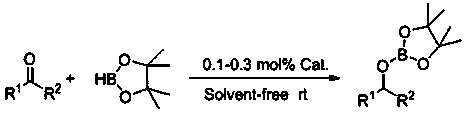Patents
Literature
530 results about "N-Butyllithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
N-Butyllithium (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.
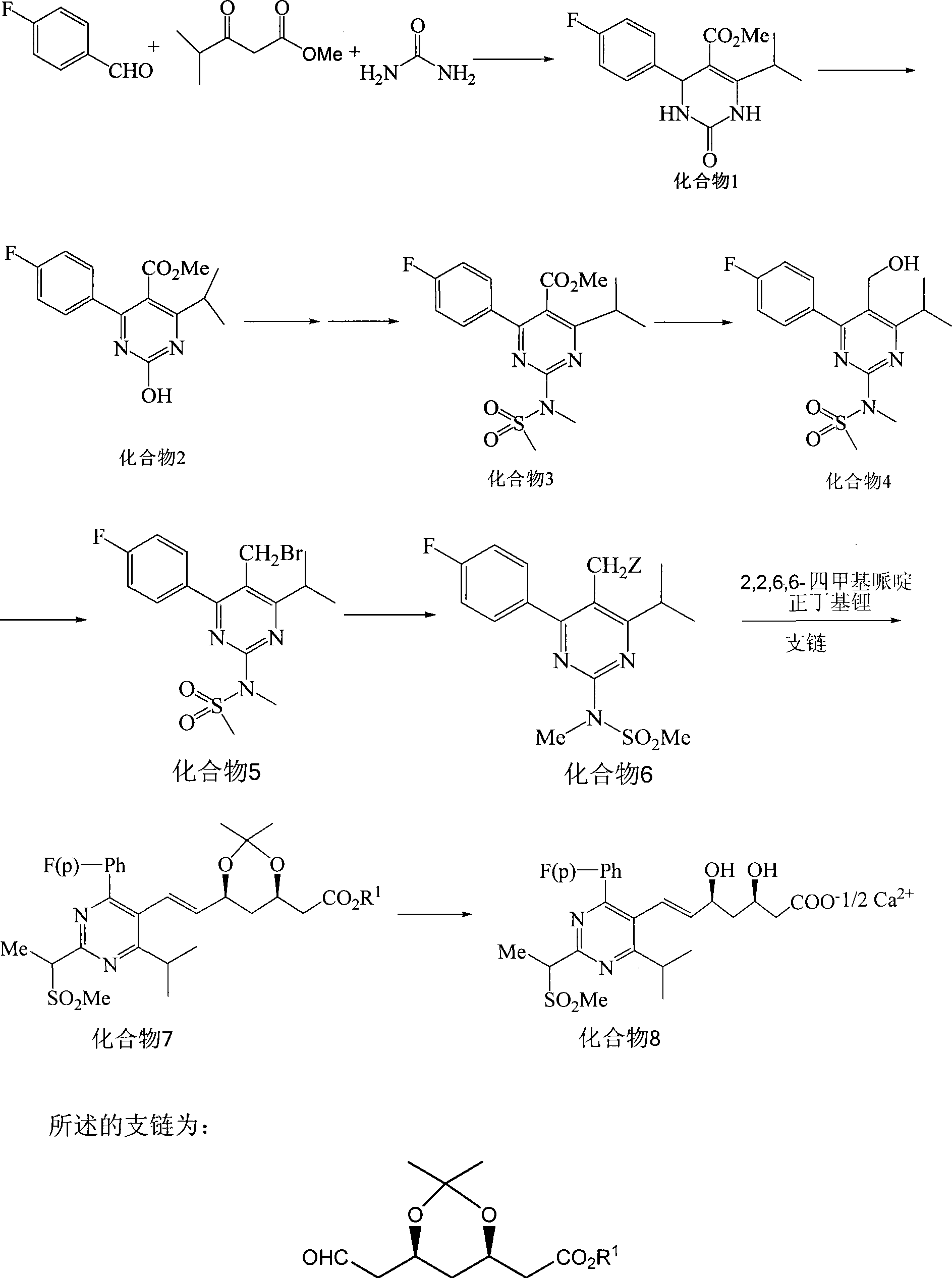
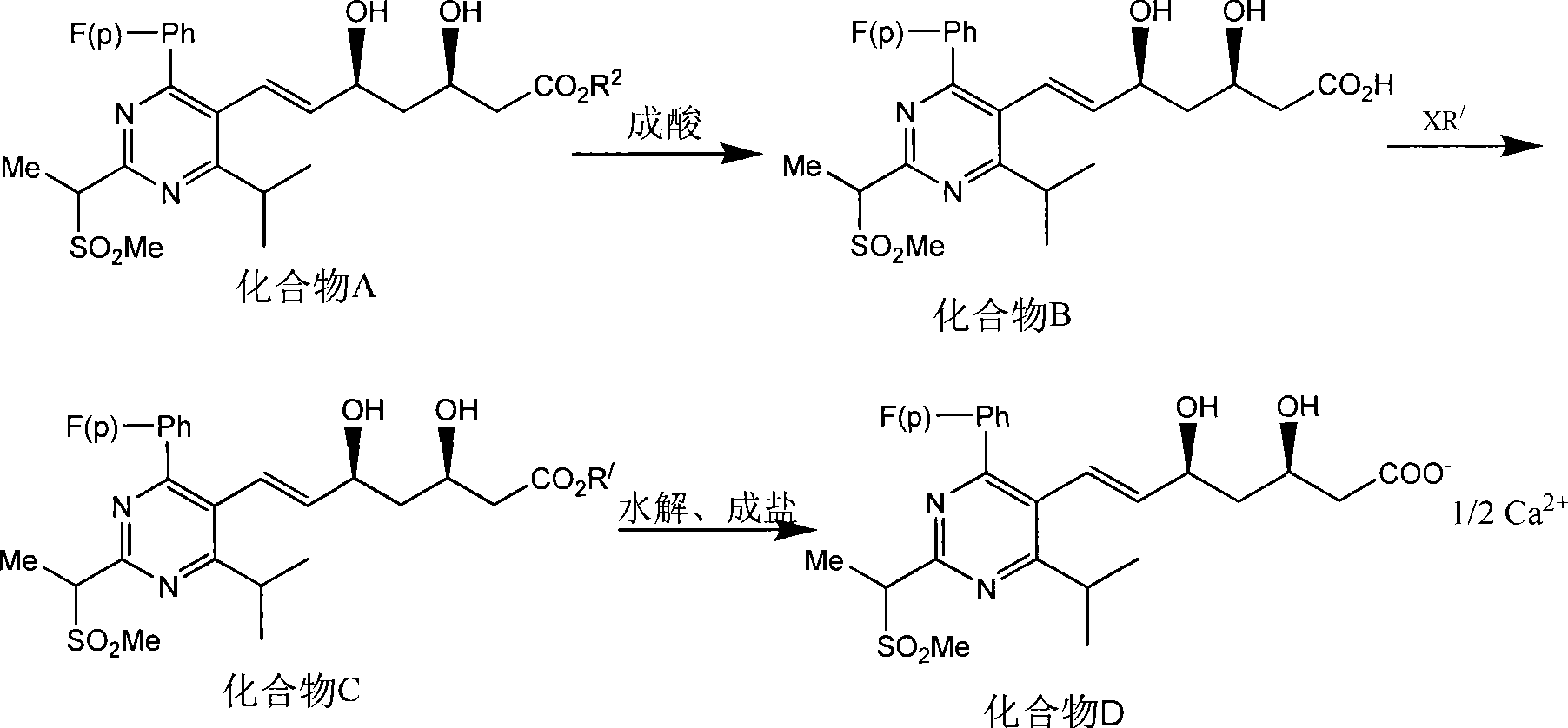
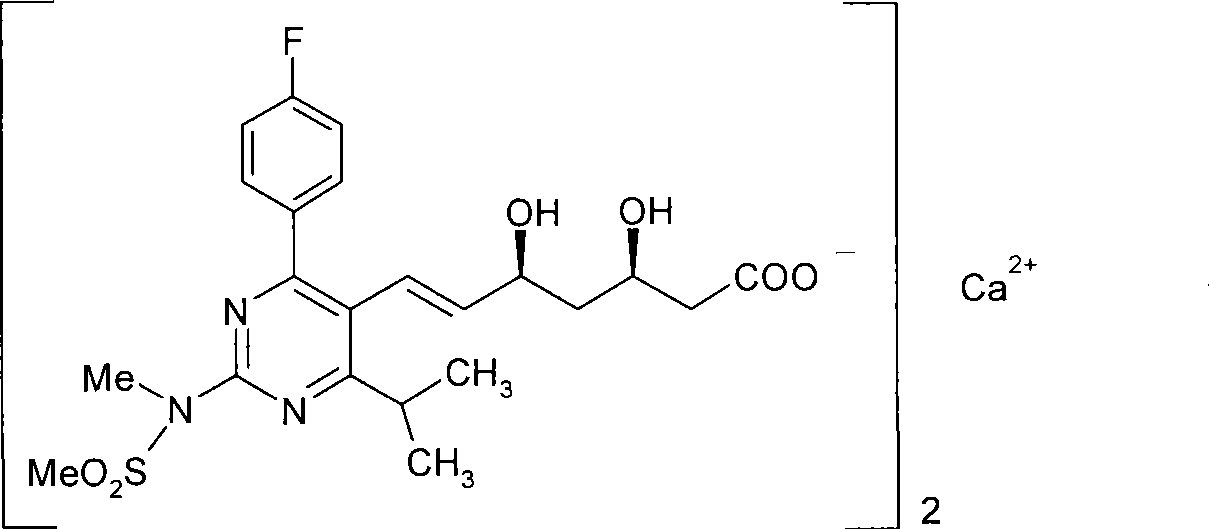
Method for synthesizing rosuvastatin intermediate and rosuvastatin
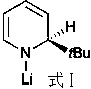
The invention provides a synthetic method of rosuvastatin calcium, which is characterized in that a compound 6 and branched chain react under the action of 2,2,6,6,-tetramethylpiperidine and n-butyl lithium to generate a compound 7, the reaction temperature is increased to minus 30 DEG C and the aspects such as reaction solvent and reaction reagent are improved so that the total reaction route has the advantages of mild condition, low device requirement and suitability for industrial production. Meanwhile, another improved synthetic route of rosuvastatin calcium comprises the following steps: converting esters into acids, synthesizing esters again, and synthesizing the rosuvastatin calcium. The rosuvastatin calcium purity is as high as 99.8%.
