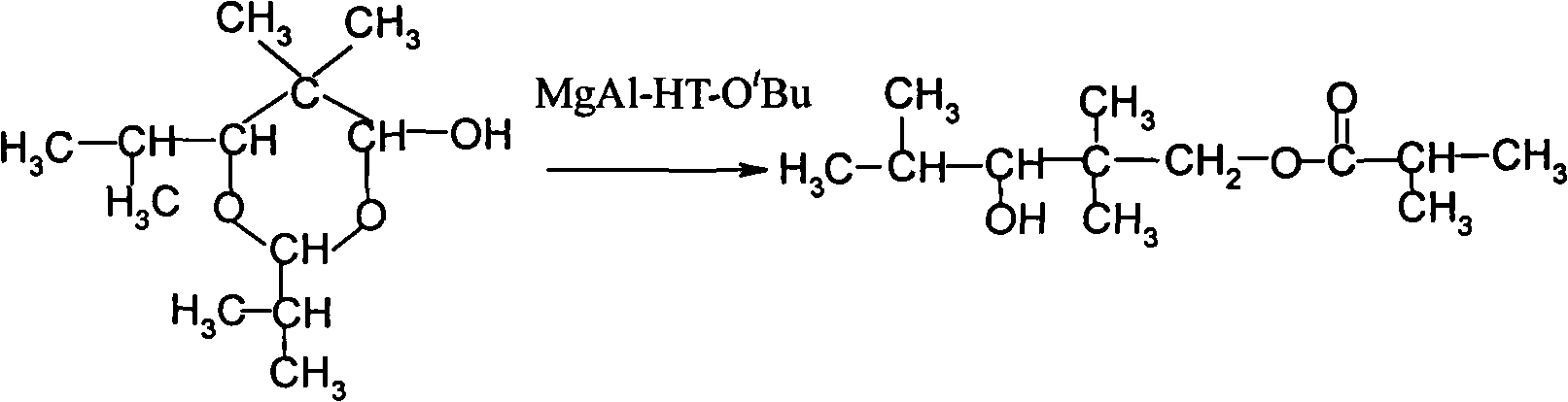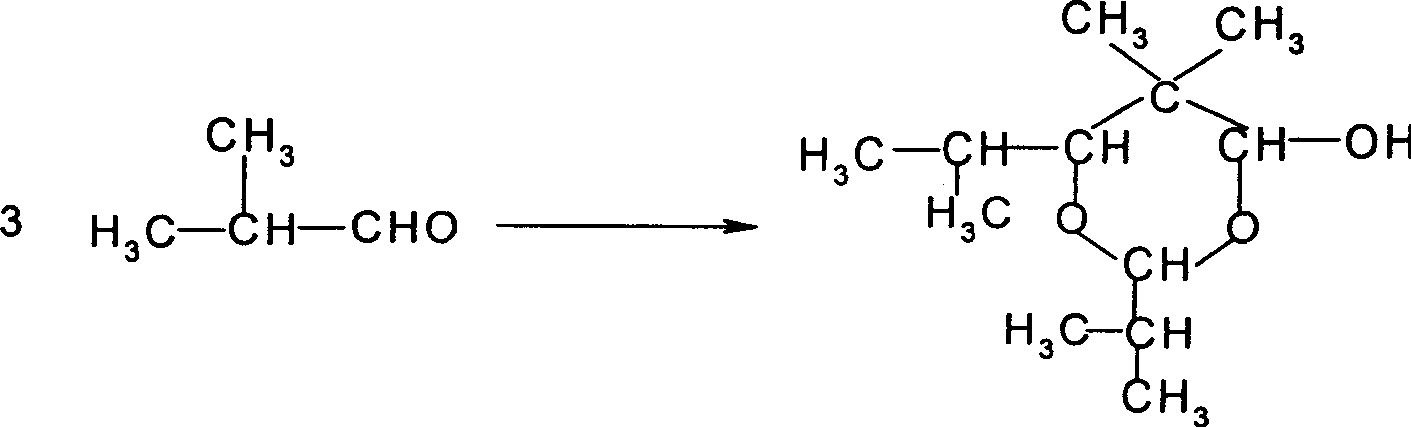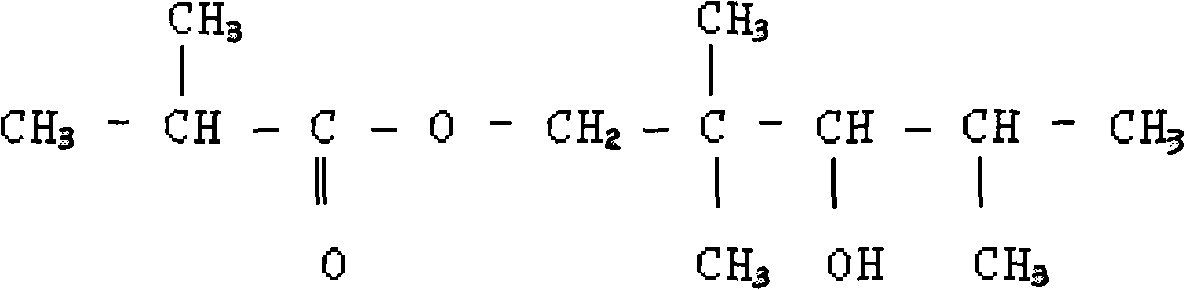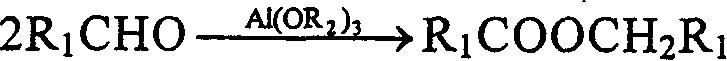Patents
Literature
154results about "Preparation by aldehyde oxidation-reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
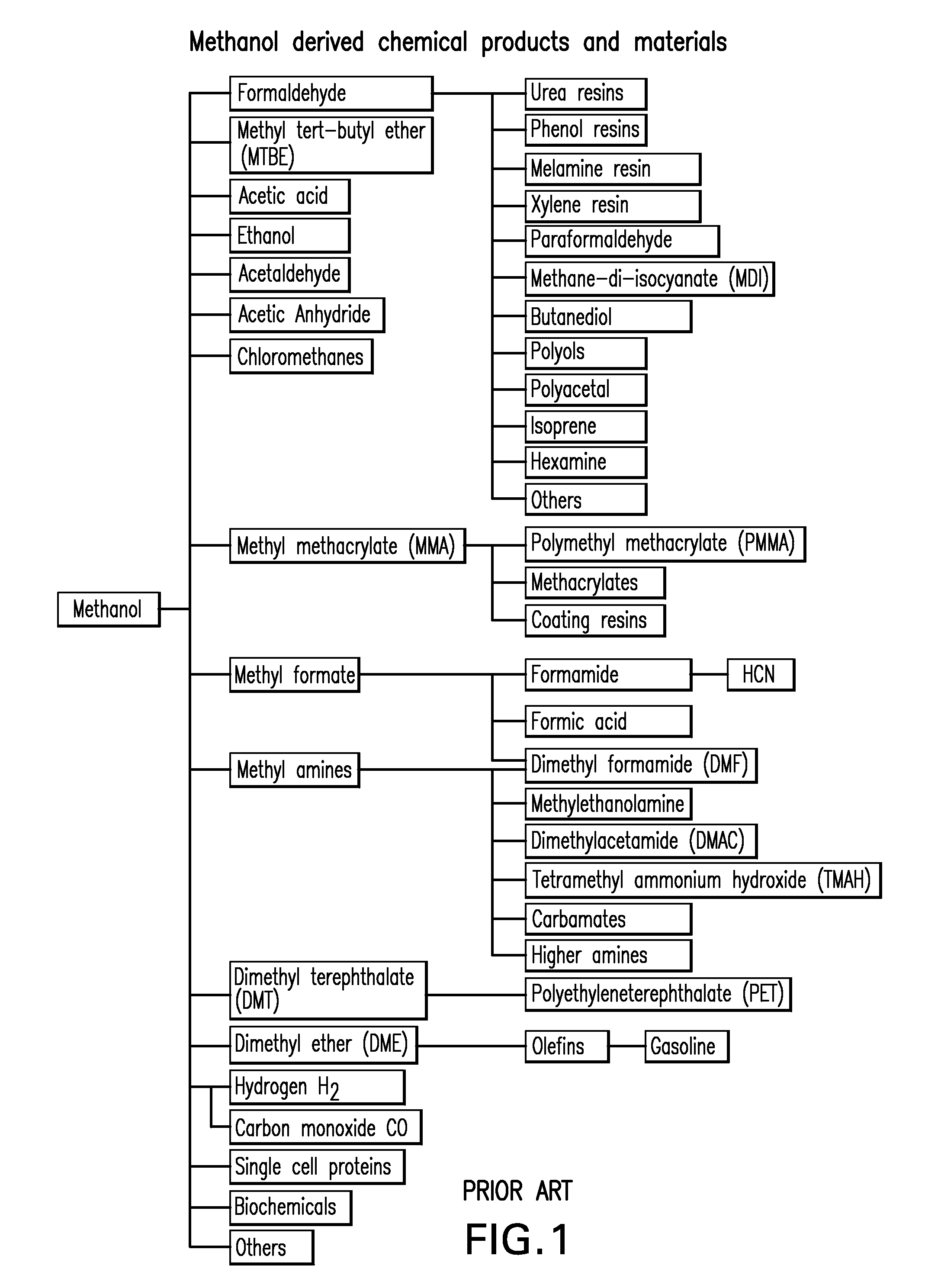
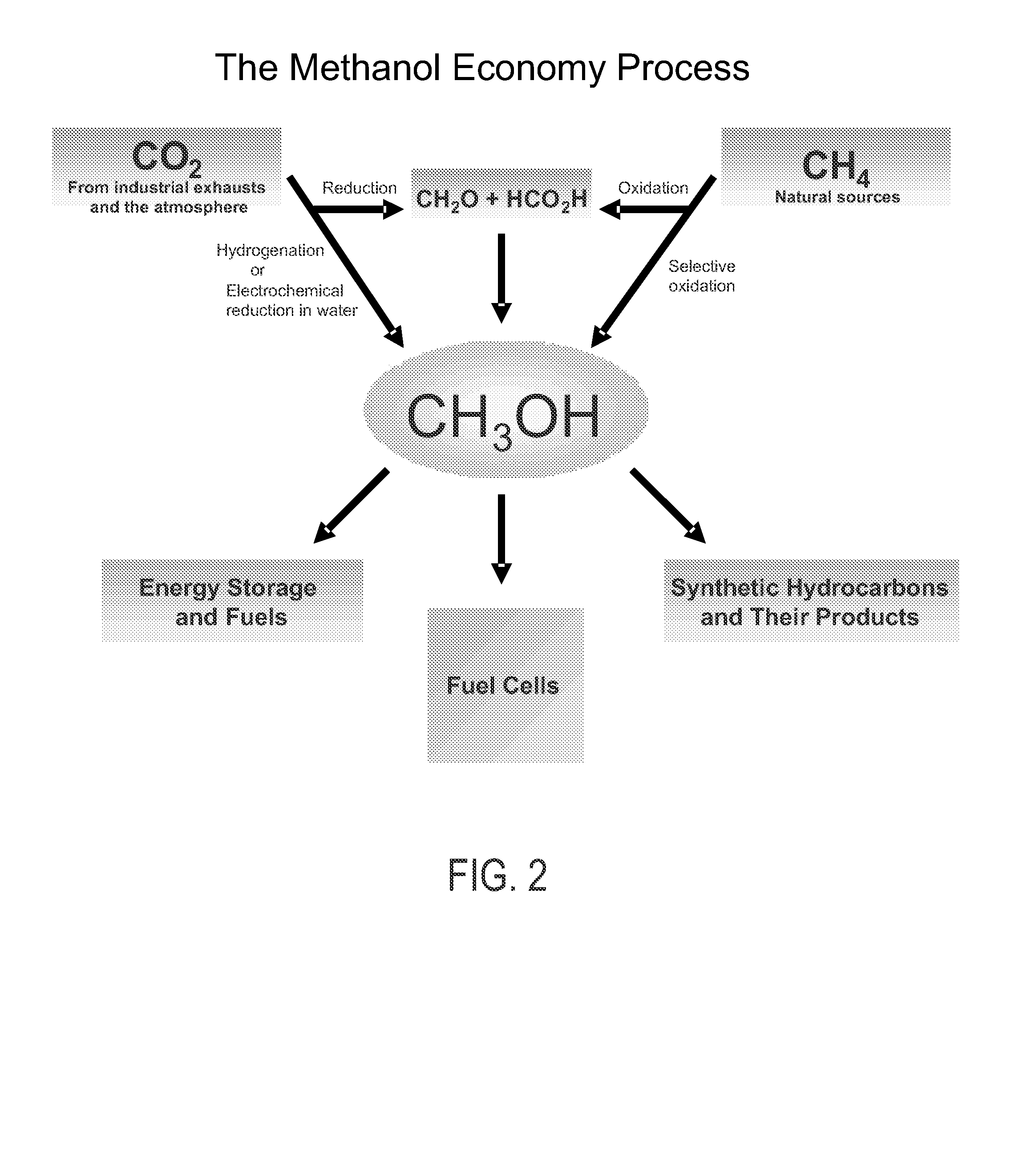
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
Production of methyl a-methacrylate with methanal as raw material
InactiveCN101074192AImprove conversion rateReduce manufacturing costPreparation by aldehyde oxidation-reductionMethacrylateDivinylbenzene
Production of methyl-methyl acrylate from methanal is carried out by reacting methanal with propanal in diethylamine muriate solution at 40-45 degree to obtain methyl-acryl, mixing methyl-acryl with methanol in proportion of 30-50:1, adding them into reactor, adding into 2.0-4.0% catalyst, inducing into oxygen at 40-60 degree and flow 10 ml / min, reacting for 4-6 hrs and synthesizing to obtain final product. The catalyst carrier is styrene-divinylbenzene polymer organic resin, which consists of palladium 2-5 wt%, bismuth 0-3 wt%, lead 0.3-1 wt% and iron 0.3-1 wt%. It's cheap, has friendly reactive environment, better selectivity and conversion rate.
Owner:TIANJIN UNIV
Catalyst for ester production and process for producing ester
InactiveUS20060224013A1Organic compound preparationPreparation by aldehyde oxidation-reductionGas phaseSilicon oxide
A catalyst for ester production which comprises zirconium oxide, copper, and at least one oxide selected from the group consisting of zinc oxide, chromium oxide, aluminum oxide and silicon oxide, and is obtainable by reducing with hydrogen a catalyst precursor prepared by the reaction of a salt containing at least one of metals constituting the oxides, a zirconium salt and a copper salt with an alkali hydroxide; and a process for producing an ester which comprises bringing either an alcohol or an alcohol and an aldehyde into contact with this catalyst in a gas phase.
Owner:CHISSO CORP
Composite particle-loaded article, method for producing the composite particle-loaded article, and method for producing compound using the composite particle-loaded article as chemical synthesis catalyst
