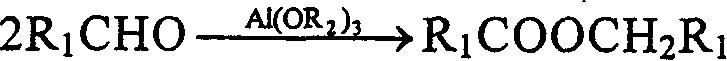Process for one-step synthesizing ester from aldehyde
A single technology for the synthesis of esters, applied in the field of ester synthesis, can solve the problems of long process routes and serious pollution, and achieve the effects of high reaction yield, easy separation, and low water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: add 35g isoamyl alcohol (analytically pure) and 1.8g aluminum powder (100-200 order, chemically pure) in 100 milliliters of there-necked flasks with electric stirrer, condenser, thermometer, add under constant stirring 1.5g anhydrous aluminum trichloride and 0.018g iodine (analytically pure), be warming up to reflux, at this moment visible reaction system color becomes gray, dark gray, black gradually, and gas is released, after stirring reaction 5h, cooling, reducing After pressure distillation removed excess isoamyl alcohol, obtain 21g aluminum isoamyl alcohol products. Then add 40g isovaleraldehyde (chemically pure) in 100 milliliters there-necked flasks with electric stirrer, condenser, thermometer, add 3.2g aluminum isoamyloxide, 1.6 aluminum isoamyloxide prepared in front in many batches in a small amount under constant stirring g anhydrous zinc chloride (analytical pure), control the temperature to 15-25 ° C, with the addition of aluminum isoamyloxi...
Embodiment 2
[0015] Embodiment 2: in 100 milliliters of there-necked flasks with electric stirrer, condenser, thermometer, add 36g propanol (analytical pure) and 2.7g aluminum powder (100-200 order, chemically pure), add 1.8 under constant stirring g of anhydrous aluminum trichloride and 0.036g of iodine (analytically pure), heated to reflux, stirred and reacted for 2 hours, cooled and distilled off under reduced pressure to remove excess propanol to obtain 23g of aluminum propoxide product. Then add 40g propionaldehyde (industrial product) in 100 milliliters of there-necked bottles with electric stirrer, condenser, thermometer, add aluminum propoxide, 2g anhydrous chlorine in many batches altogether in a small amount made before 4g under constant stirring Zinc chloride (analytical pure), the temperature is controlled to 15-25 ° C, with the addition of aluminum propoxide and zinc chloride, the system is accompanied by a phenomenon of temperature rise, after 5 hours of reaction, cooling, vac...
Embodiment 3
[0016] Embodiment 3: add 35g isoamyl alcohol (analytically pure) and 1.8g aluminum powder (100-200 order, chemically pure) in 100 milliliters of there-necked flasks with electric stirrer, condenser, thermometer, add under constant stirring 1.5g of anhydrous aluminum trichloride and 0.018g of iodine (analytically pure), heated to reflux, stirred and reacted for 5h, cooled and distilled under reduced pressure to remove excess isoamyl alcohol to obtain 21g of aluminum isoamyl alcohol product. Then add 20g isovaleraldehyde (chemically pure) and 20g propionaldehyde (chemically pure) in the 100 milliliter there-necked flask with electric stirrer, condenser, thermometer, add 3.2g front preparation altogether in many batches under constant stirring The obtained aluminum isoamyloxide and 1.6g anhydrous zinc chloride (analytical pure) are controlled at a temperature of 15-25°C. With the addition of aluminum isoamyloxide and zinc chloride, the system is accompanied by a temperature rise p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com