Owner:ENANTIOTECH CORP
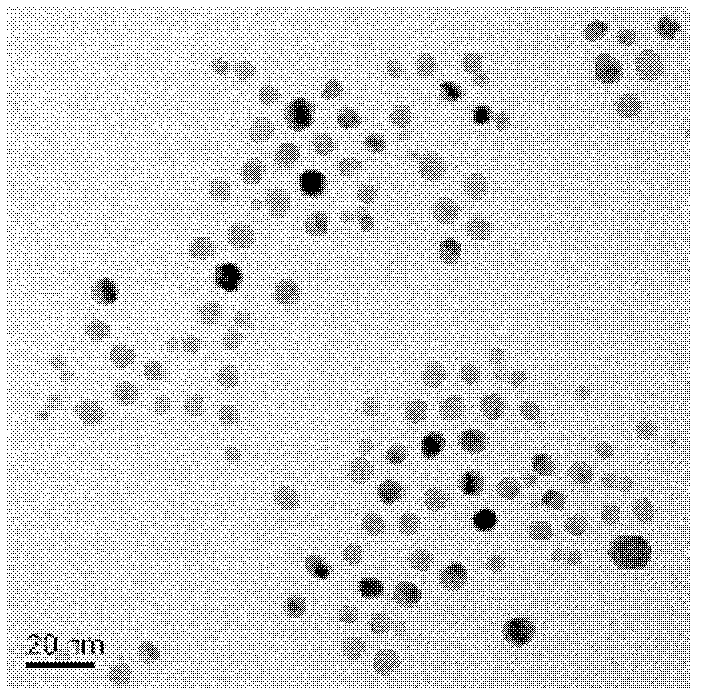
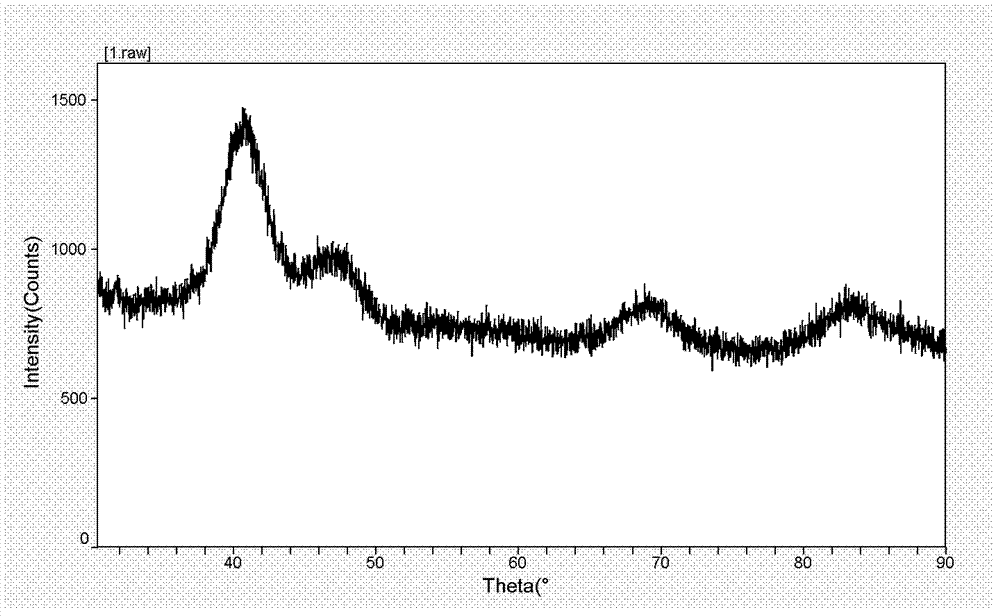
Preparation method for controllable duplex metal alloy nano particle
ActiveCN103192086AUniform and controllable sizeHas a face centered cubic structureNanotechnologyN-ButyllithiumHydrogenation reaction
The invention relates to a preparation method for a controllable duplex metal alloy nano particle. The metal alloy nano particles is a Pt / Pd-M alloy nano particle, wherein the M is selected from Ni, Fe, Co, Mn, Pd, Zn, Cu and Mo; during preparation, n-butyllithium is used as a strong reducing agent and oleylamine and tri-n-octylphosphine are used as protective agents to reduce acetylacetone, chlorate or acetate of the Pt / Pd-M (M= Ni, Fe, Co, Mn, Pd, Zn, Cu,Mo) at the same time so as to form the uniform and size-controllable Pt / Pd-M alloy nano particle without a concentration gradient. The preparation method is simple and controllable in preparation technology, and can solve the problem that when precursor reduction potentials of two metals are greatly different, one metal can be reduced in prior, so that the alloy nano particle can not be obtained easily, provides a better and universal synthetic method for the preparation of the duplex metal alloy nano particle, and provides a key precursor for the preparation of a new generation of metal-oxide nano hybridization body catalyst used for selective hydrogenation reaction.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
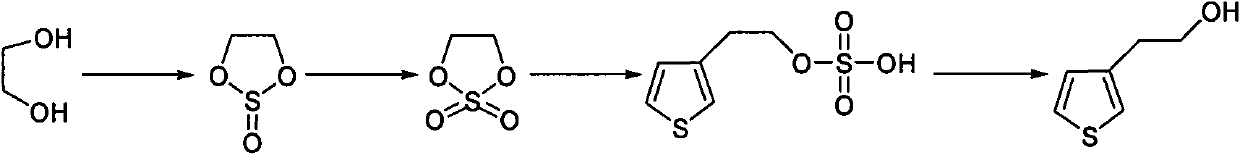
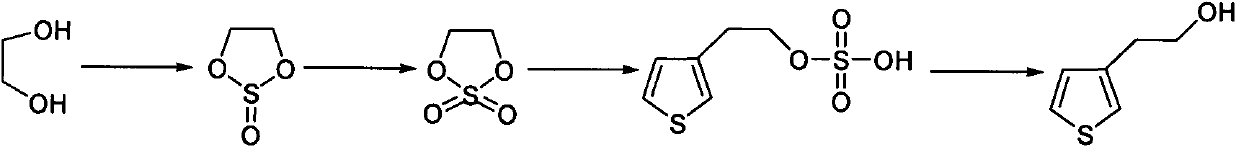
Synthetic method of thiophene-3-ethanol
The invention discloses a synthetic method of thiophene-3-ethanol. The method comprises the following steps of: adding a halogenated hydrocarbon solvent and ethylene glycol into a reaction kettle, dropwise adding thionyl chloride and preserving heat for reacting; separating liquid and extracting to obtain an organic phase containing a substance shown in the specifications; adding a ruthenium trichloride aqueous solution and a sodium bicarbonate aqueous solution in the presence of the halogenated hydrocarbon solvent and dropwise adding a sodium hypochlorite aqueous solution; after detecting that a system does not have oxidizing property, performing liquid separation, concentration, devitrification and drying to obtain a substance shown in the specifications, adding an ester solvent and butyl lithium into a reaction kettle, adding a prepared ester solution of tribromothiofuran and a prepared ester solution of the substance, separating the liquid and extracting to obtain a system containing a substance shown in the specifications; and adding a dilute sulfuric acid into the system containing the substance shown in the specifications, concentrating, neutralizing, extracting and concentrating to obtain an end product. The method has the advantages of high reaction purity and yield, stable process condition, easiness for operation and mass production capability; and the thiophene-3-ethanol is prepared from tribromothiofuran by performing low-temperature lithiation, so that the use of epoxy ethane serving as an explosive hazard is avoided, and mass production becomes possible.
Owner:ASYMCHEM LAB TIANJIN +4
Preparation method of layered molybdenum sulfide nanosheet molecular separating membrane
InactiveCN103585896ALow costIncrease productionSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisMaterials preparationFiltration
The invention discloses a preparation method of a layered molybdenum sulfide nanosheet molecule separating membrane. According to the preparation method, n-butyllithium is taken as an intercalation modular and enters a molybdenum sulfide crystal lattice after long-time stirring; water is added for decomposing n-butyllithium, so that the interlayer distances of a molybdenum sulfide sheet are enlarged; molybdenum sulfide is dispersed into monoatomic layer materials through ultrasonic treatment; redundant impurities are removed through dialysis, and a purified and clean molybdenum sulfide water solution is obtained; the obtained molybdenum sulfide water solution is treated with a vacuum filtration method, and the layered molybdenum sulfide nanosheet molecule separating membrane is obtained; and the thickness of the membrane is adjusted by adjusting the volume of the used molybdenum sulfide water solution. A molecule-selective separation membrane is obtained with a method of stacking the monoatomic layer materials to form the membrane; and the material preparation method is simple, the cost is low, and the obtained membrane has size selective separation performance on different molecules.
Owner:ZHEJIANG UNIV
Preparation method for molybdenum disulfide nanosheet with reactive group-containing surface
ActiveCN103833081AGood dispersionReduce pollutionMaterial nanotechnologyMolybdenum sulfidesN-ButyllithiumHexane
The invention discloses a preparation method for a molybdenum disulfide nanosheet with a reactive group-containing surface. The preparation method comprises the following steps: adding one part by weight of molybdenum disulfide, 2-5 parts by weight of n-butyllithium and 10-20 parts by weight of n-hexane into a hydrothermal reaction kettle, heating at 50-150DEG C for 0.5-12 hours, cooling to room temperature, performing centrifugal separation and n-hexane washing for five times, drying at 50DEG C for 12 hours so as to obtain molybdenum disulfide powder with lithium ion intercalation, adding 1 part by weight of molybdenum disulfide powder with lithium ion intercalation, 0.05-10 parts by weight of sulihydryl reagents with reactive groups, and 100-1000 parts by weight of water into a flask for performing ultrasonic treatment for one minute to five hours under 50-100 watt, to obtain molybdenum disulfide nanosheet with the reactive group-containing surface. The method is easily available, mild to react, simple to operate, and small in environmental pollution; the prepared nanosheet can be further functionalized, thus having good application prospects.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method of preparing superfine monolayer transition metal compound quantum dot solution
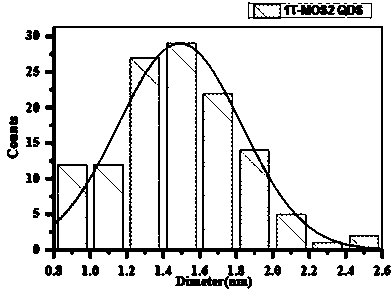
InactiveCN103880084AGood repeatabilityUniform particle size distributionLuminescent compositionsMolybdenum sulfidesProcess equipmentN-Butyllithium
The invention discloses a method of preparing a superfine monolayer transition metal compound quantum dot solution. The method comprises the following steps: (a) mixing a n-butyllithium solution with a transition metal compound solution to react to obtain an intermediate product, wherein the transition metal compound has the formula of MX2, M is a transition metal element and X is S or Se or Te; (b) drying the intermediate product to obtain a dried product; (c) adding deionized water into the dried product for ultrasonic reaction to obtain an ultrasonic product; (d) centrifuging the ultrasonic product obtained by the step c to obtain the supernate which is the superfine monolayer transition metal compound quantum dot solution. The solution prepared by the invention is uniform in distribution of particle size, has a monolayer structure, and is simple in process equipment, short in reaction period, good in repeatability and easy for industrial production.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
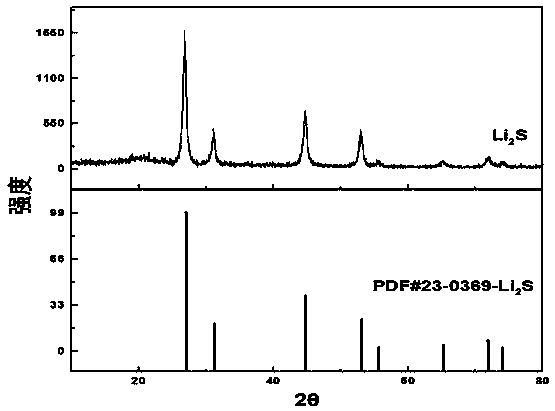
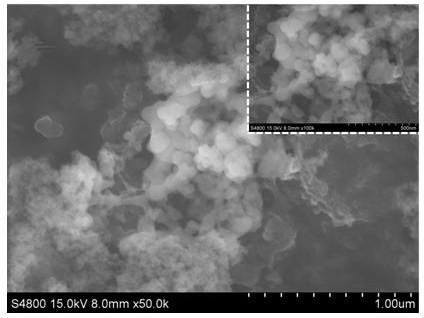
Method for synthesizing lithium sulfide
InactiveCN111517288ASynthetic raw materials are cheap and easy to obtainThe process steps are simpleHybrid capacitor electrodesPhosphorus sulfur/selenium/tellurium compoundsMetallic lithiumN-Butyllithium
The invention mainly relates to the field of lithium battery materials, and provides a method for synthesizing lithium sulfide by taking a lithiation solution and elemental sulfur as raw materials through direct reaction, which comprises the following steps: preparing the lithiation solution, an organic ether solution containing a metal lithium-aromatic compound, a lithium iodide solution and an n-butyl lithium solution; adding elemental sulfur into the lithiation solution for mixing; and sequentially carrying out a room-temperature reaction, separation and precipitation, drying and heat treatment on the mixed solution to obtain the lithium sulfide. The synthesis method of lithium sulfide has the advantages of cheap and easily available raw materials, simple process, no harmful gas generation, and mild and controllable synthesis process.