ActiveCN101835532AEfficient use ofImprove responseOrganic compound preparationOrganic chemistry methodsChemical synthesisPlatinum
Disclosed is a composite particle-loaded article which contains composite particles composed of nickel in an oxidized state and a substance X (X represents at least one element selected from the group consisting of nickel, palladium, platinum, ruthenium, gold, silver and copper), and a carrier on which the composite particles are loaded. The composite particle-loaded article has a loaded layer in which the composite particles are localized.
Owner:ASAHI KASEI KK
Synthesis of alpha,omega-dicarboxylic acids and esters thereof from unsaturated fatty acid derivatives
InactiveUS20120245375A1Inhibition formationDirect conversionOrganic compound preparationPreparation by aldehyde oxidation-reductionOzonolysisCarboxylic acid
A process for preparing an alpha, omega-dicarboxylic acid or an ester thereof, which includes (a) subjecting at least one unsaturated fatty acid or fatty acid derivative to ozonolysis to obtain an ozonolysis reaction mixture; and (b) oxidizing the ozonolysis reaction mixture with an oxidizing agent in the presence of an acid catalyst to obtain an oxidized reaction mixture comprising at least one alpha, omega-dicarboxylic acid or ester; wherein the process is performed using a solvent and the acid catalyst has a pKa of less than or equal to zero, as measured at 25° C.
Owner:EVONIK DEGUSSA GMBH
Catalyst for producing carboxylic acid esters, process for producing same and process for producing carboxylic acid esters (as amended)
ActiveUS20110184206A1Improve the level ofImprove responseOrganic compound preparationOther chemical processesOxygenPt element
Disclosed is a catalyst for use in production of carboxylic acid ester by reacting (a) aldehyde and alcohol, or (b) one or more types of alcohols, in the presence of oxygen; wherein oxidized nickel and X (wherein X represents at least one element selected from the group consisting of nickel, palladium, platinum, ruthenium, gold, silver and copper) are loaded onto a support within the range of the atomic ratio of Ni / (Ni+X) of from 0.20 to 0.99.
