Method for preparation of methyl formate
A technology for methyl formate and methanol, applied in the field of preparing methyl formate, can solve the problems of difficulty in obtaining high yield, decreased selectivity of methyl formate, etc., and achieves cheap raw materials, high yield of methyl formate, and adjustable wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1) Prepare formaldehyde gas according to method one:
[0062] Paraformaldehyde (Sinopharm Chemical Reagent Beijing Co., Ltd.) was decomposed at 90° C., and the formaldehyde gas produced by the decomposition was mixed with N2 to obtain a mixed gas composed of formaldehyde and nitrogen with a molar concentration of formaldehyde of 4%.
[0063] 2) Place catalyst B industrial ZrO in reactor B 2 , feed a mixture of formaldehyde and nitrogen with a formaldehyde content of 4% to carry out the Tishchenko reaction, the temperature is 120 ° C, catalyst B is diluted with quartz sand to eliminate the thermal effect of the exothermic reaction, and the reaction gas flow rate is 30ml / min. In order to avoid product condensation, all pipelines connecting the reactor and the chromatograph are kept above 120°C.
[0064] The conversion rate of formaldehyde was 98%, the selectivity of methyl formate was 96%, the yield of methyl formate was 95%, and the yield of methyl formate remained stab...
Embodiment 2
[0066] 1) Prepare formaldehyde gas according to method two:
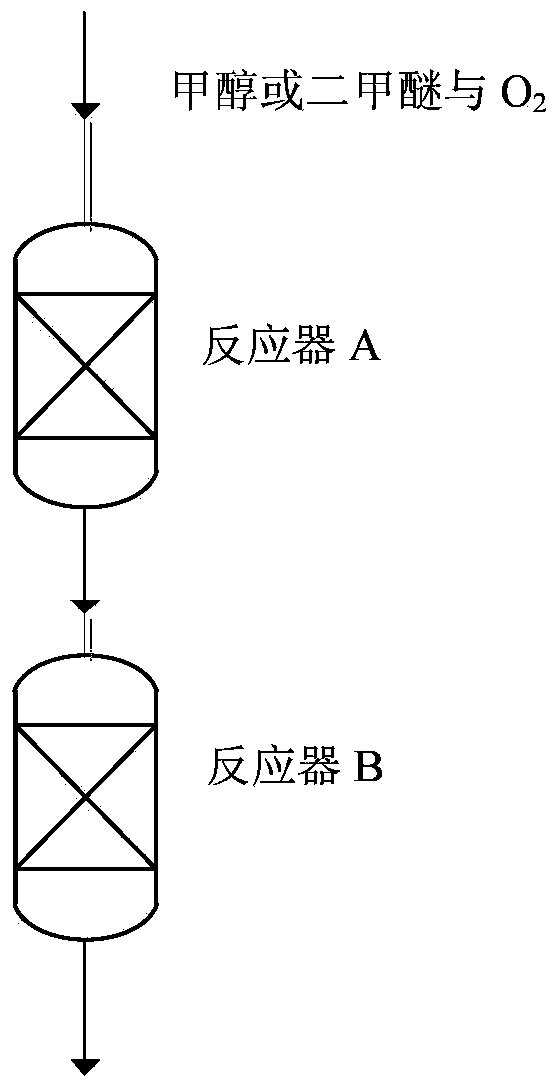
[0067] Use attached figure 1 of the reactor.
[0068] The reaction raw material gas is a mixed gas composed of methanol and air with a methanol molar concentration of 4%, and the reaction raw material gas is figure 1 Selective oxidation reaction is carried out in the reactor A (reaction tube inner diameter is 14mm) in the reactor A, and catalyst A is industrial iron-molybdenum composite metal oxide catalyst (with Mo and Fe mol ratio being 2.8 with ammonium molybdate and iron nitrate) : 1 After co-precipitation, the precipitate is collected and roasted in the air at 500°C), and the catalyst A is diluted with quartz sand to eliminate the thermal effect of the exothermic reaction, the reaction temperature T1 is 320°C, and the resulting formaldehyde gas is passed into the reactor B for follow-up response;
[0069] 2) Place catalyst B industrial ZrO in reactor B 2 , the formaldehyde produced by passing into reactor A...
Embodiment 3
[0072] 1) prepare formaldehyde gas according to the method of embodiment 1 step 1);
[0073] 2) Place catalyst B industrial ZrO2 in reactor B, and dilute catalyst B with quartz sand to eliminate the thermal effect of exothermic reaction, pass into formaldehyde to carry out Tishchenko reaction, in order to test the stability of catalyst under more severe reaction conditions, reduce the amount of catalyst , and the reaction temperature was increased to 150° C., and the reaction gas flow rate was 50 ml / min. In order to avoid product condensation, all pipelines connecting the reactor and the chromatograph are kept above 120°C.
[0074] The conversion rate of methanol was 93%, the selectivity of methyl formate was 96%, and the yield of methyl formate was 88%. Under the reaction conditions, the reaction activity did not decrease significantly after 80 hours, indicating that the catalyst had good stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com