Owner:TIANMU LAKE INST OF ADVANCED ENERGY STORAGE TECH CO LTD +2
Method for preparing high vinyl solution polymerized butylbenzene by high temperature continuous polymerization technique
The invention discloses a method for preparing high-vinyl solution styrene butadiene through a high-temperature continuous polymerization process. Butyl lithium is taken as an initiator of anionic polymerization, an alkyl tetrahydrofurfuryl ether is taken as a polarity regulator, and the continuous polymerization process is adopted to synthesize high-vinyl solution butadiene styrene at a polymerization temperature of between 100 and 130 DEG C. The vinyl content can reach more than 30 percent, thereby meeting the practical production requirements. The method has the advantages that the alkyl tetrahydrofurfuryl ether is taken as the anion regulator for the homopolymerization of alkadiene or the copolymerization of alkadiene and other olefins; under the condition of the high-temperature continuous polymerization, higher activity is still maintained so as to effectively regulate the vinyl content in the alkadiene.
Owner:DALIAN MARITIME UNIVERSITY
Triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material and its preparation method and use
ActiveCN105924472ABalance injectionBalanced transmissionGroup 5/15 element organic compoundsSolid-state devicesElectron donorTriplet state
The invention relates to a fluorescent blue light guest material and its preparation method and use and especially relates to a triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material and its preparation method and use. The triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material solves the problem that because of large difference of an electron donor and an acceptor, when the electron donor is increased, guest emission wavelength produces red shift so that stable and efficient blue guest luminescence cannot be realized. The blue light guest material has a structural formula shown in the description. The preparation method comprises 1, preparing PXZPhBr, PXZPhBr, PTZPhBr and DMACPhBr, 2, mixing the products obtained through the step 1 and tetrahydrofuran, adding n-butyllithium and diphenylphosphinous chloride into the mixture, adding hydrogen peroxide into the mixture, carrying out stirring and carrying out chromatography and recrystallization. The triphenyl phosphorus oxide-based thermal excitation delayed fluorescent blue light guest material is used for preparation of a thermal excitation delayed fluorescent electroluminescent device. The material utilizes short-axis modification strategy, effectively keeps a matrix triplet state energy level, has a donor-acceptor (D-A)-type molecular structure and balances carrier injection and transmission. The preparation method belongs to the field of fluorescent material preparation.
Owner:HEILONGJIANG UNIV
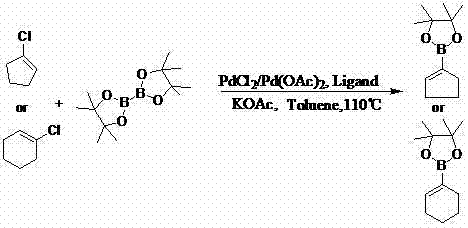
Method for preparing cyclopentene/cyclohexene-1-boronic acid pinacol ester
InactiveCN103044469AReduce dosageLow costGroup 3/13 element organic compoundsCyclopentenePtru catalyst
The invention discloses a method for preparing cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method comprises the step of carrying out coupling reaction on 1-chlorine-cyclopentene / cyclohexene serving as a raw material in an organic solvent A in the presence of monophosphate ligand and a palladium catalyst based on potassium acetate as alkali so as to prepare the cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method is characterized in that the physicochemical reaction at the n-butyllithium and ultralow temperature condition in the literature is avoided, and the palladium catalyst is adopted for catalyzing, so that the amount of the used catalyst is reduced, and the cyclopentene / cyclohexene-1-boronic acid pinacol ester can be prepared in relatively high yield at the acceptable temperature; the boronizing agent n-butyllithium / isopropoxy boronic acid pinacol ester system is replaced by the boronizing agent palladium catalyst / bis(pinacolato)diboron system, and therefore, the cost of the raw materials is greatly reduced, the reaction condition is relatively easy to realize, amplification is easy, and the technical operation is simpler and more convenient.
Owner:DALIAN NETCHEM CHIRAL TECH
Preparation method for 9,9-diaryl thiophene xanthene-10,10-dioxide
InactiveCN104557856AFlexible designEasy to operateOrganic chemistryNonlinear opticsOrganic synthesis
The invention discloses a preparation method for 9,9-diaryl thiophene xanthene-10,10-dioxide, and belongs to the field of fine organic synthesis and organic semiconductor material preparation. The preparation method comprises the following steps: using diphenylsulphone as a starting material and tetrahydro furan as a solvent, conducting ortho metalation reaction on diphenylsulphone under the action of n-butyllithium within a temperature range of -78-0 DEG C to obtain a corresponding aryl lithium salt; allowing the aryl lithium salt to react with an added aryl formic ether ester derivative, and performing hydrolysis to obtain a corresponding tertiary alcohol; dissolving the tertiary alcohol and electron-rich aromatic with an acetate or methylene chloride solution, using Lewis acid as a catalyst, and carrying out friedel-crafts reaction to obtain the 9,9-diaryl thiophene xanthene-10,10-dioxide. The preparation method has the advantages that the reactions are easy to control, the operation is simple, the cost is low, the repeatability is good, the productivity is high, and the product quality is high; an organic modular unit and an organic functional semiconductor can be conveniently made; the 9,9-diaryl thiophene xanthene-10,10-dioxide is used in the fields of organic light emitting diodes, organic transistors, organic laser, and organic nonlinear optics, and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
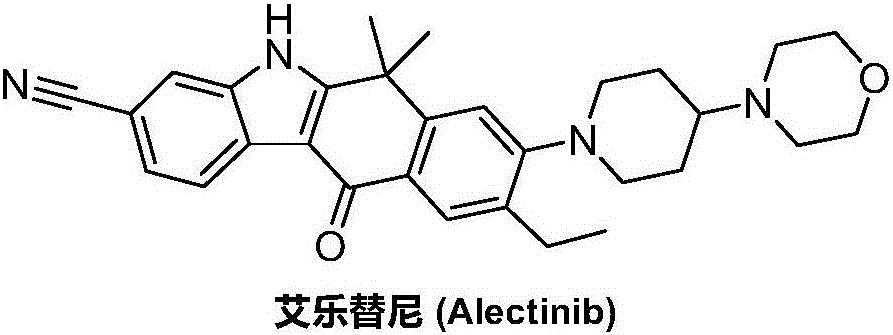
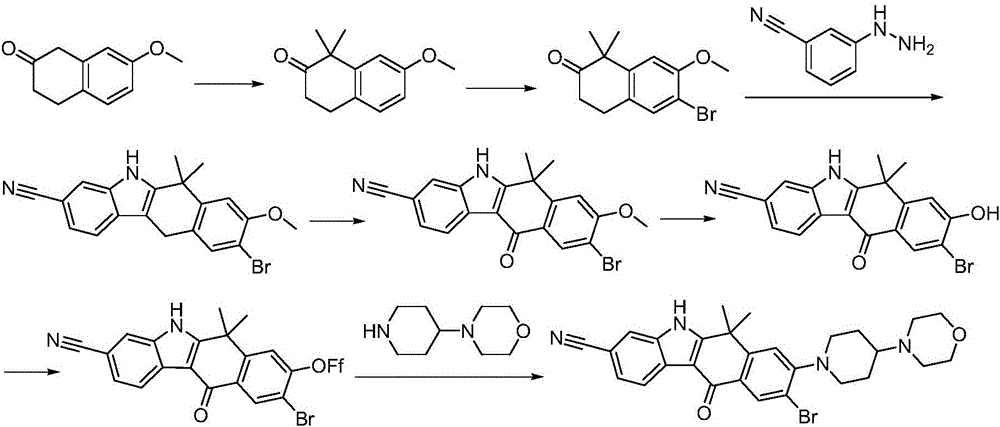
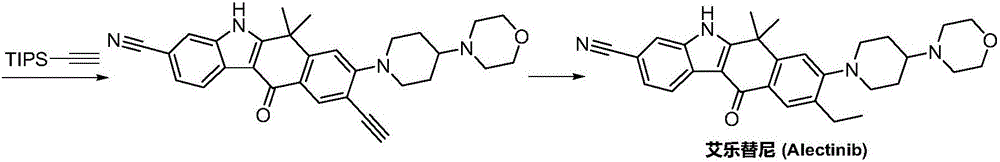
Synthesis method of Alectinib
ActiveCN105777710AMeet the needs of useApplicable generationOrganic chemistryButyl lithiumN-Butyllithium
The invention discloses a synthesis method of Alectinib. The method comprises the following steps: carrying out a borating reaction between 6-bromo-3,4-dihydro-2-naphthalenone and n-butyl lithium and then an organic boron reagent; carrying out a catalytic coupling reaction between the obtained 3,4,-dihydro-2-naphthalenone-6-boric acid and bromoethane; carrying out a dimethylation reaction between the obtained 6-ethyl-3,4-dihydro-2-naphthalenone and iodomethane; carrying out a bromination reaction between the obtained 1,1-dimethyl-6-ethyl-3,4-dihydro-2-naphthalenone and a bromination reagent; carrying out a substitution reaction between the obtained 1,1-dimethyl-6-ethyl-7-bromo-3,4-dihydro-2-naphthalenone and 4-(4-piperidyl)morpholine; carrying out a cyclization reaction between the obtained 1,1-dimethyl-6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone and 3-cyanophenylhydrazine; and carrying out an oxidation reaction between the obtained 9-ethyl-6,6-dimethyl-8-[4-(morpholine-4-yl)piperidine-1-yl]-6,11-dihydro-5H-benzo[b]carbazole-3-formonitrile and dichlorodicyanobenzoquinone to obtain a finished product of Alectinib. The synthesis method has the advantages of relatively short route, simplified operation and relatively low cost and is a green and environment-friendly method suitable for industrial production.