Owner:ASAHI KASEI KK
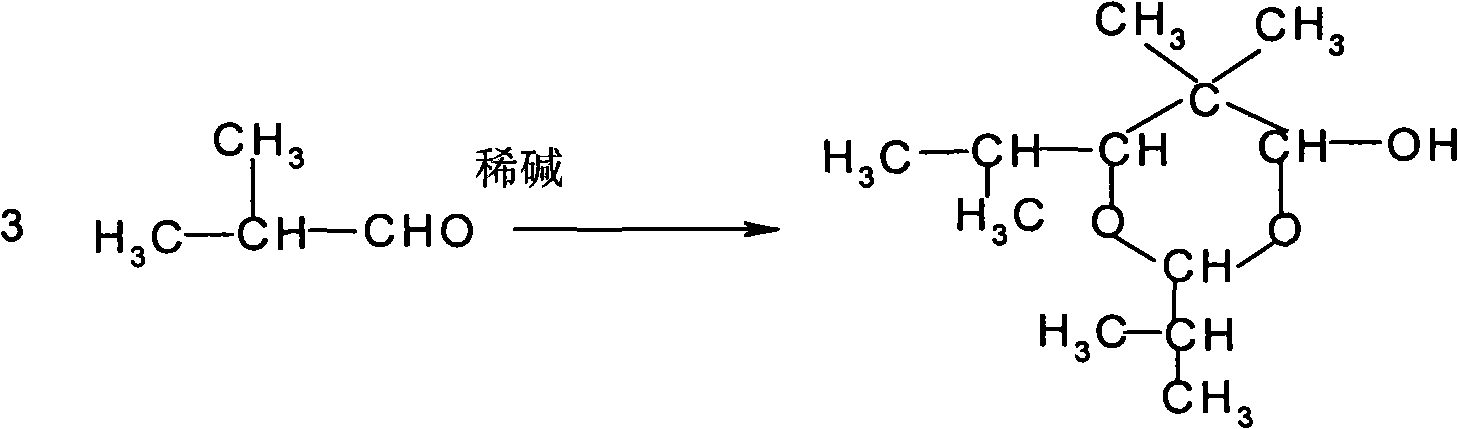
Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
ActiveCN101948386AAvoid process problemsAvoid energy consumptionPreparation by aldehyde oxidation-reductionAlcoholOrganic synthesis
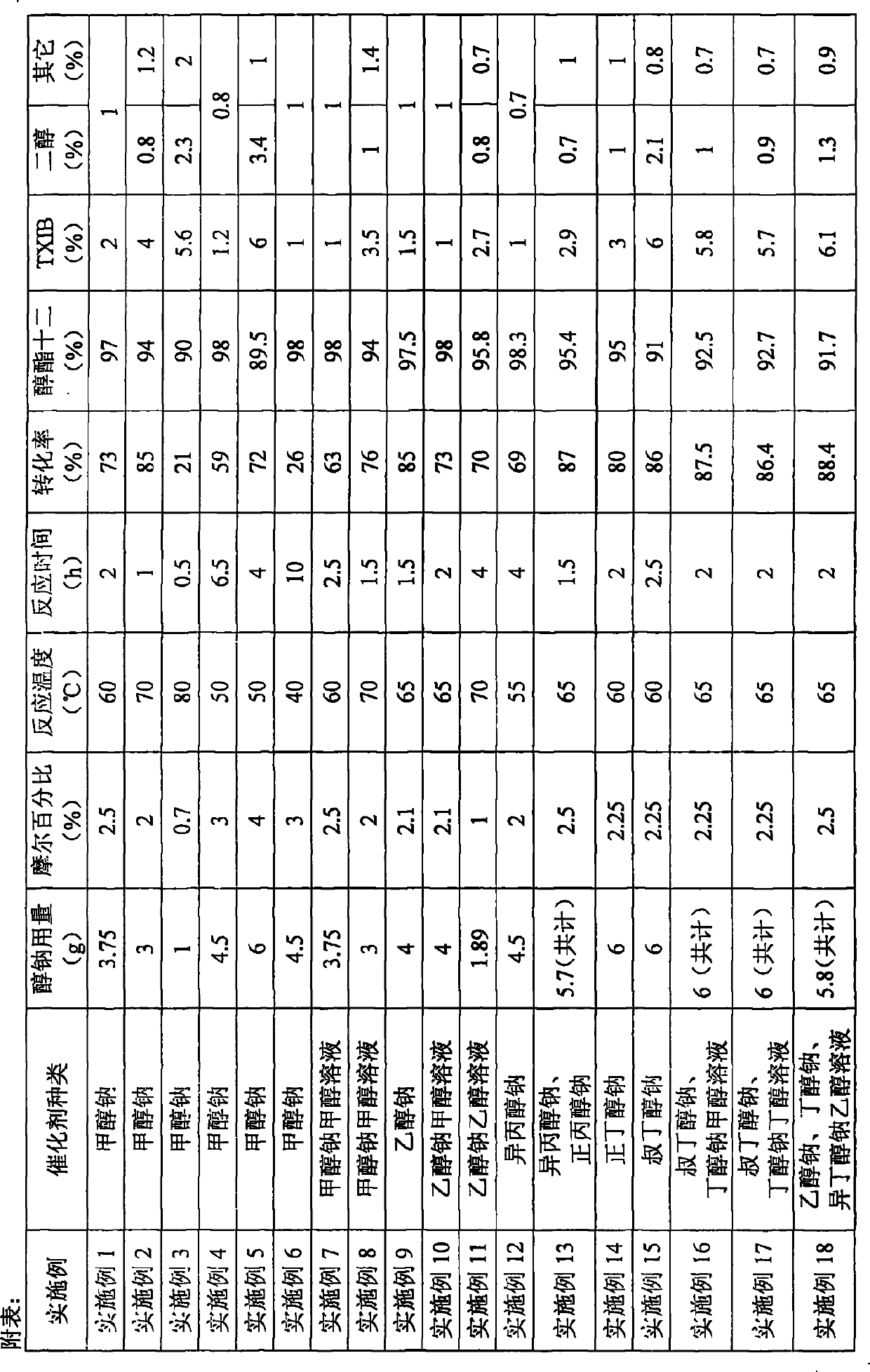
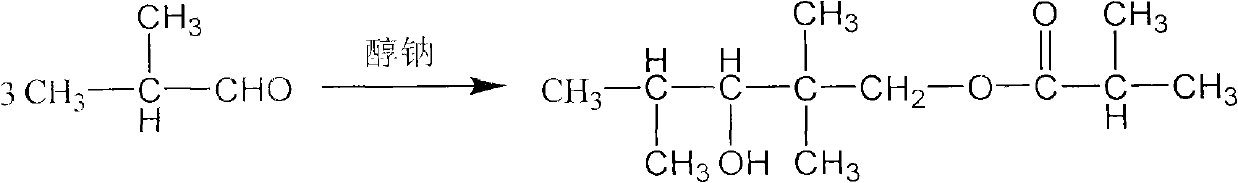
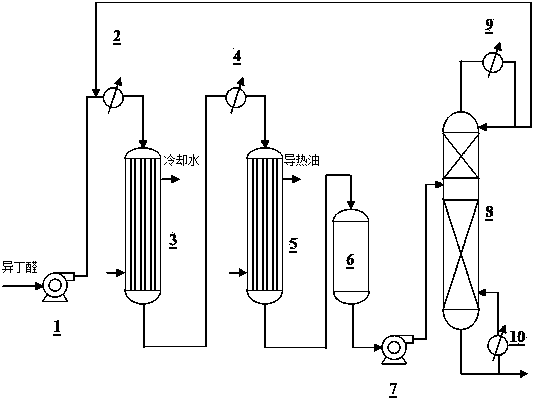
The invention discloses a method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, which relates to the technical field of organic synthesis. In the invention, isobutylaldehyde is used as a raw material, aldol condensation and Cannizzaro reactions are completed by a one-step reaction process in the presence of a catalyst, and the process adopts sodium alcoholate as the catalyst; and the isobutylaldehyde is used to form alcohol ester-12 in the presence of the sodium alcoholate catalyst, so the complex and high-energy consumption drawbacks of a two-step reaction process using an inorganic alkali as a catalyst are overcome, the drawbacks of toxic catalyst and high quality requirement on the isobutylaldehyde raw material of a one-step reaction process using Ba(OH)2 as a catalyst are overcome, and simple process, high single-pass yield, small catalyst dosage, low energy consumption, safety and environmental protection are realized.
Owner:YIXING HENGXING FINE CHEM
Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
InactiveCN101863762AImprove one-way yieldHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by aldehyde oxidation-reductionTert-butoxyTishchenko reaction
The invention provides an environmental-friendly method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, which comprises a condensation reaction and a Tishchenko reaction of isobutyraldehyde, wherein condensation products of the isobutyraldehyde are subjected to the Tishchenko reaction under the catalytic action of tert-butoxy anion-pillared layered magnesium-aluminum anionic clay to generate 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate. The preparation method has the advantages of high single-pass yield, high selectivity of target products, a few by-products, mild reaction conditions, catalyst recycling and environmental-friendly synthetic process.
Owner:江苏天音化工有限公司 +1
Production of 2,2,4-trimethyl-1,-3-pentadiol mono-isobutyric acid
InactiveCN1817850AImprove one-way yieldMild reaction conditionsPreparation by aldehyde oxidation-reductionHydrotalciteHomogeneous catalysis
Production of 2,2,4-trimethyl-1,3-pentanediol monoester isobutyrate is carried out by condensation pre-treating isobutyl aldehyde, and Knigcharlo reacting in molecule under the action of hydrotalcite-like solid catalyst to obtain the final product. It has high single-pass recovery rate, gentle reactive condition and no corrosion.
