Continuous production method for 2,2,4-trimethyl-1,3-pentanediol isobutyrate
A technology of pentanediol monoisobutyrate and production method, which is applied in the field of continuous preparation of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, and can solve the problem of reaction temperature Low, low selectivity, complicated process, etc., to achieve the effect of simple reaction operation, high product selectivity, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Solid base catalyst Cs-K / SiO 2 preparation of
[0030] 1. Preparation of solid base catalyst carrier
[0031] Step (1) Adhesive molding: Grind and sieve commercially available coarse-porous type II microporous silica gel (produced in Qingdao) that has not been roasted to obtain 200-300 mesh silica gel powder as the raw material for rolling balls. The above-mentioned silica gel powder is placed on the stainless steel turntable of the turntable granulator, and the turntable rotates at a speed of 36 rpm. Spray JA-25 silica sol with a concentration of 25% (calculated as SiO2) (the average particle size of the colloidal particles is 10-20nm) on the powder through a spray gun to make rolling ball seeds. Use 0.5-0.7 liters of silica sol per kilogram of silica gel powder. Then slowly add raw material powder and JA-25 silica sol. When the average diameter of the rolled balls (take the average value of 20 grains) reaches 2-3mm, stop adding silica sol, but continue to...
Embodiment 2
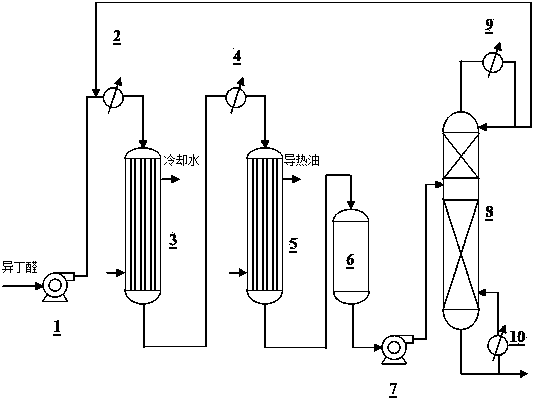
[0050] Adopt described two-step method fixed-bed continuous reaction technique to prepare 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, technological process is as follows figure 1 shown, but the removed unreacted isobutyraldehyde is not recycled. Using two series tubular fixed bed reactors, the solid base catalyst Cs-K / SiO in each reactor 2 The total filling volume is 20 liters.
[0051] The first step reaction process is: the raw material of isobutyraldehyde passes through the delivery pump 1 sent to heat exchanger 2 Preheat, then at a space velocity of 0.3 h -1 From the first tubular fixed bed reactor 3 The top enters the catalyst bed for aldol condensation reaction. The reactor inlet temperature was 35°C. Due to the relatively large heat release of the reaction, cooling water was introduced into the shell side to obtain heat, and the reactor outlet temperature was 50°C.
[0052] The second step reaction process is: from the first tubular fixed bed reactor ...
Embodiment 3
[0058] Except that unreacted isobutyraldehyde reclaims and recycles, other conditions are identical with embodiment 2, and the technological process of present embodiment is as follows figure 1 shown.
[0059] The first step reaction process: dealdehyde tower 8 The removed unreacted isobutyraldehyde is returned to the heat exchanger 2 before, and with the transfer pump 1 The conveyed fresh isobutyraldehyde raw material is mixed and enters the heat exchanger 2 warm up. The preheated material is at a space velocity of 0.3h -1 From the first tubular fixed bed reactor 3 The top enters the catalyst bed for aldol condensation reaction. The reactor inlet temperature was 35°C. Due to the intense heat release of the reaction, cooling water was introduced into the shell side of the reactor, and the outlet temperature of the reactor was controlled at 50°C.
[0060] The second step reaction process: from the first tubular fixed bed reactor 3 The aldol condensate disch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com