Owner:湖南欧亚药业有限公司
Synthesis of anticancer medicine Raltiprexed
InactiveCN1486985AShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
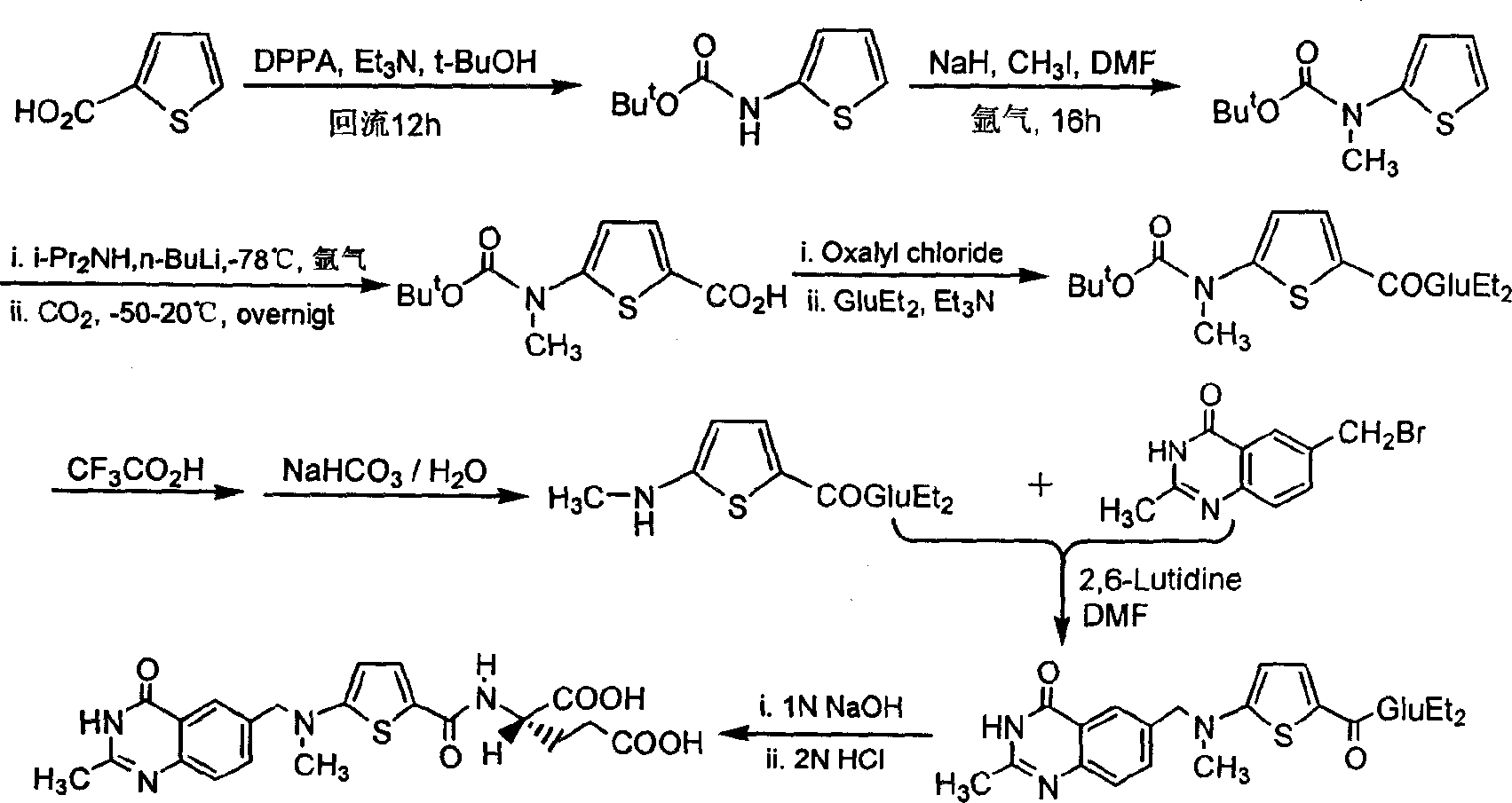
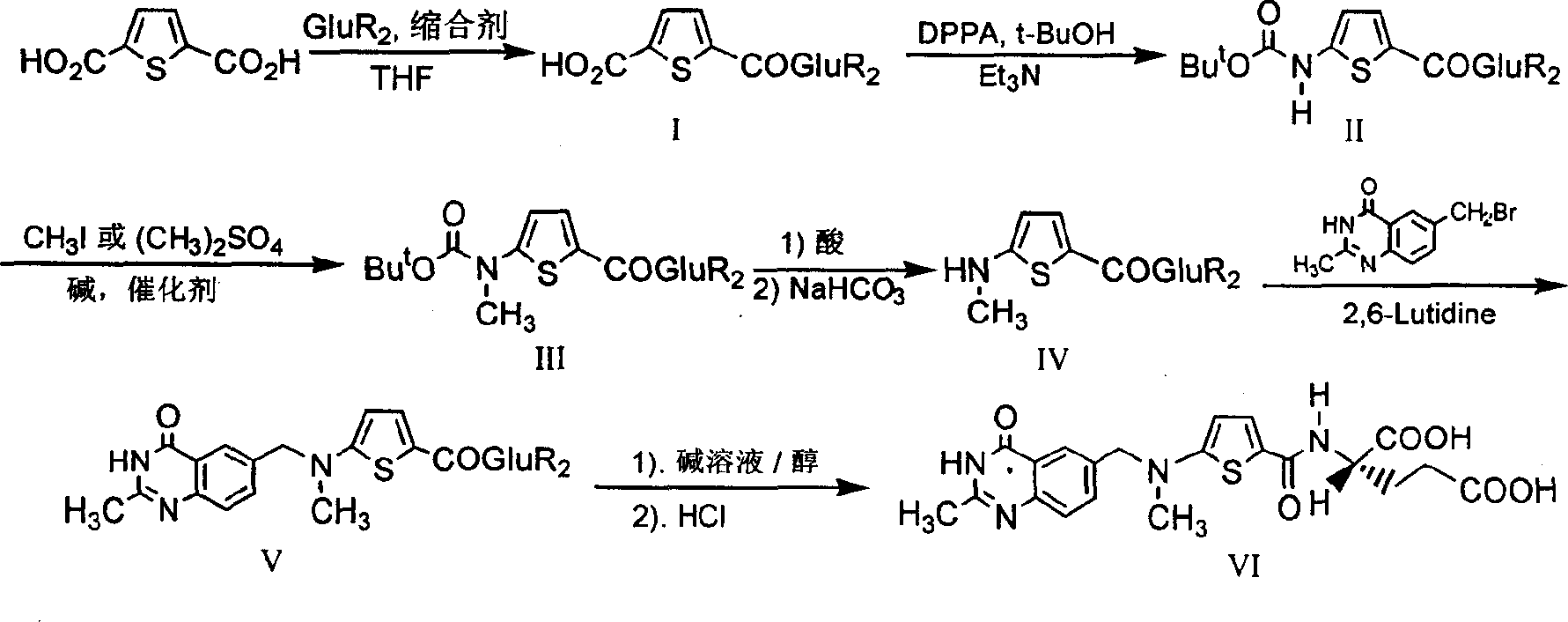
The synthesis of anticancer medicine Raltitrexed with 2, 5-thienyl diformic acid and diethyl glutamate as initial material and through six reaction steps of monocondensation, rearrangement, N-methylation, e;limination of tert-butoxy carbonyl group, condensation with 6-bromomethyl-2-methyl-4-quinbolone and saponification. The present invention has total yield of 18.1%, higher than that of available synthesis line, less reaction steps, mild condition and simple operation, and is suitable for mass production.
Owner:CAPITAL NORMAL UNIVERSITY
Application of n-butyllithium in catalytic hydroboration of ketone and borane
InactiveCN108654692AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsN-ButyllithiumKetone
The invention discloses an application of n-butylllithium in catalytic hydroboration of ketone and borane. The n-butyllithium is a commercial n-butyl lithium reagent, and the method includes: adding borane to a reaction flask subjected to dehydration and deoxygenation treatment without oxygen and water at inert gas atmosphere; adding a catalyst n-butyllithium with even mixing prior to adding ketone for hydroboration. The catalyst has good universality to aromatic ketones with different substitution positions and different electron effects as well as heterocyclic ketone and aliphatic ketone, and more choices for borate compounds with different substituent structures are provided.
Owner:SUZHOU UNIV
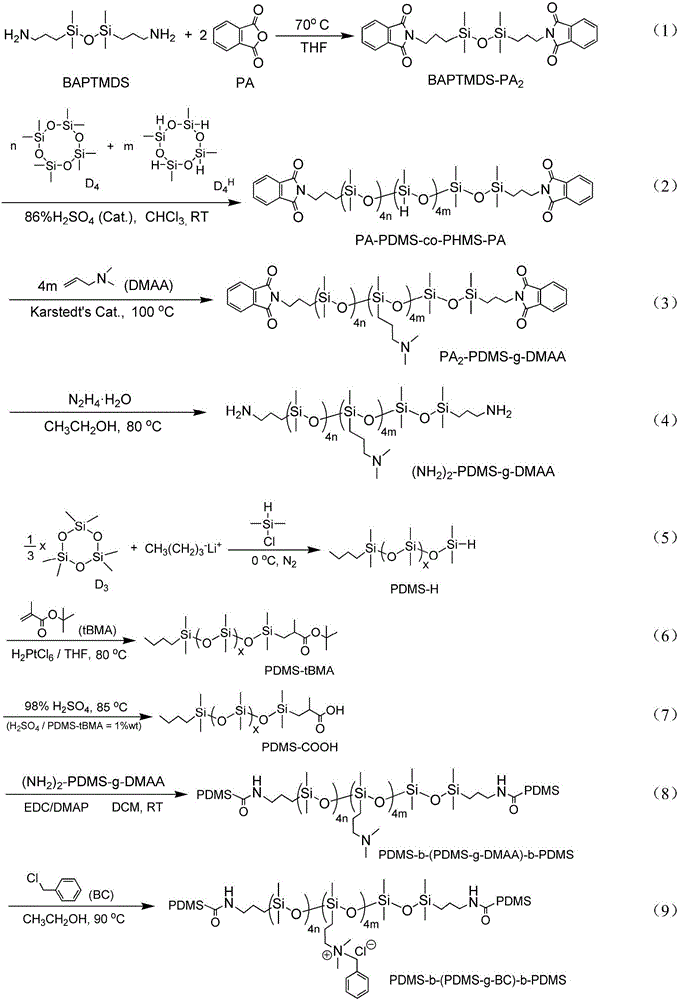
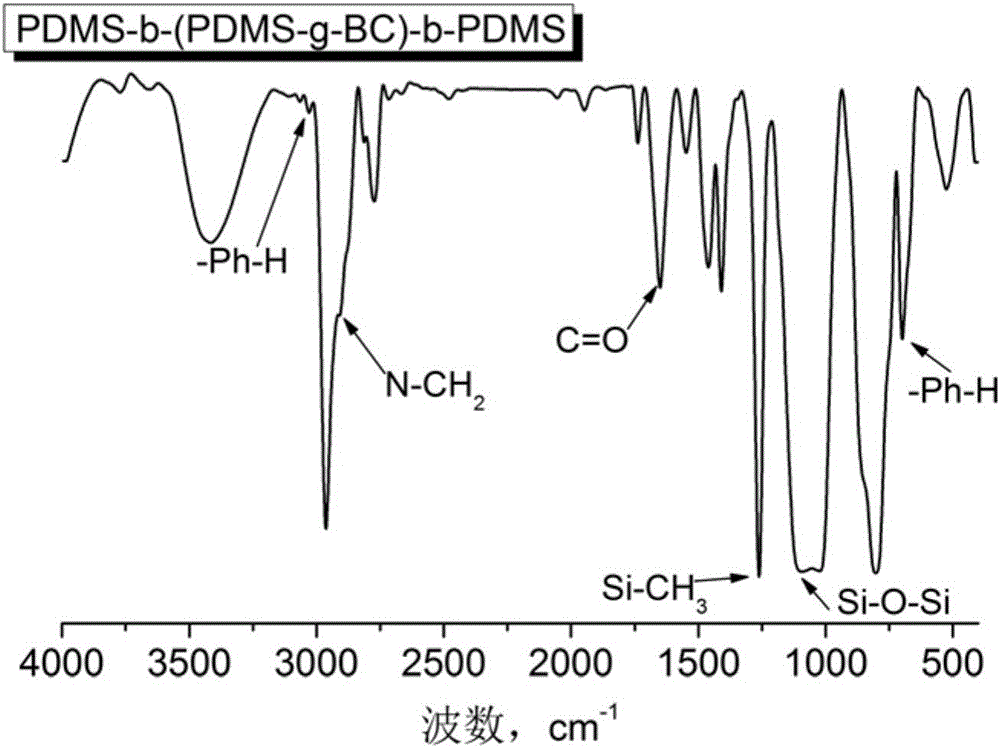
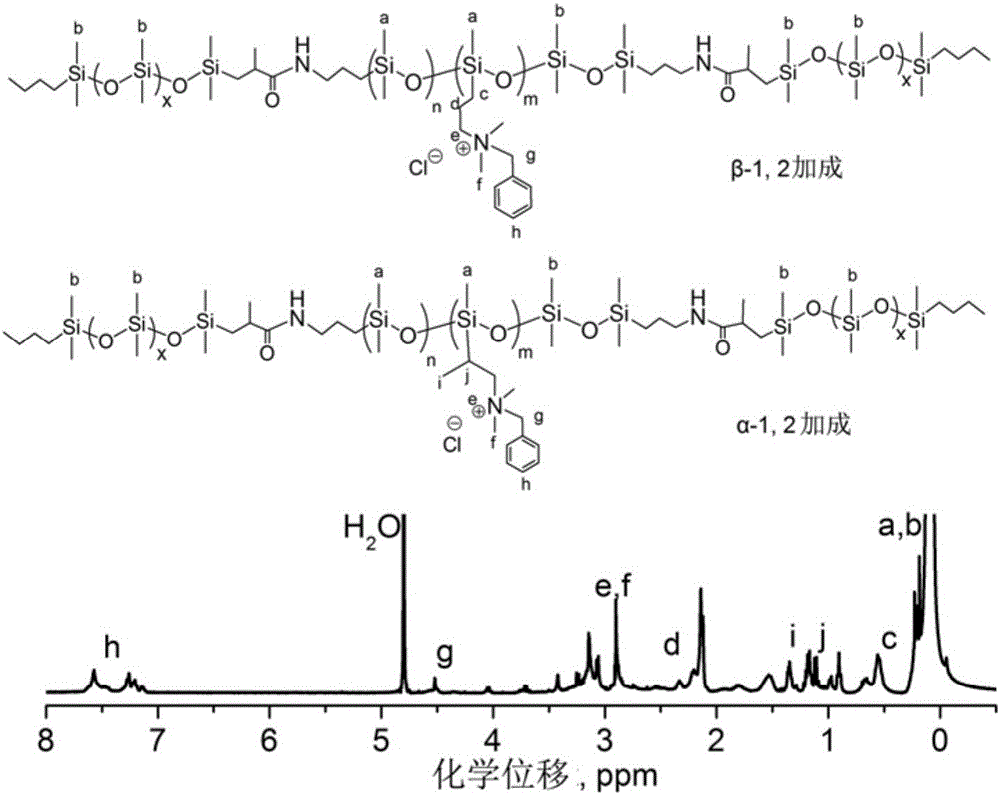
Quaternary ammonium salt group containing polysiloxane block copolymer and preparation method and application
ActiveCN105885054AGood water solubilityEnhanced inhibitory effectBiocideFungicidesQuaternary ammonium cationHalohydrocarbon
The invention discloses a quaternary ammonium salt group containing polysiloxane block copolymer and a preparation method and application. The primary amino in BAPTMDS is protected, and the obtained BAPTMDS-PA2 reacts with D4 and D4H to obtain hydrogen containing polysiloxane; the hydrogen containing polysiloxane reacts with DMAA to obtain tertiary amino polysiloxane; the tertiary amino polysiloxane reacts with hydrazine hydrate to obtain tertiary amino polysiloxane with terminated amino; D3 reacts with an n-butyl lithium solution to obtain single-ended hydrogen containing polysiloxane; the single-ended hydrogen containing polysiloxane reacts with tBMA to obtain single-ended ester-based polysiloxane; the single-ended ester-based polysiloxane reacts with sulfuric acid to obtain single-ended carboxyl polysiloxane; the single-ended carboxyl polysiloxane reacts with the tertiary amino polysiloxane with terminated amino and then reacts with halohydrocarbon to obtain the quaternary ammonium salt group containing polysiloxane block copolymer. The copolymer can effectively adhere to the hydrophobic surfaces of plant leaves and the like and plays the role of preventing plant fungi diseases.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of stable type compound nano anti-wearing agent
The invention discloses a preparation method of a stable type compound nano anti-wearing agent, and belongs to the technical field of preparation of lubricating oil additives. The preparation method comprises the following steps: firstly, enabling nano molybdenum disulfide to react with n-butyl lithium to obtain pretreated molybdenum disulfide; carrying out ultrasonic dispersion on the pretreated molybdenum disulfide and mercaptoethylamine; inserting the n-butyl lithium into a molybdenum disulfide sheet layer; stripping the molybdenum disulfide sheet layer in an ultrasonic treatment process, so as to expose an active group of the molybdenum disulfide; loading the mercaptoethylamine, and reacting with maleic anhydride and ethanol, so as to increase the lipophilic property of the molybdenum disulfide; coating the surface of the molybdenum disulfide with maleate, so that molybdenum disulfide powder is difficultly gathered, and the stability and uniformity are improved; finally, stirring with modified nano boron nitride, dispersing, and drying to obtain the anti-wearing agent. The anti-wearing agent prepared by the method has relatively good anti-wearing performance, and also has excellent stability and dispersity; the anti-wearing agent has no layered precipitation and no nano agglomeration phenomenon; after the anti-wearing agent is added into lubricating oil, the lubricating performance can be remarkably improved; the anti-wearing agent has relatively good application prospect.