Owner:EAST CHINA NORMAL UNIV
Preparation method of catalyst for producing methyl methacrylate and application thereof
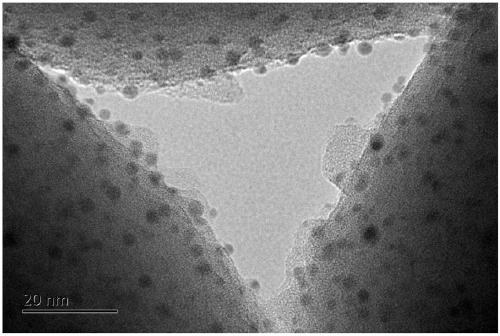
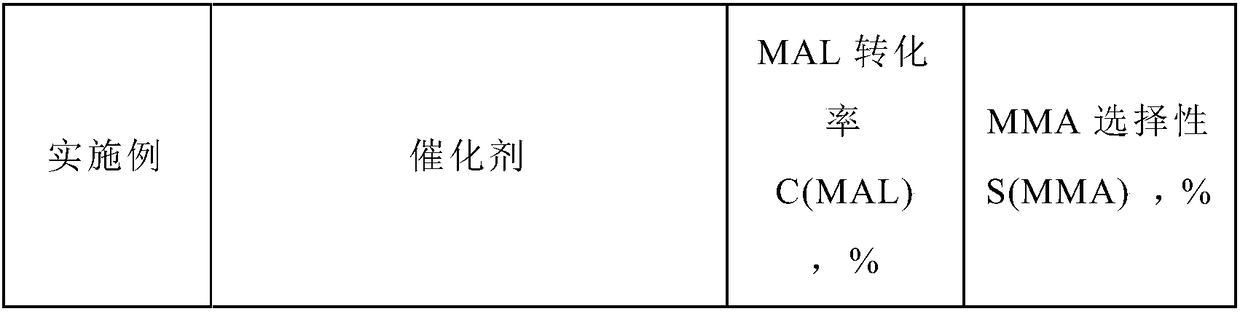
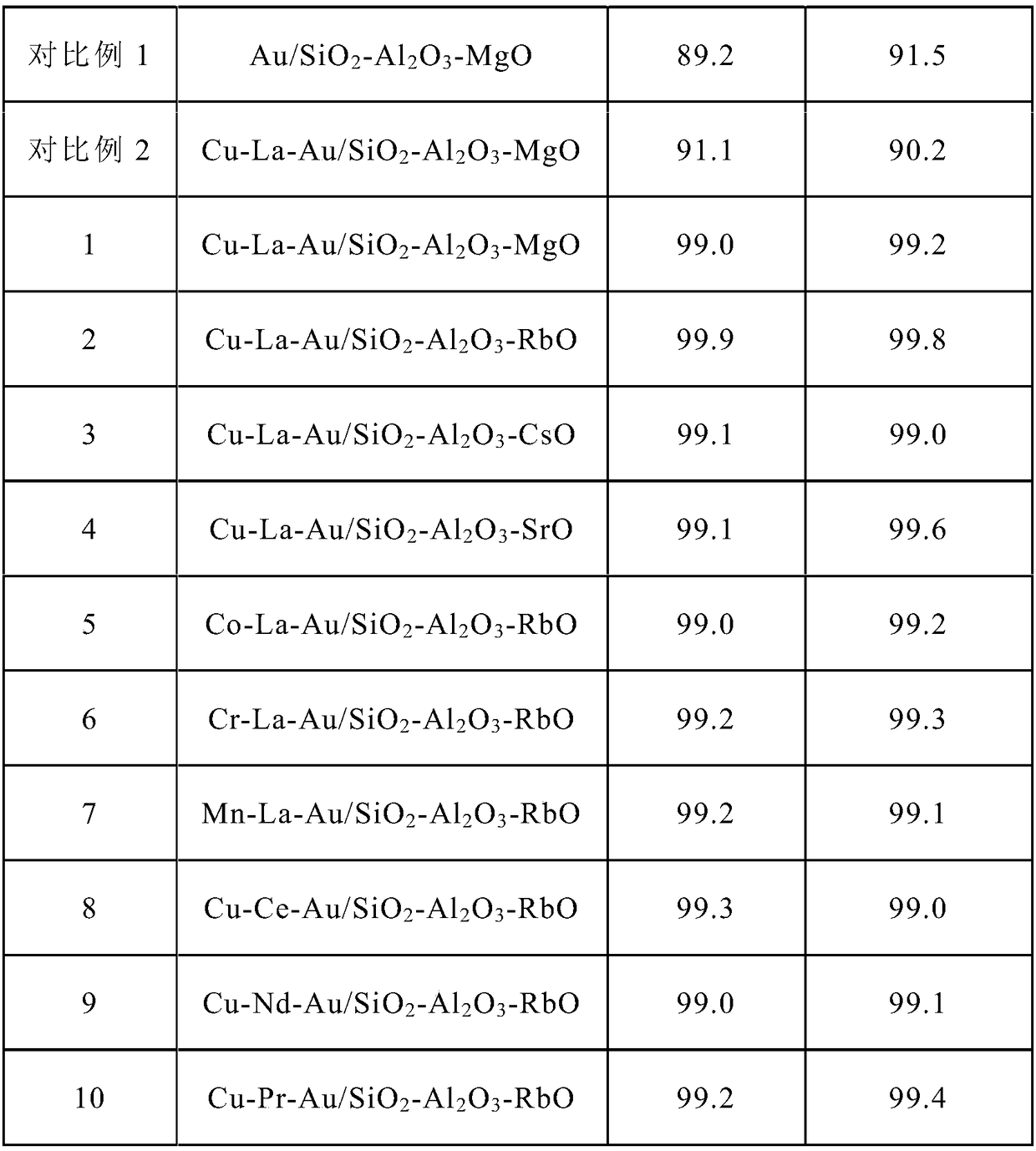
ActiveCN109331839AHigh selectivityImprove conversion rateHeterogenous catalyst chemical elementsPreparation by aldehyde oxidation-reductionFiltrationRoom temperature
The invention discloses a preparation method for a catalyst for producing methyl methacrylate. The preparation method comprises the following steps: fully mixing a gold precursor with a reducing agentand deionized water under stirring by adopting a a macromolecular protection method, so as to obtain stable and uniformly single gold sol in a higher dispersion state; sequentially adding a lanthanide metal and a transition metal in the presence of a macromolecular protection agent, then adding a carrier, continuously stirring for 2 to 20 hours, slowly heating the mixture to 65 to 85 DEG C, cooling the product to room temperature after the stirring is ended, leaving to stand for filtration, washing the product with the deionized water till no chloride ions are detected, drying the product, and calcining the product in air to obtain the catalyst. The catalyst has outstanding performance in the reaction for producing the methyl methacrylate. According to the preparation method, the gold carrying amount is low; the preparation process is simple and easy to operate; the catalyst is excellent in activity, extremely high in stability and low in cost; the methylacrolein conversion rate and the MMA (methyl methacrylate) selectivity are high; the preparation method is suitable for industrial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
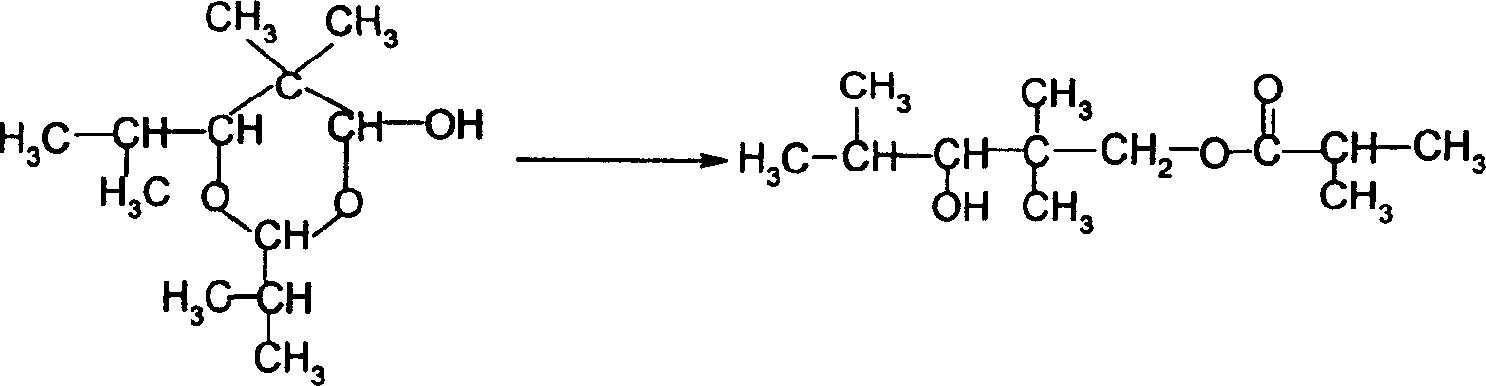
Method for preparing 2,2,4-trimethyl-1,3-pentanediol single-isobutyrate
InactiveCN101838197AEasy to separate and recycleReduce corrosionPreparation by aldehyde oxidation-reductionAldehydeCorrosion
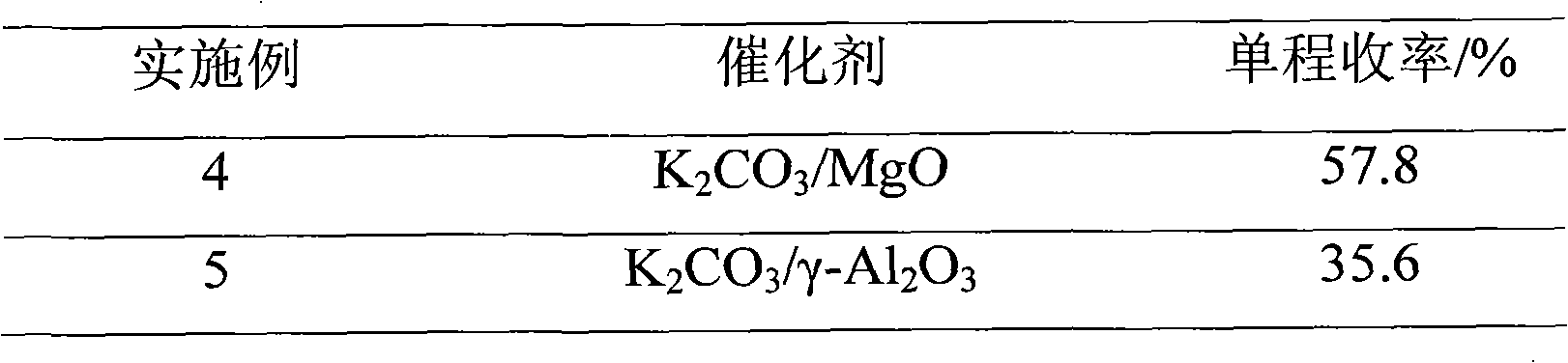
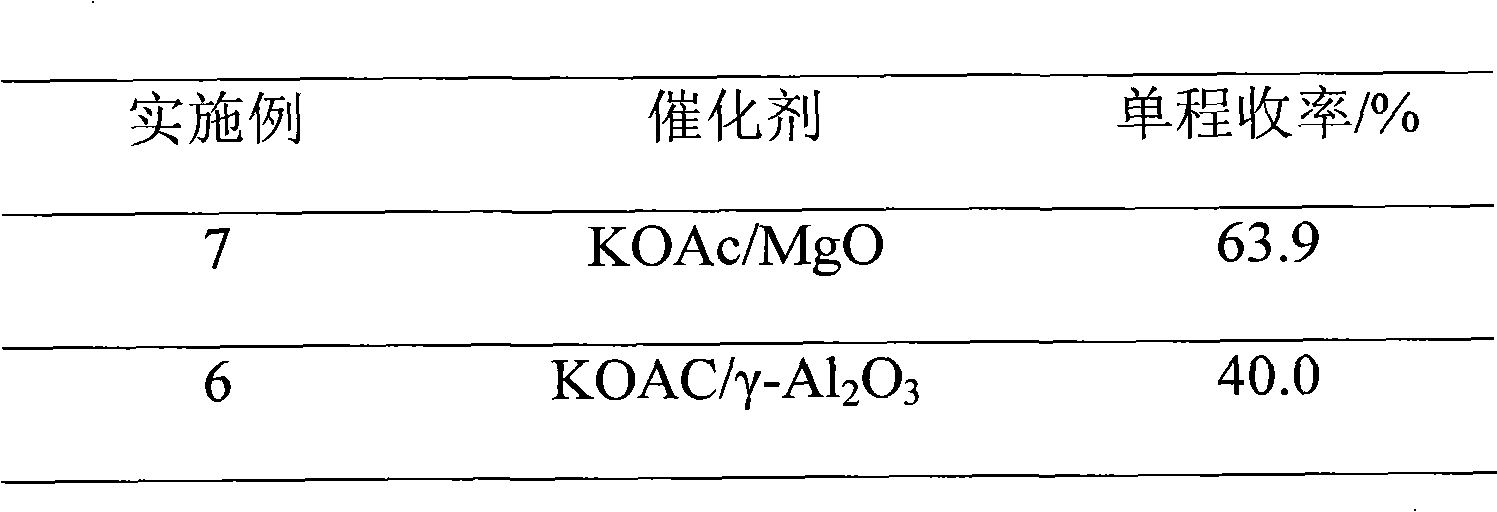
The invention discloses a method for preparing 2,2,4-trimethyl-1,3-pentanediol single-isobutyrate, which comprises the step of performing an intra-molecular Cannizzaro reaction on an aldol condensation product, which is prepared from isobutyraldehyde at a low temperature, under the action of a catalyst to synthesize the 2,2,4-trimethyl-1,3-pentanediol single-isobutyrate, wherein the catalyst is ametallic oxide heterogeneous catalyst modified by KF, K2CO3 or KOAc which is used as an active center base. The catalyst in the method is simple and easy to prepare, is easy to separate and reclaim and has small corrosion to equipment; and the method is simple to operate and has a mild reaction condition.
