Synthesis of anticancer medicine Raltiprexed
A synthetic process, a drug mine technology, applied in the field of new synthetic process of the anticancer drug raltitrexed, which can solve the problems of long reaction time and high price, and achieve the effect of long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
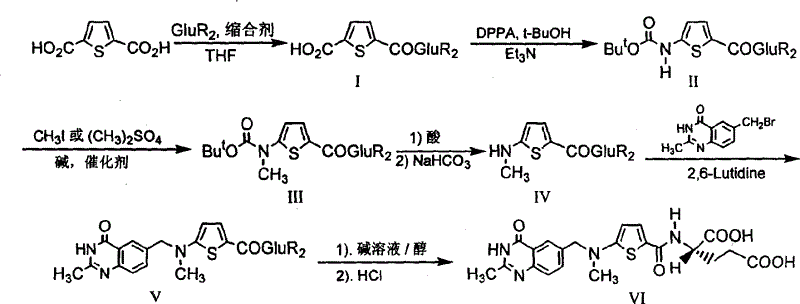
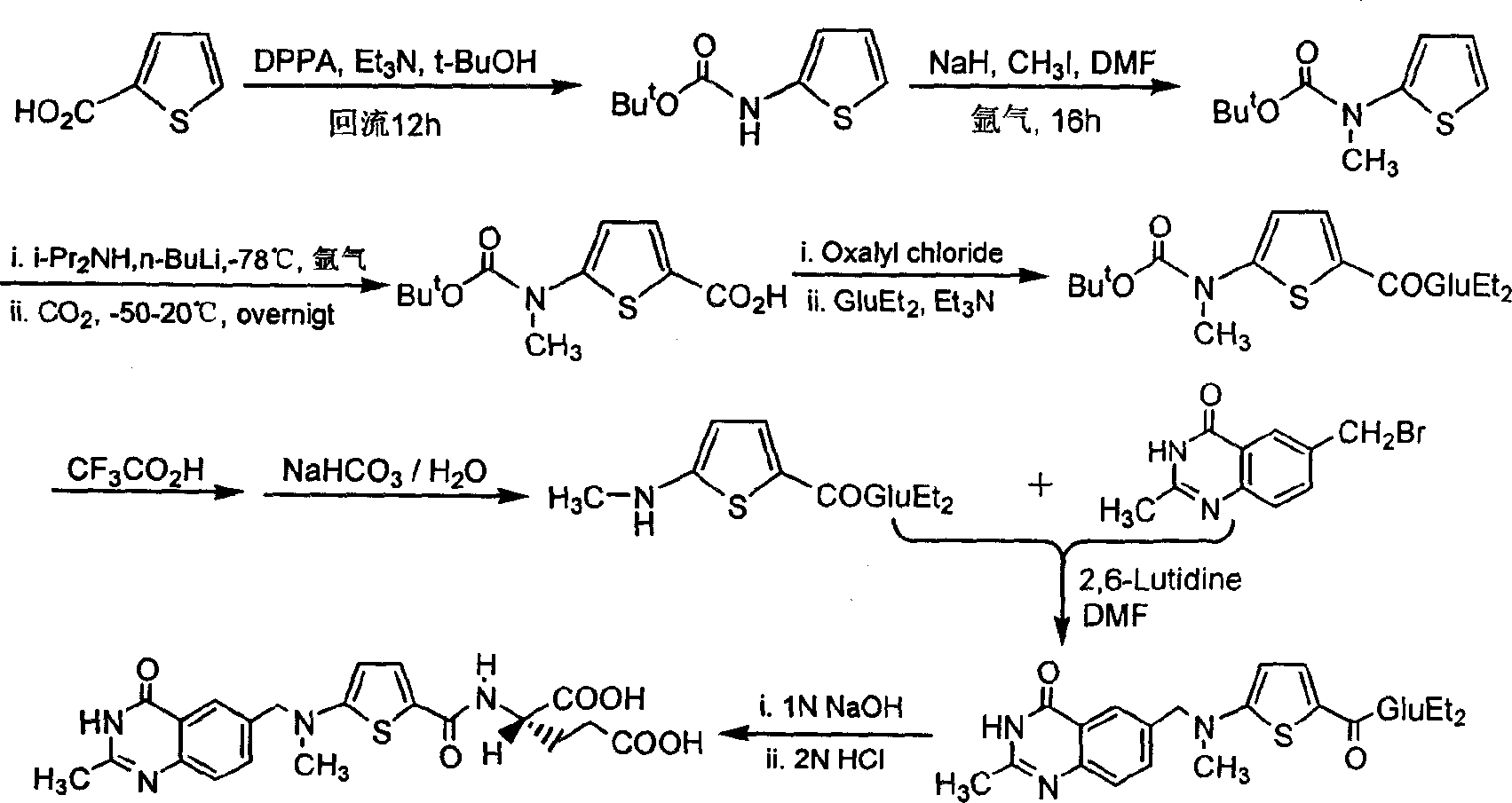
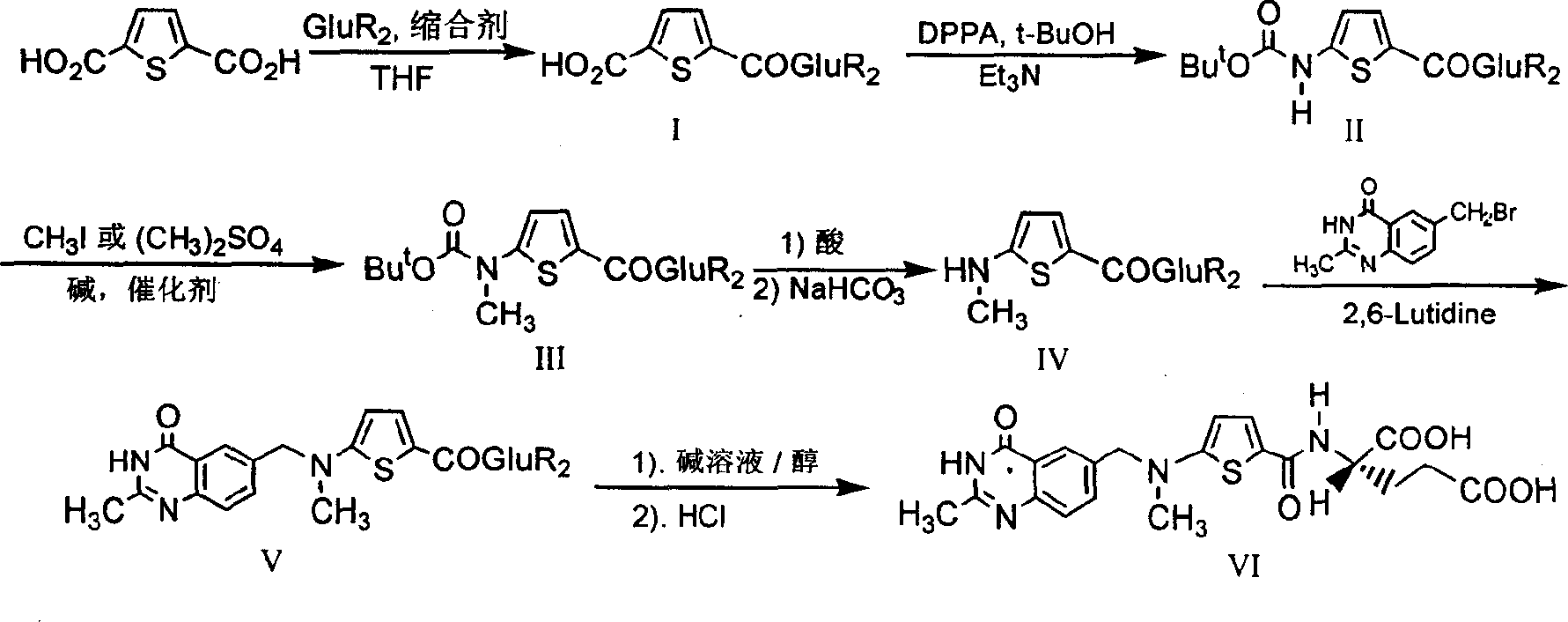
[0022] The synthetic route is as follows:
[0023]
[0024] The first step: the synthesis of N-(5-carboxy-2-thiophenoyl)-L-diethyl glutamate (I):
[0025] Suspend 2.93 g (17.2 mmol) of 2,5-thiophenedicarboxylic acid in 30 mL of dry tetrahydrofuran (THF), and add N, N'-dicyclohexylcarbodiimide (DCC) 3.55 g (17.2 mmol). After stirring for a while, 3.5 g (17.2 mmol) of L-diethyl glutamate dissolved in 20 mL of dry THF was added dropwise, and the reaction was stirred overnight at room temperature. Filter, wash the filter cake with THF, combine the filtrate and washings, and remove THF by rotary evaporation. The residue was dissolved in 15 mL of chloroform, then petroleum ether was added until cloudy, and after standing at room temperature, the precipitated solid was removed by filtration. The filtrate was rotary evaporated to remove the solvent to obtain 3.67 g of viscous liquid with a yield of 59.8%.
[0026] The second step: the synthesis of N-[5-[N-(tert-butoxycarbonyl)a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com