Owner:唐林元
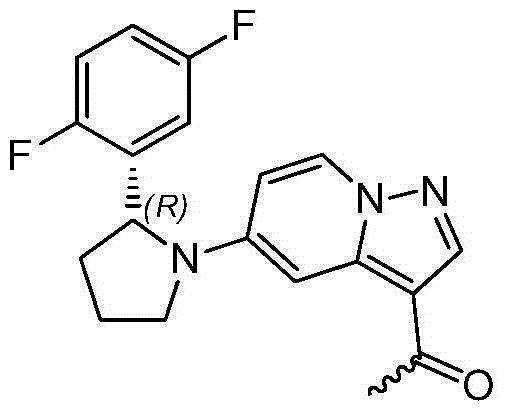
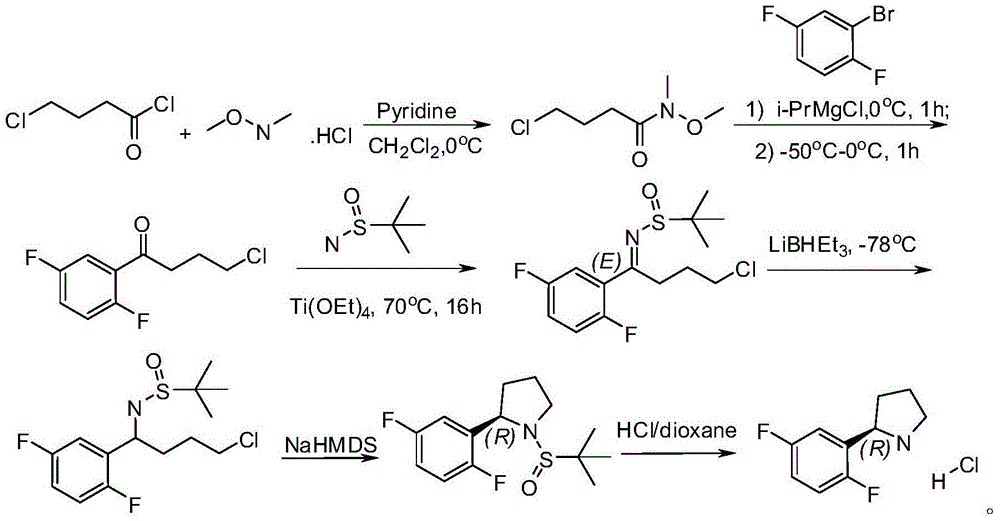
Method for preparing 2R-(2, 5-difluorophenyl) pyrrolidine hydrochloride
The invention discloses a method for preparing 2R-(2, 5-difluorophenyl) pyrrolidine hydrochloride. The method comprises the following steps: (a) reacting a compound (1) with the N, O-dimethyl hydroxylamine hydrochloride to obtain a compound (2); (b) reacting the 1, 4-difluorobenzene and n-butyllithium for 1-3h at -70 DEG C to -50 DEG C in an organic solvent, adding the compound (2) and reacting at the temperature of -70 DEG C to -50 DEG C to obtain a compound (3); (c) reacting the compound (3), a compound (7) and the Ti(OEt)4 in the organic solvent under refluxing to obtain a compound (4); (d) reacting the compound (4) with the sodium borohydride to obtain a compound (5); (e) reacting the compound (5) with the alkali to obtain a compound (6); and (f) reacting the compound (6) in an organic solvent of hydrogen chloride to obtain the 2R-(2, 5-difluorophenyl) pyrrolidine hydrochloride. By adopting the method provided by the invention, the cost is greatly reduced, the yield is obviously increased and the method is safe with a good application future. The reaction route of the method is as shown in the specification.
Owner:PHARORGSYN LAB
Method for synthesizing pentafluorophenol by using microchannel reactors
ActiveCN104774140AShort reaction timeShorten the production cycleOrganic chemistryOrganic compound preparationSulfite saltOil phase
The invention relates to a method for synthesizing pentafluorophenol by using microchannel reactors, which has the advantages of high reaction speed, short production cycle, low cost and high product purity. The method comprises the following steps: connecting three microchannel reactors in series, and dissolving pentafluorobromobenzene in an ether solvent to obtain a homogeneous mixed solution; respectively pumping the homogeneous mixed solution and an n-butyllithium n-hexane solution into a reactor I to react, thereby obtaining a reaction solution I; respectively pumping borate and the reaction solution I in a volume flow ratio of 1:(5-15) into a reactor II to react, thereby obtaining a reaction solution II; adding dilute hydrochloric acid into the reaction solution II for neutralization until the pH value is 1-3, standing, carrying out oil-water separation, and obtaining a pentafluorobenzene boric acid solution in the oil phase; pumping a 30 mol% oxydol solution and the pentafluorobenzene boric acid solution in a volume flow ratio of 1:(0.5-5) into a reactor III, and reacting to obtain a pentafluorophenol crude product solution; adding a 10 wt% sodium sulfite solution, removing excessive oxydol, washing with water, drying, and rectifying under vacuum to obtain the pentafluorophenol.
Owner:RUICHENG COUNTY SIPULUNDI BIOLOGICAL ENG CO LTD
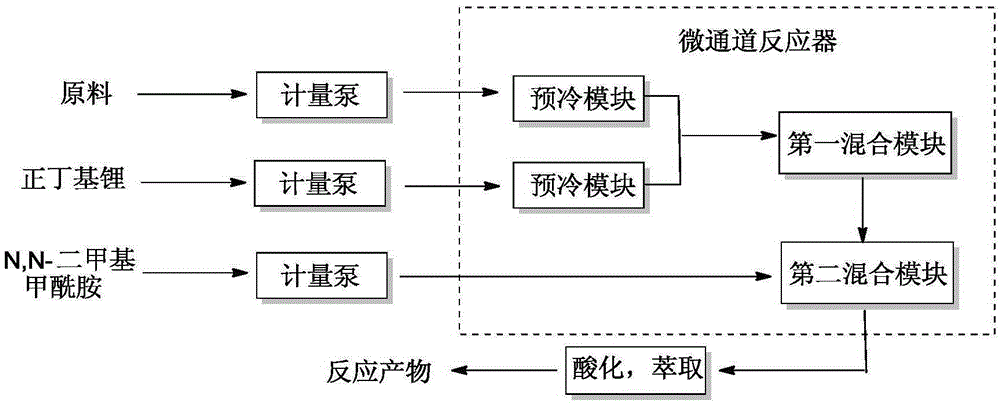
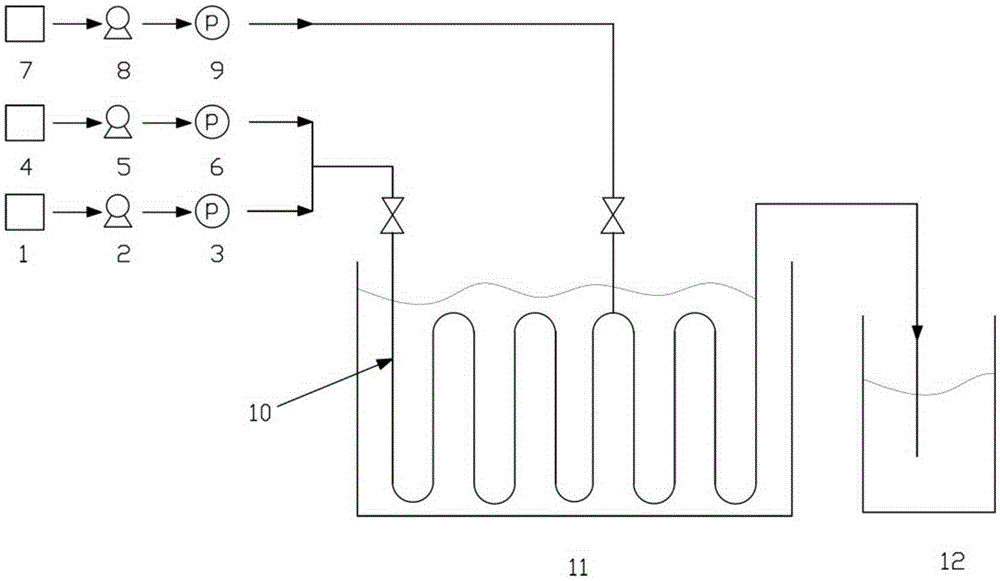
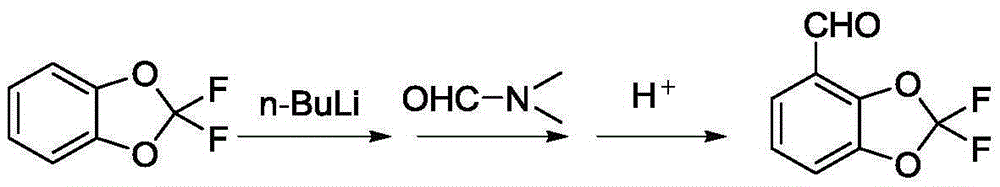
Method for preparing difluoro piperonal by utilizing continuous flow microchannel reactor
ActiveCN105254610AShort reaction timeImprove reaction efficiencyOrganic chemistryOrganic solventN-Butyllithium
The invention discloses a method for preparing difluoro piperonal by utilizing a continuous flow microchannel reactor. The method for preparing the difluoro piperonal by utilizing the continuous flow microchannel reactor comprises the following steps: respectively introducing difluoro piperidine organic solvent solution and n-hexane solution of n-butyllithium into a precooling module of a microchannel reactor by virtue of a metering pump to be precooled, then enabling the difluoro piperidine organic solvent solution and n-hexane solution of n-butyllithium to enter a first mixing module for reacting, after reaction is finished, enabling reaction products to enter a second mixing module for reacting with N,N-dimethyl formamide, acidifying, extracting, and distilling, so that the difluoro piperonal is obtained. The method for preparing the difluoro piperonal by utilizing the continuous flow microchannel reactor has the advantages of short reaction time, easiness and safety in operation and low production energy consumption.