Owner:EAST CHINA NORMAL UNIVERSITY
Method for preparation of methyl formate
ActiveCN104016857ASave raw materialsWide range of operationsPreparation by aldehyde oxidation-reductionTishchenko reactionMixed gas
The invention discloses a method for preparation of methyl formate. The method comprises the following steps: conducting a Tishchenko reaction on formaldehyde gas or mixed gas containing formaldehyde gas in a reactor to obtain the methyl formate after the reaction. The method can efficiently convert methanol or dimethyl ether for synthesis of methyl formate, and the yield of methyl formate can reach more than 90%. The method employs cheap catalyst and simple equipment, can be applied to the current common industrial production conditions, and has good application prospect.
Owner:PEKING UNIV
Alcohol ester-12 production process
InactiveCN107698451AImprove performanceGood film formingOrganic compound preparationPreparation by aldehyde oxidation-reductionAlcoholBoiling point
The invention discloses an alcohol ester-12 production process which includes the steps: condensing isobutyraldehyde under alkaline conditions to generate 2,2,4-trimethyl-3-hydroxyl-1-valeraldehyde; continuing to perform polycondensation reaction on the isobutyraldehyde and the 2,2,4-trimethyl-3-hydroxyl-1-valeraldehyde generated by reaction to generate the alcohol ester-12. The production processhas the advantages that the process is continuous and simple, convenient to operate, small in occupied area and leas in investment, and products prepared by the process are stable. The alcohol ester-12 prepared by the method is a dihydric alcohol ester solvent with high boiling point and poly-functional groups, a novel environmental-friendly green chemical and mainly used for special coalescing agents of waterborne architectural coatings, and the waterborne architectural coatings made of the coalescing agents cannot emit substances toxic for people into an atmospheric environment.
Owner:ANHUI PROVINCE CHEM IND DESIGN INST
Green preparation process for synthesizing 12-carbon alcohol ester by double catalytic system
InactiveCN106631776AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholWastewater
The invention relates to a preparation method of 12-carbon alcohol ester. The method is characterized in that the 12-carbon alcohol ester is directly prepared from isobutyraldehyde by one step under the action of a catalyst; the catalyst is a double catalytic system consisting of supported type solid alkali and alkaline ionic liquid. The process effectively overcome the defect that the catalyst which is sodium alcoholate or sodium hydroxide is removed from the reaction system difficultly, and has the advantages that the catalyst is easy to separate and recover, the selectivity is high, the yield is high and waste water is basically not generated in production.
Owner:GUANGZHOU YINTIAN NEW MATERIAL CO LTD
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS7705059B2Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
Hydroxypivalyl hydroxypivalate ester plasticizer composition and method of preparing the same
ActiveCN101103063AHigh tensile strengthHigh elongationPreparation by aldehyde oxidation-reductionPlasticizerPolyvinyl chloride
Provided are a plasticizer composition including a hydroxypivalyl hydroxypivalate ester and a neopentylglycol ester, and a method of preparing the plasticizer composition. The plasticizer composition provides a polyvinyl chloride resin having excellent properties of heat loss, migration resistance and plasticization efficiency, and tensile strength, elongation, etc.
Owner:LG CHEM LTD
Gold-based catalyst for oxidative esterification of aldehydes to carboxylic acid esters
ActiveUS20180326400A1Improve bindingReduce mechanical wearOrganic compound preparationHeterogenous catalyst chemical elementsAcroleinCarboxylic acid
The present invention relates to novel catalysts for oxidative esterification, by means of which, for example, (meth)acrolein can be converted to methyl (meth)acrylate. The catalysts of the invention are especially notable for high mechanical and chemical stability even over very long periods. This especially relates to an improvement in the catalyst service life, activity and selectivity over prior art catalysts which lose activity and / or selectivity relatively quickly in continuous operation in media having even a small water content.
Owner:ROHM GMBH
Gold-based catalyst for the oxidative esterification of aldehydes to obtain carboxylic esters
ActiveUS20180001307A1Reduce wearExtended service lifeOrganic compound preparationHeterogenous catalyst chemical elementsAcroleinChemical stability
Catalysts for oxidative esterification can be used, for example, fro converting (meth)acrolein to methyl (meth)acrylate. The catalysts are especially notable for high mechanical and chemical stability even over very long time periods, including activity and / or selectivity relatively in continuous operation in media having even a small water content.
Owner:ROHM GMBH
Solid catalyst for preparing methylmethacrylate
InactiveCN101332427APreparation by aldehyde oxidation-reductionMetal/metal-oxides/metal-hydroxide catalystsMethacroleinPalladium
The invention provides a solid catalyst used for preparing methyl methacrylate, and relates to a solid catalyst, in particular to a solid catalyst which takes oxides of palladium, plumbum and metal as the main components and is mainly used for preparing methyl methacrylate by further oxidizing and esterifying methylacrolein. The invention provides a solid catalyst used for preparing methyl methacrylate. The components and compounding ratio are Alpha-Pd-Beta-Pb-Gamma-M / carrier, wherein, Pd is palladium, Pb is plumbum, and M is Fe or Bi; Alpha, Beta and Gamma respectively represents the mass percentage of each component in the catalyst, Alpha equals 4% - 7%, Beta equals 1% - 3.5%, Gamma equals 0% - 3%, and the rest is the carrier, which is at least one of MgO, ZnO orCo2O3.
Owner:XIAMEN UNIV
Process for converting bio-oil
ActiveUS20140256965A1Reduce acidityImprove stabilityOxygen-containing compound preparationOrganic compound preparationHeating oilAlcohol
Disclosed is a process for converting bio-oil, wherein the process includes the steps, where a feedstock including bio-oil selected from bio-oils, any fractions of bio-oils and any combinations thereof is subjected to azeotropic distillation with at least one alcohol to yield a liquid component, and subjecting the liquid component to alcoholysis whereby converted bio-oil is obtained. The invention also relates to the use of converted bio-oil, obtainable by the process, as heating oil, as starting material in processes for producing fuels, fuel components, fine chemicals, chemical building-blocks, and solvents.