Owner:XIAN MODERN CHEM RES INST
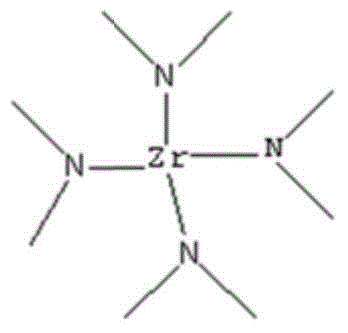
Synthetic method of tetra(dimethylamino)zirconium
InactiveCN103910640ALow toxicityEasy to operateOrganic compound preparationAmino compound preparationArgon atmosphereDistillation
The invention relates to a synthetic method of tetra(dimethylamino)zirconium, which comprises the following steps: 1)under argon atmosphere, adding dimethylamine and hexane in a three-necked bottle according to a proportion that 100-300 milliliters n-hexane is added in 100g dimethylamine, uniformly stirring, and placing the reaction bottle between the temperature of -20--60 DEG C, adding a n-butyllithium solution drop by drop in the reaction bottle, stirring after dropping, and reacting for 10 hours; 2)adding zirconium tetrachloride in the above reaction system, keeping the temperature of the reaction system between -20-0 DEG C, after adding zirconium tetrachloride, stirring the reaction system under the protection of inert gas to react for 24-30 hours; and 3)after the reaction is finished, removing a solvent of the reaction under one atmospheric pressure, after solvent n-hexane is completely removed, performing underpressure distillation, and collecting the fraction at 110-112 DEG C / 4mmHg which is the tetra(dimethylamino)zirconium compound. According to the invention, the reaction employs the raw material dimethylamino lithium and hafnium tetrachloride which are simple and easy to obtain, the operation is simple, and the cost is reduced.
Owner:NANJING UNIV
Organic field effect transistor material based on oxa-condensed ring, and synthetic method and application thereof
ActiveCN106749318AImprove solubilityImprove mobilityOrganic chemistrySolid-state devicesSolubilityTrimethyltin chloride
Owner:SUZHOU JOYSUN ADVANCED MATERIALS CO LTD
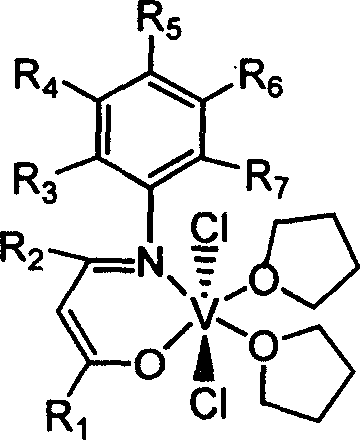
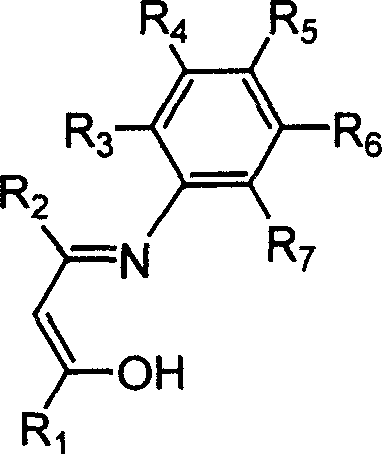
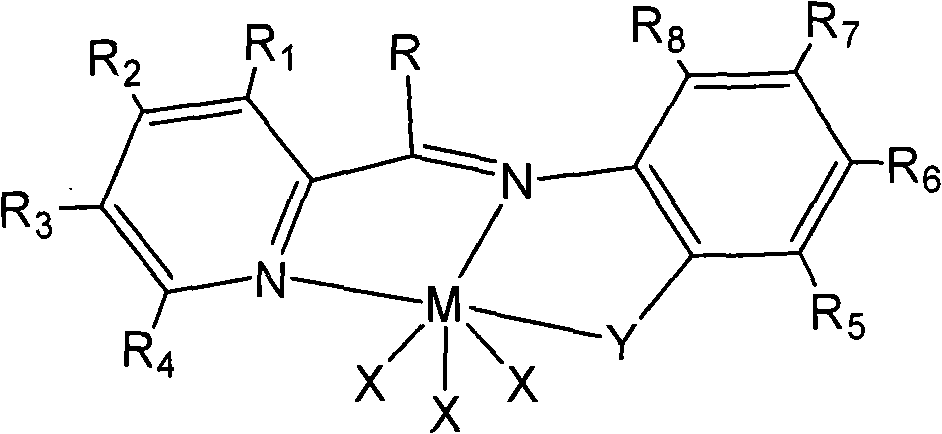
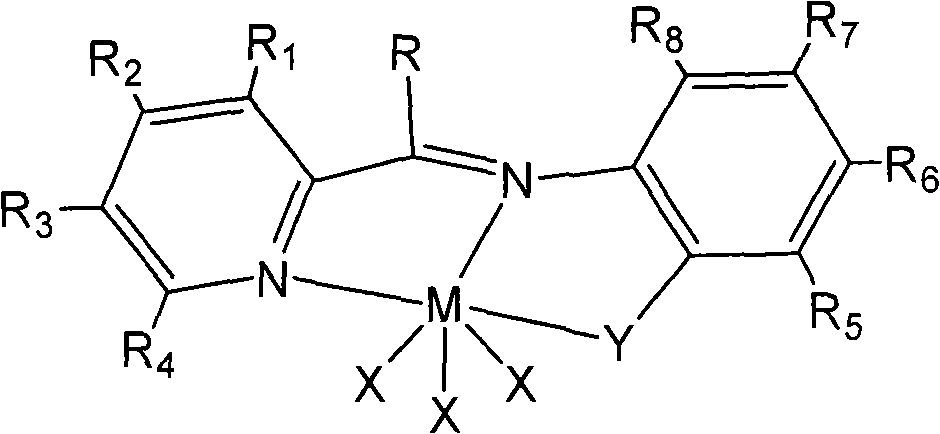
Beta-diketo mono imine vanadium olefinic polymerization catalyst, and its preparing method and use
The invention discloses a beta-diketone mono-imide vanadium olefinic polymerization catalyst and the manufacture method and the application of ethylene polymerizing, ethylene and norborene polymerizing, ethylene and alpha-alkene or norborene copolymerization. Under the catalysis of formic acid, beta-diketone compound and aniline or the ramification of aniline taking condensation reaction in methanol solution to gain Schiff base; under the non water and non oxygen condition, the Schiff base taking reaction with butyl lithium to gain negative ion ligand; under the non-water, non oxygen condition, the negative ion ligand taking coordination reaction with VCl3, the beta-ketimine vanadium alkene polymerization catalyst could be gained. The invention could catalyze ethylene polymerization, and the copolymerizing of ethylene and alpha-alkene or norborene.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Method for preparing medium-high vinyl block-free solution-polymerized butylbenzene and product thereof
The invention discloses a method for preparing medium-high vinyl block-free solution-polymerized butylbenzene and a product thereof. The method mainly comprises the following steps of: A, undergoing a polymerization reaction on a vinyl aromatic hydrocarbon and an insufficient reacting dose of conjugated diene; and B, continually reacting the remaining reacting dose of conjugated diene. In a medium-high vinyl block-free solution-polymerized butylbenzene product, the content of a styrol block is zero, and the content of the vinyl is between 25 percent and 60 percent. In the method, n-butyl lithium is taken as an initiator for anionic polymerization, a compound product of alkyl tetrahydrofurfuryl ether and sodium alkyl benzene sulfonate is taken as a structure modifier, the alkyl tetrahydrofurfuryl ether can effectively adjust the content of vinyl in the product, the sodium alkyl benzene sulfonate can always keep high reaction activity, and further tail end functionalization is promoted; and high-temperature continuous polymerization is adopted, so that the produce efficiency of the product can be increased, and the product with wide molecular weight distribution can be produced; and the styrol block in the product is eliminated through stepwise reaction, so that practical requirements on production can be met.