Owner:UPM-KYMMENE OYJ
Method for making methyl methacrylate from propionaldehyde and formaldehyde via oxidative esterification
InactiveUS20140206897A1Organic compound preparationPreparation by aldehyde oxidation-reductionHydrogenOxygen
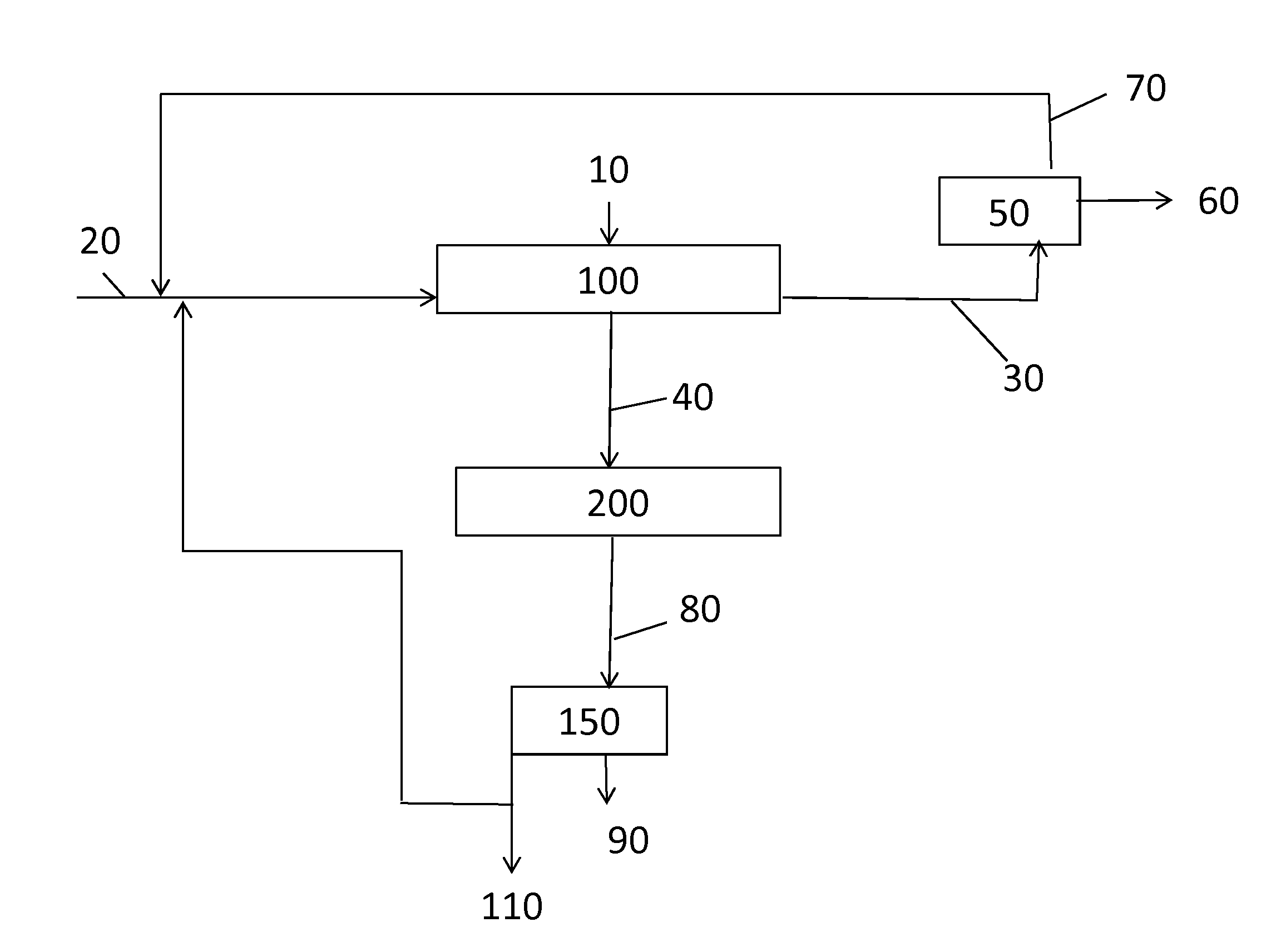
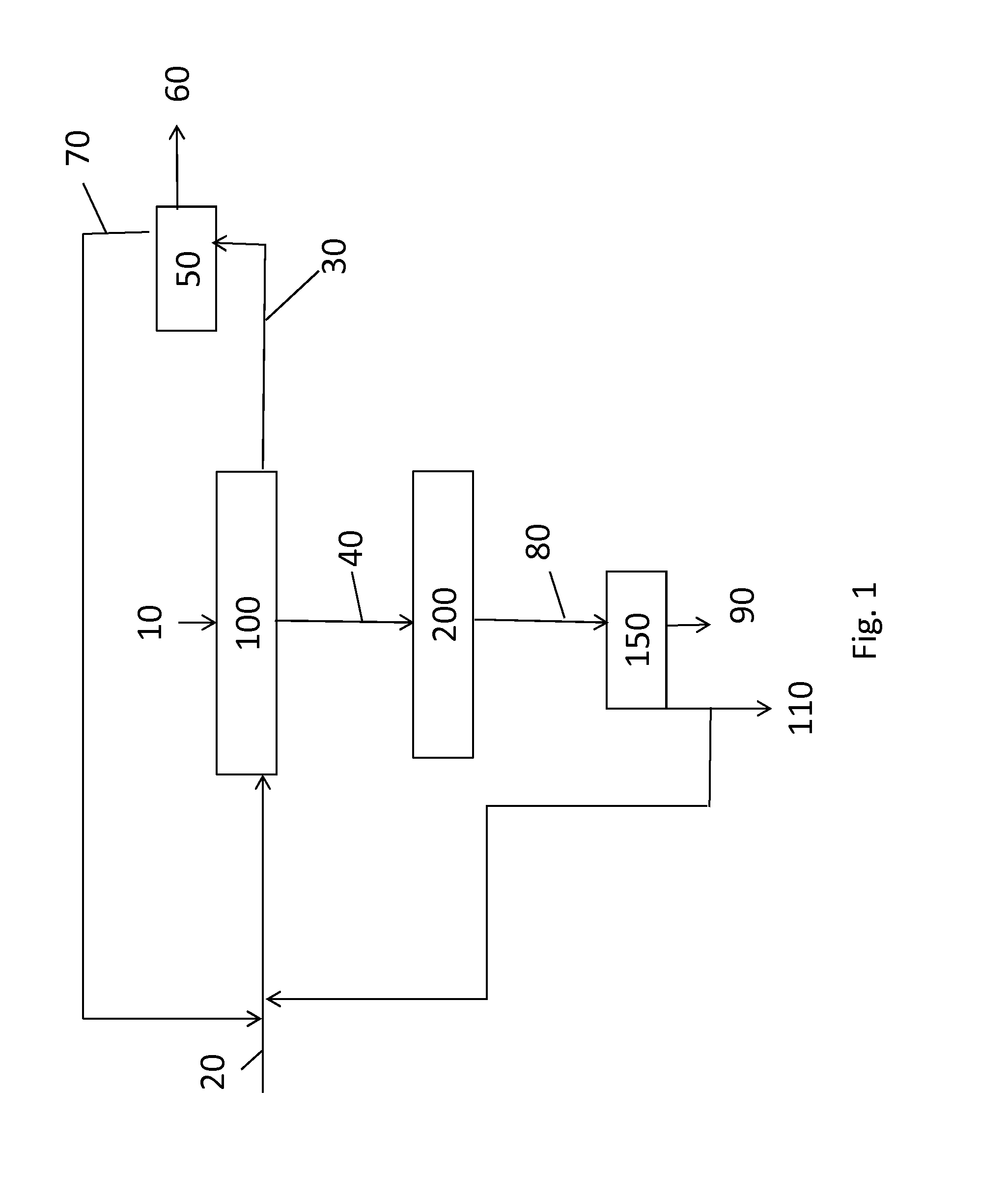
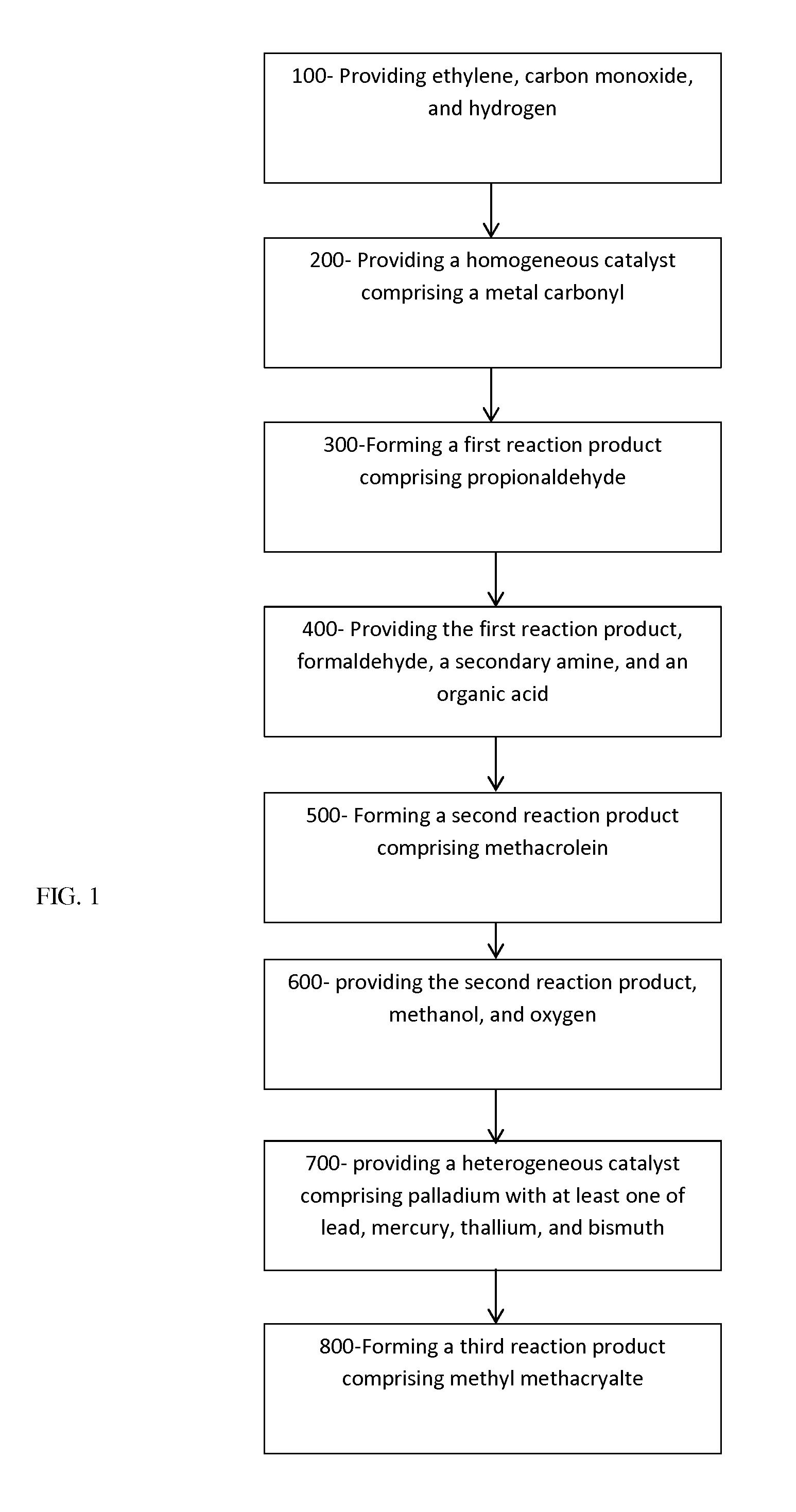
A process for forming methyl methacrylate can comprise: reacting ethylene, carbon monoxide, and hydrogen, in the presence of a first catalyst comprising a metal carbonyl; removing a first reaction product comprising propionaldehyde; reacting the first reaction product with formaldehyde; removing a second reaction product comprising methacrolein; reacting the second reaction product with oxygen and methanol in the presence of a second catalyst to form a third reaction product comprising methyl methacrylate. Another process for forming methyl methacrylate can comprising: reacting ethylene with carbon monoxide to form propionaldehyde; reacting the propionaldehyde with formaldehyde to form methacrolein; and reacting the methacrolein with methanol and oxygen to form the methyl methacrylate.
Owner:SAUDI BASIC IND CORP SA
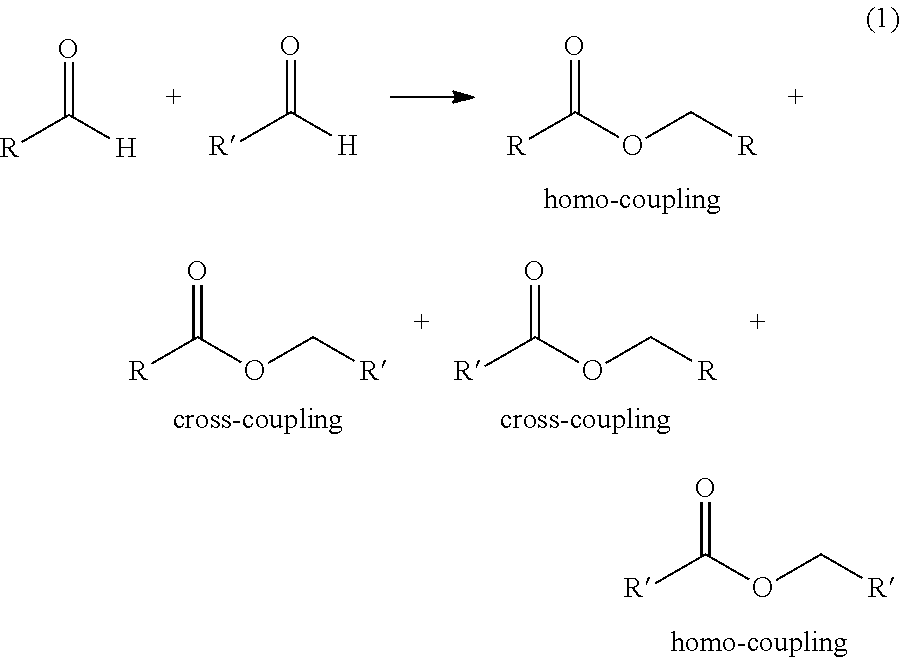
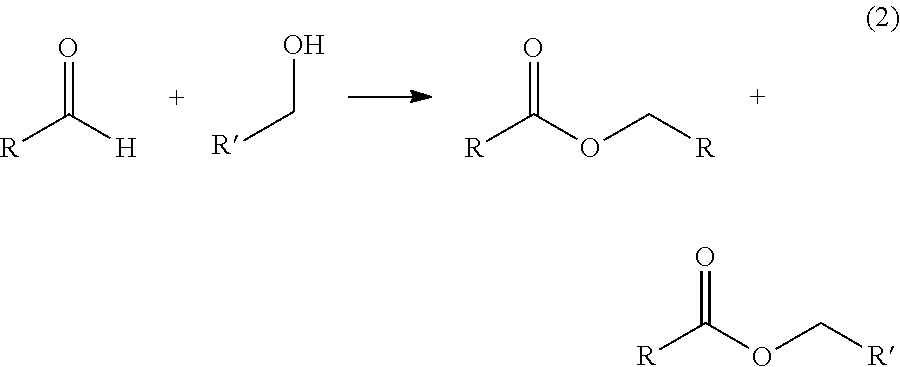
Process for one-step synthesizing ester from aldehyde
InactiveCN1566066AEasy to separateHigh purityPreparation by aldehyde oxidation-reductionAlcoholChloride
The invention provides a single step process for synthesizing esters, wherein esters are synthesized from aldehydes in one step at the presence of alkoxy aluminium catalyst and waterless zinc chloride catalyst promoter. Compared with the conventional alcohol acid esterification method, the invention realizes high yield, low moisture content in synthesized product, and readily releasable high purity esters.
Owner:PETROCHINA CO LTD
Metallized mesoporous silicate and method of oxidation with the same
InactiveUS20050090688A1Preparation by oxidation reactionsMolecular-sieve silicatesOrganic synthesisVanadium atom
A metallized mesoporous silicate which is obtained by (i) reacting (a) either a metal peroxide obtained by the reaction of an aqueous hydrogen peroxide solution with at least one metal or metal compound selected from the group consisting of the following 1) to 4) 1) tungsten 2) molybdenum 3) vanadium 4) compounds comprising 4a) any of tungsten, molybdenum, and vanadium and 4b) at least one element selected from Groups 13 to 16 (excluding oxygen) or a solution of the metal peroxide with (b) a silicon compound in the presence of an alkylamine or a quaternary ammonium salt and separating the resultant silicate; and a process for producing the metallized mesoporous silicate. Also provided is a method of organic synthesis with the silicate.
Owner:SUMITOMO CHEM CO LTD
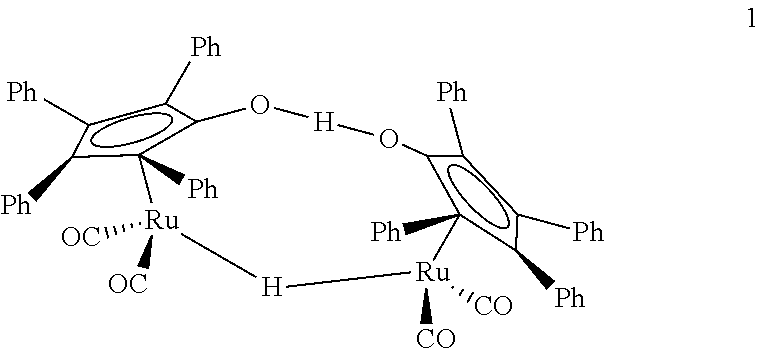
Catalyst for preparing n-Butyl butyrate by n-butanal and preparation method thereof
ActiveCN107649180AImprove stabilityReusableOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by aldehyde oxidation-reductionPolymer scienceN-Butyllithium
The invention relates to a catalyst for preparing n-Butyl butyrate by n-butanal and a preparation method thereof. The catalyst is prepared by the following steps: using a hydroxy-enriched porous organic polymer as a substrate, and forming a complexation structure of Ru on the surface of the substrate; firstly preparing a hydroxy-enriched porous organic polymer material POP; adding tert-butyl lithium or n-butyl lithium, and preparing a polymer POP-Li; and adding ruthenium salt to obtain a polymer POP-Ru. The catalyst has the characteristics of good stability, repeated use, and higher reaction conversion rate and selectivity. The preparation method is capable of creatively fixing Ru ions on the surface of the catalyst, a new idea is provided for the preparation of the catalyst, and the catalyst has the significant application value.
Owner:TIANJIN BOHUA YONGLI CHEM IND
Method for continuously producing neopentyl glycol
ActiveCN108623437AGuaranteed uptimeReduce generationOrganic compound preparationPreparation by aldehyde oxidation-reductionHydrogenation reactionFixed bed
The invention discloses a method for continuously producing neopentyl glycol. The method comprises the following steps: performing a condensation reaction on isobutyraldehyde and formaldehyde under the catalysis of tertiary alkylamine to obtain hydroxypivalaldehyde (HPA); condensing the HPA into a mixed hydroxylpivalic neopentyl glycol monoester (HPNE) liquid through a Tishchenko reaction under the action of an alkali catalyst in a fixed-bed reactor; continuously adding the synthesized mixed HPNE liquid into a loop reactor containing a hydrogenation catalyst and an NPG solution for a hydrogenation reaction, separating a reaction product by using a cross-flow filter, refluxing a circulating liquid containing the catalyst into the loop reactor, and rectifying a clear liquid to obtain the neopentyl glycol (NPG). According to the continuous production method of the neopentyl glycol, the HPA is basically converted into the more stable HPNE, cyclic hydrogenation is performed by using the loop reactor, and byproduct production is reduced, therefore, the product quality is improved and the product yield is increased; by the method, the stable operation of a continuous industrial productiontechnology and a continuous industrial production device of the neopentyl glycol can be guaranteed.
Owner:LIHUAYI GROUP CO LTD +1
Catalyst for the oxidative esterification of aldehydes to carboxylic esters
ActiveUS20190084914A1Promote formationOrganic compound preparationPreparation by aldehyde oxidation-reductionAlcoholAcrolein
The present invention relates to a novel process for oxidative esterification, generally for reaction of aldehydes with alcohols in the presence of oxygenous gases directly to give the corresponding ester in the presence of a heterogeneous catalyst, by means of which, for example, (meth)acrolein can be converted to methyl (meth)acrylate. The new catalyst has titanium dioxide as the main component of the support material. The catalysts are especially notable for high mechanical and chemical stability and for good catalytic performance even over very long periods. The process is an improvement in the catalyst service life, activity and selectivity over prior art catalysts which lose activity and / or selectivity relatively quickly in continuous operation in media having even a small water content.