Owner:周铁生
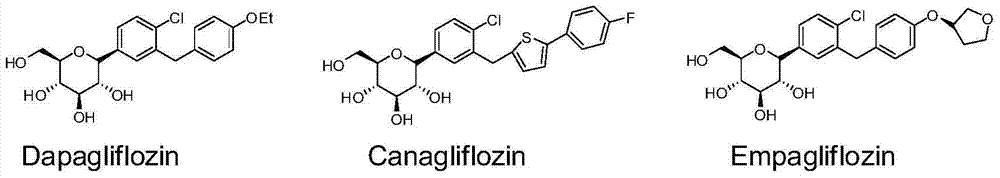
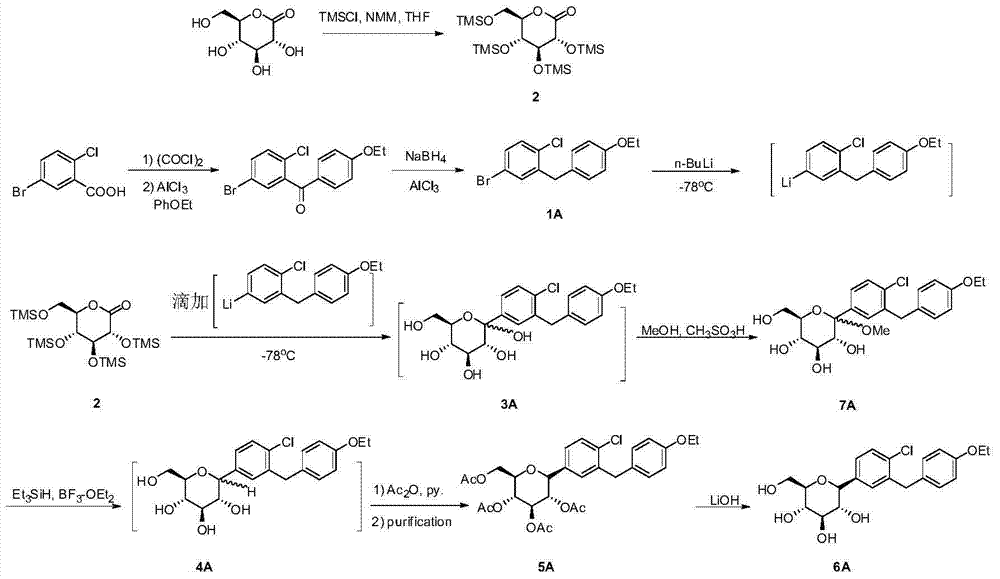
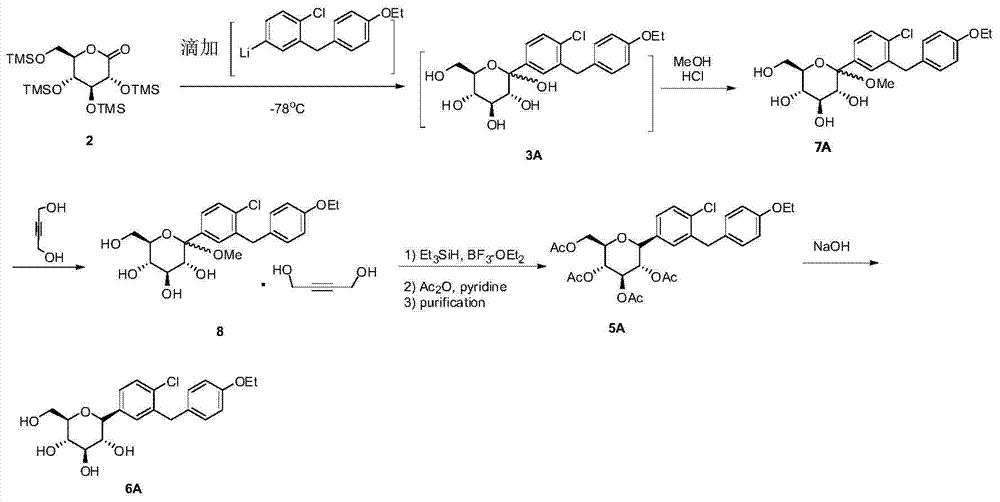
Method for synthesizing SGLT2 inhibitor drugs
InactiveCN104710486AHigh purityFew reaction stepsSugar derivativesSugar derivatives preparationLithium chlorideAcetic anhydride
The invention provides a method for synthesizing SGLT2 inhibitor drugs. The method comprises the following steps: (a) reacting a compound (1) with n-butyllithium in an organic solvent for 0.5-2 hours, and then dripping an organic solvent dissolved with a compound (2) to react for 1.5-4 hours to obtain a compound (3); or, in a nitrogen atmosphere, reacting the compound (1) with isopropylmagnesium chloride and lithium chloride in the organic solvent for 0.5-1 hour, and then dripping the organic solvent dissolved with the compound (2) to react for 1.5-4 hours to obtain the compound (3); (b) in the organic solvent, carrying out a reduction reaction on the compound (3) to obtain a compound (4); (c) in the organic solvent, carrying out an acetylation reaction on the compound (4), pyridine and acetic anhydride to obtain a compound (5); and (d) carrying out a hydrolysis reaction on the compound (5) to obtain a compound (6), namely the SGLT2 inhibitor drugs. The method provided by the invention is simple in steps, low in cost, high in yield and is suitable for industrial production. The reaction route is as follows in the specification.
Owner:ARROMAX PHARMATECH
Synthesis of end amino phenylethylene/butadiene copolyer by tndcapping process and its method for preparing storage-stable modifie asphalt
InactiveCN1775823ASimple preparation processLow costBuilding insulationsPolymer modifiedPolymer science
The invention relates to an end capping method composing end amidogen cinnamene / butadiene copolymer and its preparation stockpile stable modified asphalt method. It belongs to polymer modified asphalt technique field. It uses butyl lithium as the anionic polymerization initiator, and 1, 5-dinirogen bicyclo caproylhydride as the anionic polymerization terminator. It makes omega-amidogen cinnamene / butadiene / cinnamene ternary block polymer. It adopts that end function SBS and the asphalt are mixed. This can effectively deduce the emanation degree of the SBS modified asphalt and obviously improve storage stability. It applies in modified asphalt manufacture field.
Owner:CHINA PETROLEUM & CHEM CORP
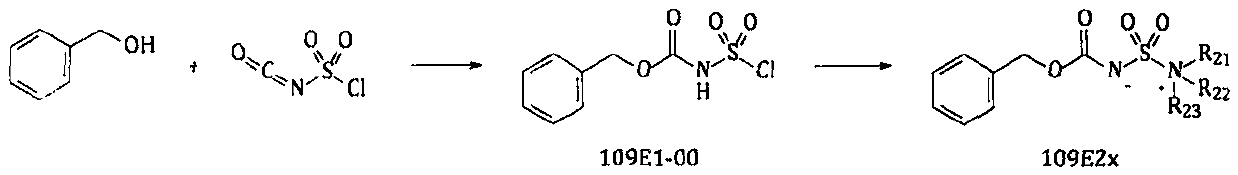
Preparation method of edoxaban
ActiveCN109942600AStable structureHigh yieldOrganic chemistryBulk chemical productionSulfurN-Butyllithium
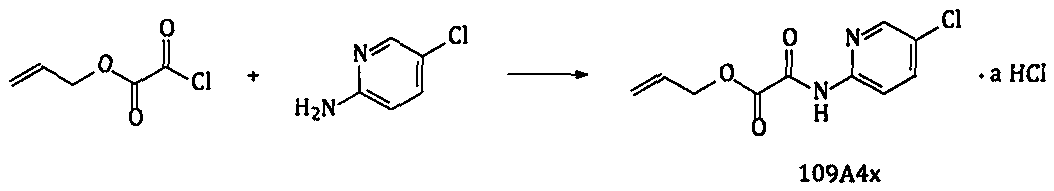
The invention relates to a new preparation route and a new method for a p-toluenesulfonic acid edoxaban hydrate and intermediates thereof. The new method comprises the steps that a high-reactivity compound 109A4x is prepared; a compound 109C6x is prepared by using a new synthesizing method; new compounds 109E8-01, 109E9x and 109T7-01 are prepared; the p-toluenesulfonic acid edoxaban hydrate is prepared by using the intermediates. By using the new method and the new route, the reaction step of copious cooling is omitted, and dangerous elemental sulfur, high-risk n-butyllithium and high-risk azides are prevented from being used. In a word, by means of the method, the p-toluenesulfonic acid edoxaban hydrate and the key intermediates thereof are more easily and safely prepared at a lower coston an industrialization scale.
Owner:内蒙古京东药业有限公司 +1
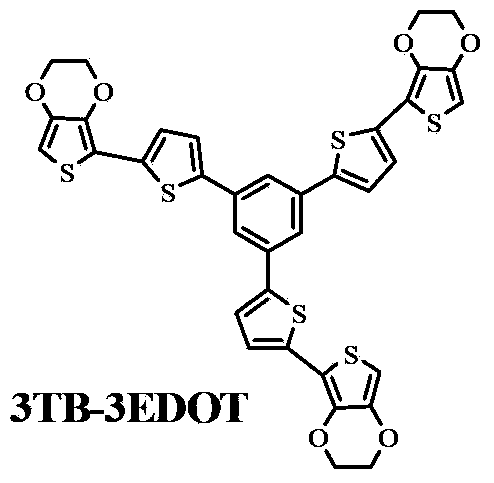
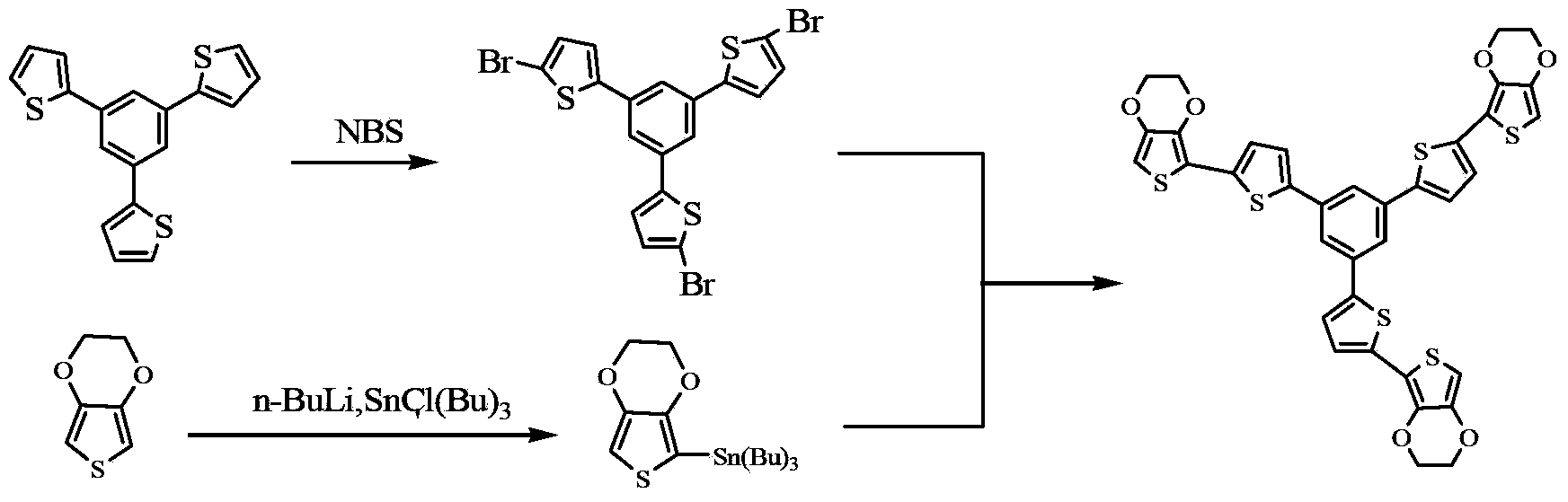
Oligothiophene derivative and preparation method thereof
The invention relates to an oligothiophene derivative and a preparation method thereof. The preparation method of the oligothiophene derivative mainly comprises the following steps of: dissolving 3TB into dichloromethane, adding NBS (N-bromosuccinimide), stirring and reacting so as to obtain 3TB-3Br; adding EDOT (3,4-ethylenedioxythiophene) and tetrahydrofuran into a three-neck flask, controlling the reaction temperature to be minus 78 DEG C, dropwise adding n-butyllithium, gradually raising to be room temperature after the dropwise adding, and continuously reacting; subsequently decreasing the reaction temperature to be minus 78 DEG C, dropwise adding tributyl stannic chloride, gradually raising to be the room temperature after the dripping, and reacting to obtain an EDOT tin reagent; under the protection of nitrogen, adding methylbenzene into the three-neck flask, further adding the 3TB-3Br and the EDOT tin reagent, carrying out backflow reaction so as to obtain a 3TB-3Br crude product, thus obtaining pure 3TB-3EDOT through separation. As cation (or anion) free radicals generated in electrochemical doping are stable, the oligothiophene derivative can be used as a novel organic electrochromic material.
Owner:SOUTH CHINA UNIV OF TECH
Non-metallocene catalyst and preparation method and application thereof
The invention discloses a non-metallocene catalyst and a preparation method and the application thereof. The general structural formula of the non-metallocene catalyst is as shown in formula I. The preparation method of the catalyst comprises the following steps: (1) pyridine or pyridone formaldehyde containing a substituent and amino phenol or amino thiophenol containing the other substituent are put into organic solvent to have reflux reaction and obtain a ligand; and (2) first the organic solution of the ligand obtained in step (1) reacts with butyl lithium, is added with metal halide to react and obtain the non-metallocene catalyst after reaction. When the non-metallocene catalyst is used for ethylene polymerization, not only dichloromethane with polarity but also non-polar toluene and heptane can be selected as the solvent during the ethylene polymerization process, and higher activity is also achieved during the process; and the polyethylene has high molecular weight, and the weight-average molecular weight is 800,000 to 1 million(Formula I is shown as the accompanying drawing.).