Owner:ROHM GMBH
Continuous production method for 2,2,4-trimethyl-1,3-pentanediol isobutyrate
ActiveCN104072367AHigh selectivityIncrease productivityOrganic compound preparationHeterogenous catalyst chemical elementsIsobutyratesPtru catalyst
The invention discloses a continuous production method for 2,2,4-trimethyl-1,3-pentanediol isobutyrate. A two-step method fixed bed continuous reaction process and a solid catalyst are adopted, wherein the catalyst is a solid catalyst Cs-K / SiO2 which is prepared by taking spherical silicon oxide as a carrier to carry caesium-potassium double-alkali metal active components. The method comprises the following steps: adopting two serial tubular type fixed bed reactors, firstly feeding a preheated isobutyraldehyde raw material into a first tubular type fixed bed reactor for an aldol condensation reaction, subsequently feeding an obtained an aldol condensation product into a second tubular type fixed bed reactor for intramolecular Cannizzaro reaction, and continuously reacting so as to obtain a target product. Due to adoption of a two-step method fixed bed continuous reaction process, the method is not only high in isobutyraldehyde conversion rate and product selectivity, but also high in production efficiency, simple in reaction operation, beneficial for energy conservation and discharge reduction and has a large-scale continuous industrial production prospect.
Owner:德纳化工滨海有限公司 +1
Method for generating low-carbon ester by condensation of low-carbon aldehyde
InactiveCN110283076ALow priceEasy to separatePreparation by aldehyde oxidation-reductionChemical recyclingReaction temperatureWastewater
The invention relates to a new method for preparing low-carbon ester by condensation of low-carbon aldehyde through catalysis of acetylacetone metal salts. The method is characterized in that the acetylacetone metal salts are taken as a catalyst, the low-carbon ester is prepared by performing a reaction for 2-4 h under the conditions that the mass ratio of aldehyde to the catalyst is (10:1) to (200:1), the mass ratio of the aldehyde to a solvent is (1:1) to (10:1), the reaction temperature is 80 to 250 DEG C, and the reaction pressure of 0.1 to 1.0 MPa, and the catalyst is recovered and recycled. Compared with the prior art, the defects that a traditional catalyst is not easy to separate, is liable to inactivate, cannot be recycled, and the like are solved; the dosage of the catalyst is less, the yield is high, no waste water is generated basically during production, and a green synthesis process can be realized.
Owner:RUNTAI CHEM TAIXING CO LTD +1
Production of two esters using homogeneous catalyst
ActiveUS9493395B2Speed up the conversion processOrganic compound preparationPreparation by aldehyde oxidation-reductionPtru catalystEthyl group
Owner:EASTMAN CHEM CO
Magnetic nanogold catalyst for synthesis of ester through one-step oxidative esterification of aldehydes as well as preparation method and application of catalyst
ActiveCN107824199AEasy to recycleImprove performanceOrganic compound preparationHeterogenous catalyst chemical elementsActive componentSolid solution
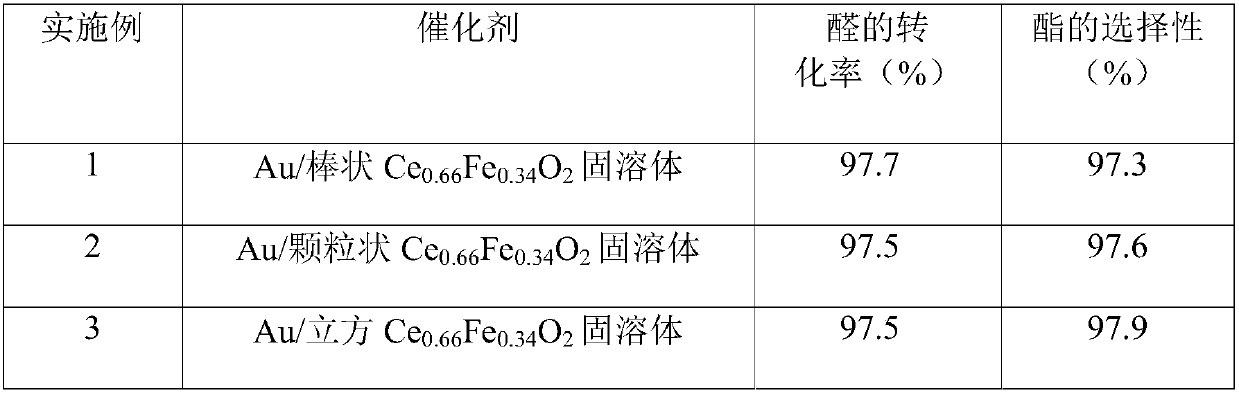
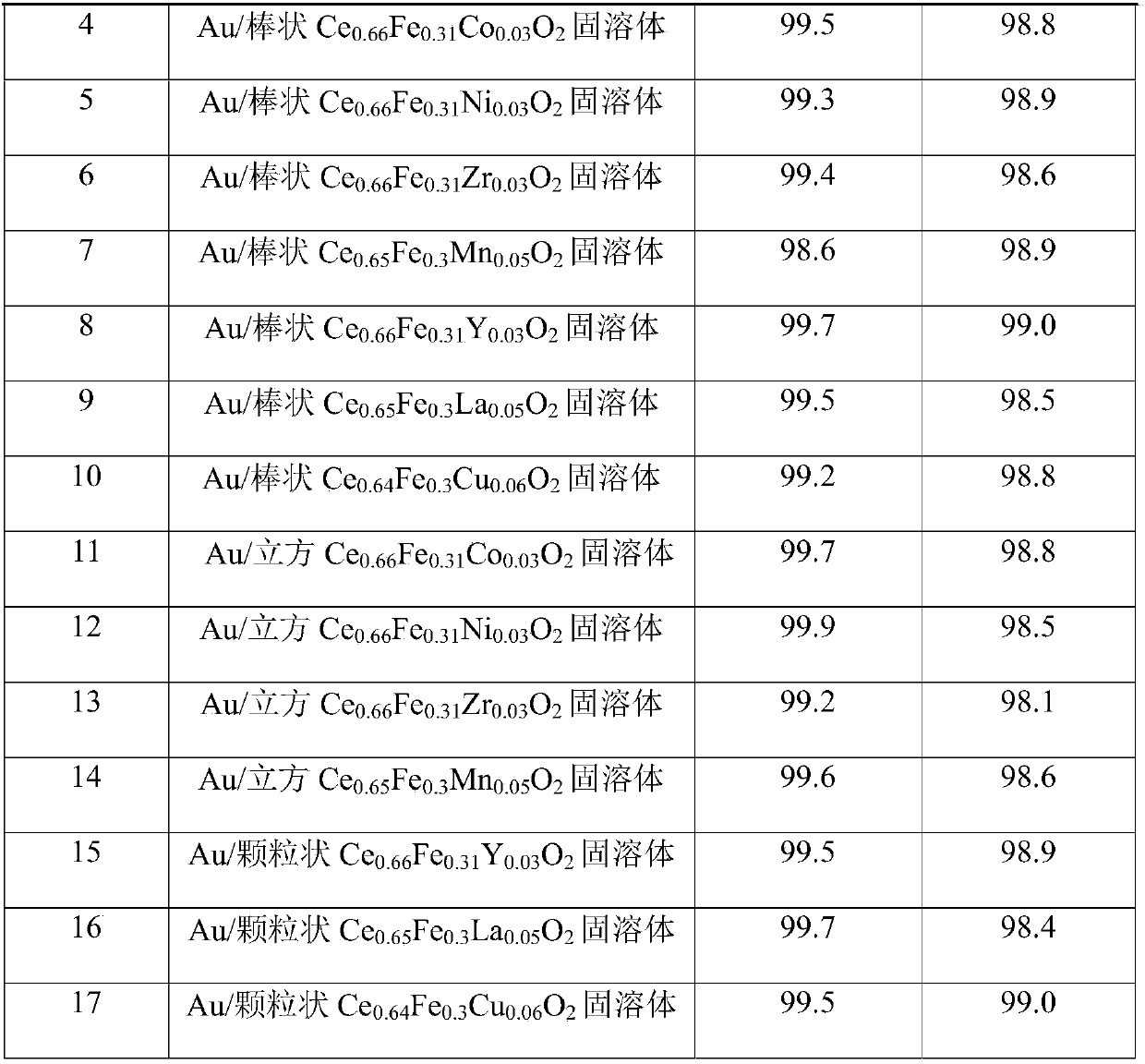
The invention relates to the technical field of chemical catalysis, in particular to a magnetic nanogold catalyst for synthesis of ester through one-step oxidative esterification of aldehydes as wellas a preparation method and an application of the catalyst. The magnetic nanogold catalyst takes Au as an active component and a CeaFebXcO2 solid solution as a carrier, wherein the supporting amount of Au accounts for 0.1wt.%-2wt.% of the total mass of Au and the CeaFebXcO2 solid solution; in the CeaFebXcO2 solid solution, a is larger than 0 and smaller than 1, b is larger than 0 and smaller than1, c is larger than or equal to 0 and smaller than 1, the sum of a, b and c is equal to 1, and X is Co, Ni, Zr, Mn, Y, La, Cu or Zn. The aldehyde conversion rate is 97.5%-99.9%, the ester selectivityis 97.3%-99.0%, the catalyst has the characteristics of being simple to operate, higher in conversion rate and better in product selectivity, the catalyst is convenient to recover, and the reaction stability and the process economy are greatly improved.
Owner:山东达民化工股份有限公司
Popular searches
Ethylene production Bio-feedstock Preparation by oxygen reduction Hydrocarbon oils treatment products Oxygen compounds preparation by reduction Liquid carbonaceous fuels Carbonyl compound preparation by oxidation Carboxylic compound preparation Electrolytic organic reduction Ether preparation by compound dehydration
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com