Owner:INST OF CHEM CHINESE ACAD OF SCI
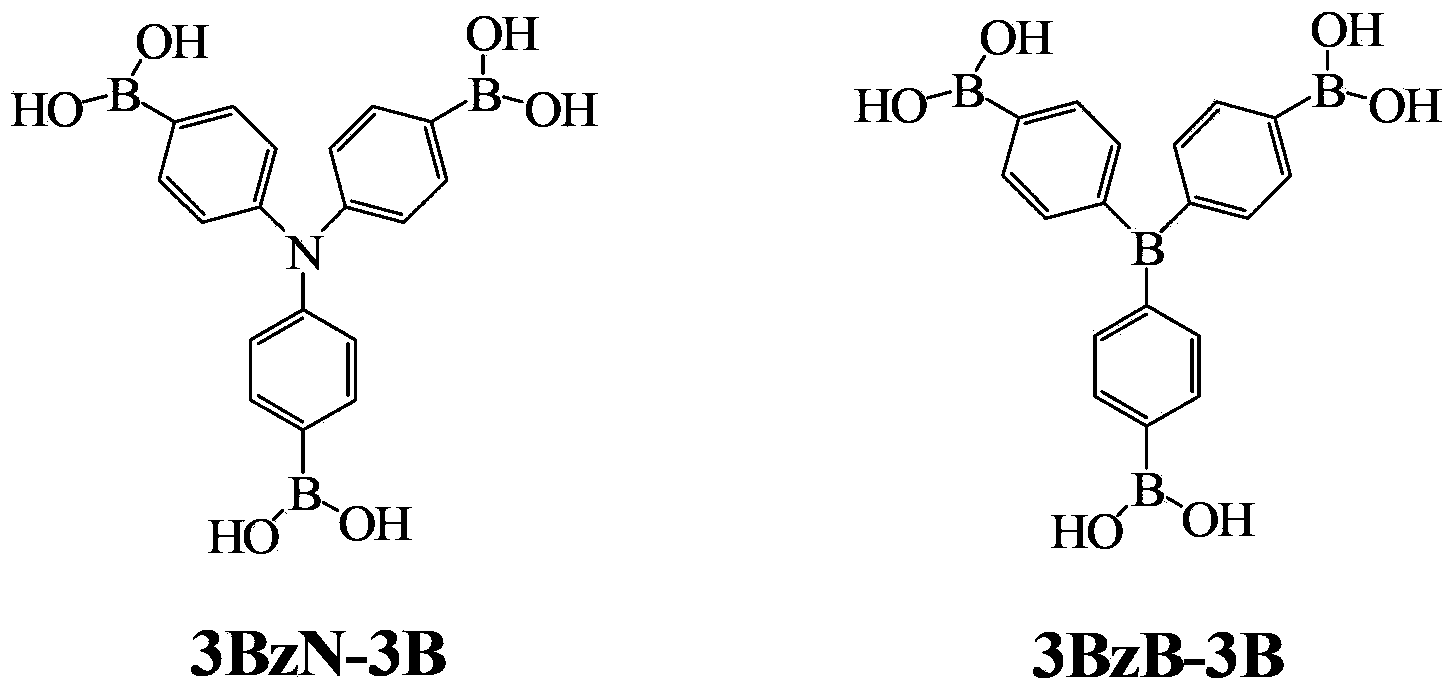
Arylboronic acid derivatives and preparation method thereof
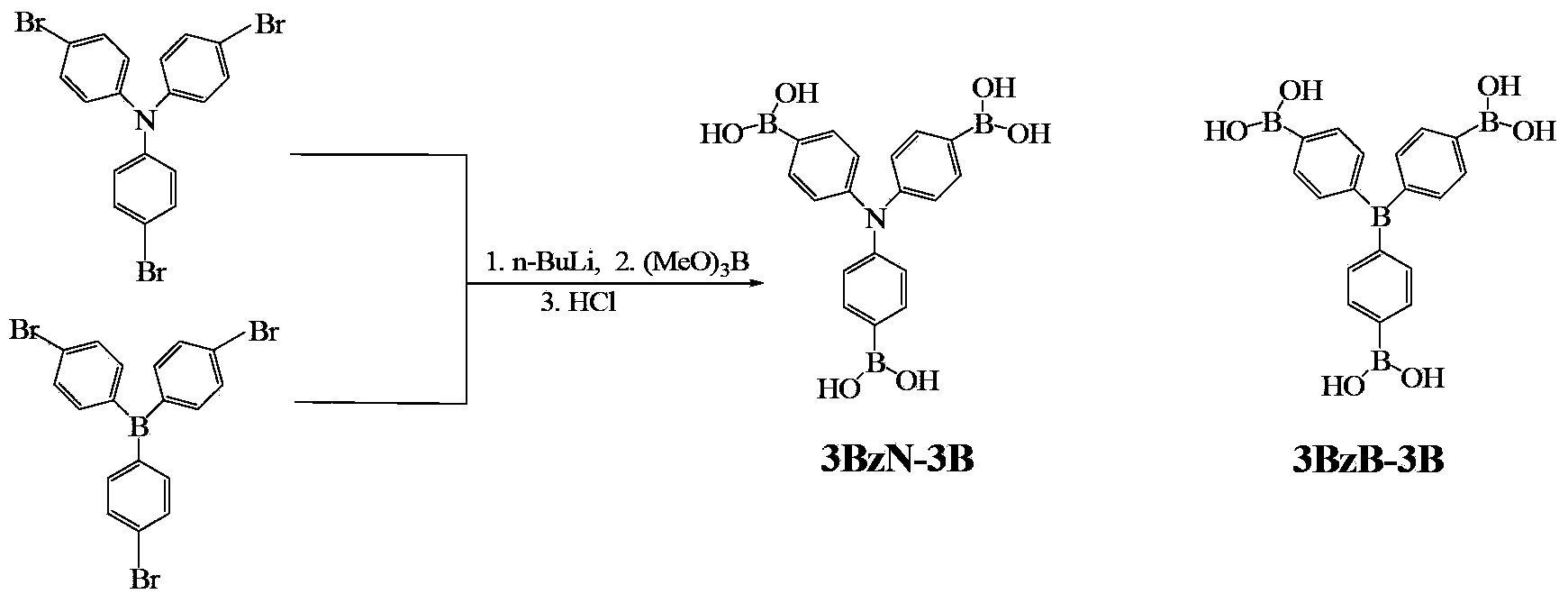
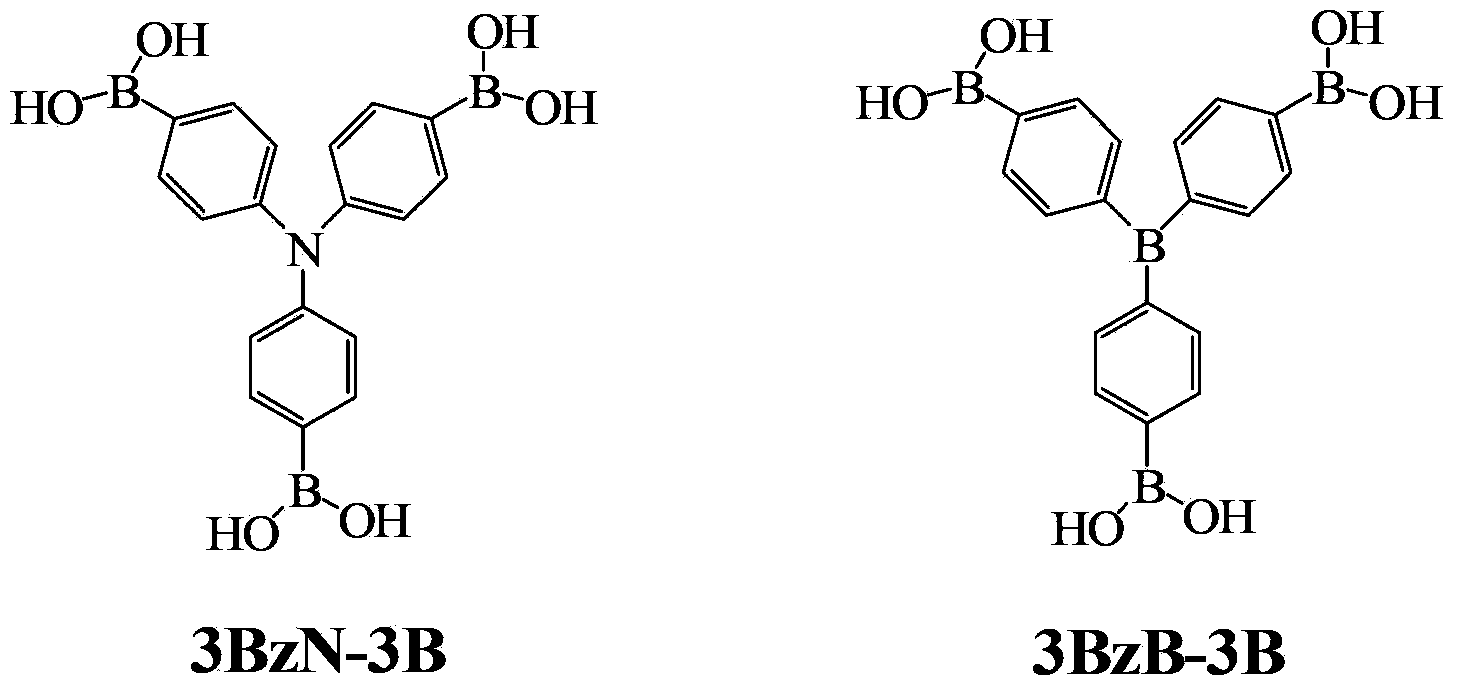
ActiveCN103374024AStop burningTo solve the toxicGroup 3/13 element organic compoundsArylN-Butyllithium
The invention provides arylboronic acid derivatives and a preparation method thereof. The two arylboronic acid derivatives are tri(4,4',4'-triboric acid-phenyl)amino(3BzN-3B) and tri(4,4',4'-triboric acid-phenyl)boron(3BzB-3B), and the molecular structures are as shown in the specification. 3BzN-3B and 3BzB-3B are prepared by firstly, respectively reacting tri(4,4',4'-triboric acid-phenyl)amino and tri(4,4',4'-triboric acid-phenyl)boron with n-butyllithium, secondly, respectively carrying out nucleophilic substitution reaction with boric acid ester, and finally carrying out acidolysis by using diluted hydrochloric acid. The products of the arylboronic acid derivatives, namely, the 3BzN-3B and the 3BzB-3B are expected to be excellent environment-friendly organic fire retardants.
Owner:SOUTH CHINA UNIV OF TECH
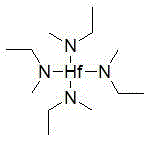
Tetrakis(ethylmethylamino)hafnium synthesis method
InactiveCN103601750AEasy to operateSimple and fast operationGroup 4/14 element organic compoundsAlkaneN-Butyllithium
The invention relates to a tetrakis(ethylmethylamino)hafnium synthesis method, which comprises the following synthesis steps: (1) under an inert atmosphere, adding methylethylamine and an alkane solvent to a three-necked bottle, carrying out mechanical stirring, placing the reaction bottle into an environment with a temperature of -10 to -80 DEG C, adding a n-hexane solution of n-butyl lithium to the reaction bottle in a dropwise manner, and carrying out a stirring reaction after completing the addition; (2) adding hafnium tetrachloride to the reaction system, maintaining the temperature of the reaction system to not more than 60 DEG C, carrying out a stirring reaction on the reaction system under the protection of the inert gas after completing the addition; and (3) after completing the reaction, removing the reacting solvent under an atmospheric pressure, carrying out vacuum distillation after completely removing the solvent, and collecting the 110-115 DEG C / 4-5 mmHg fraction, wherein the fraction is the tetrakis(ethylmethylamino)hafnium compound. According to the present invention, the reaction adopts the simple and easily available materials such as methylethylamine, butyl lithium and hafnium tetrachloride as the raw materials, the operations are simple, and the cost is reduced.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com