Patents
Literature
55 results about "N methylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
N -methylation is a powerful technique to modulate the physicochemical properties of peptides by introducing one or more methyl groups into the peptidic amide bonds. Together with peptide cyclization, this procedure confers unprecedented pharmacokinetic properties to the peptides, including metabolic stability,...
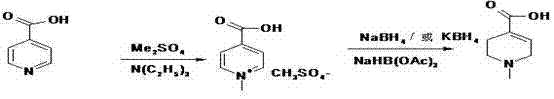
New synthesis technology of anti-cancer drug Raltitrexed
InactiveCN102127063ALow costOptimize the synthetic routeOrganic chemistryAntineoplastic agentsBenzoic acidTert-Butyloxycarbonyl protecting group
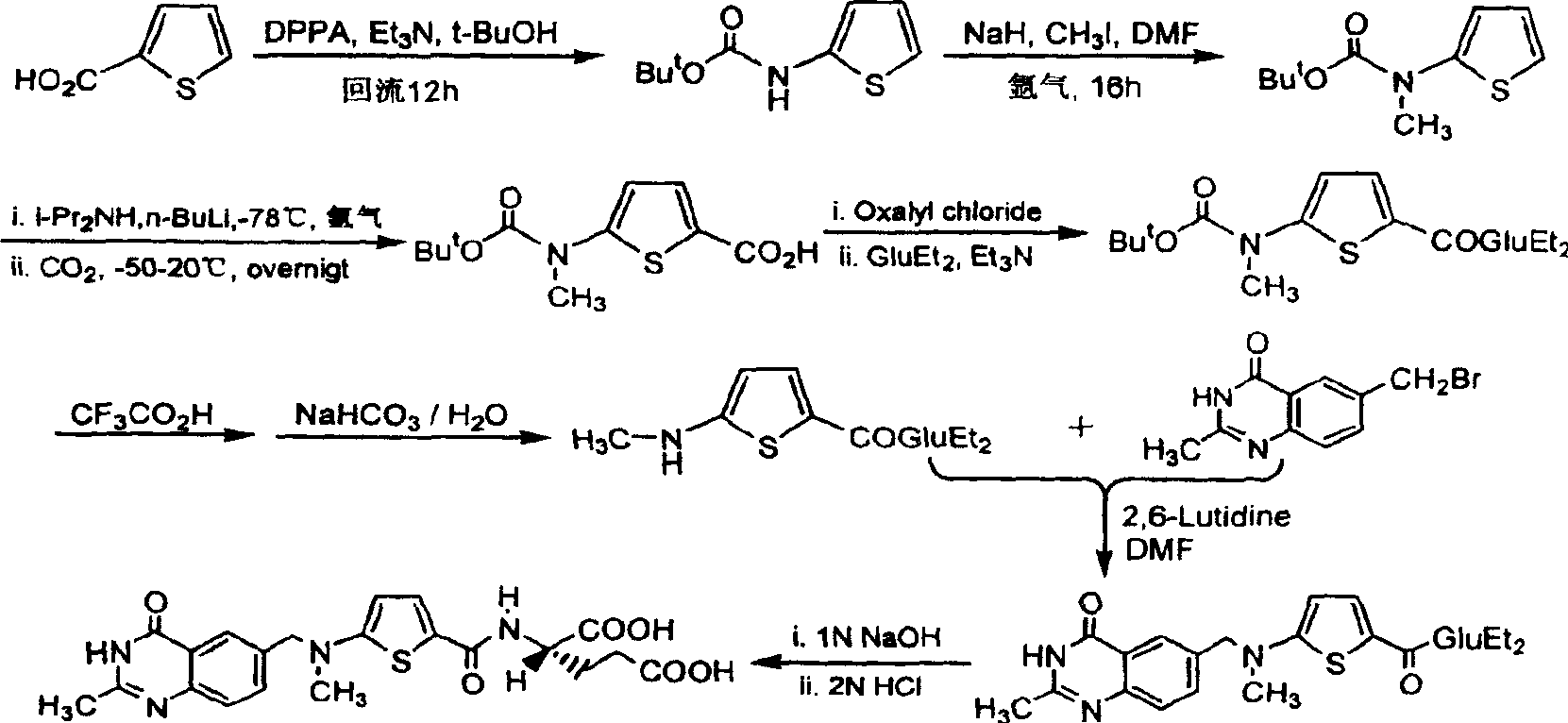
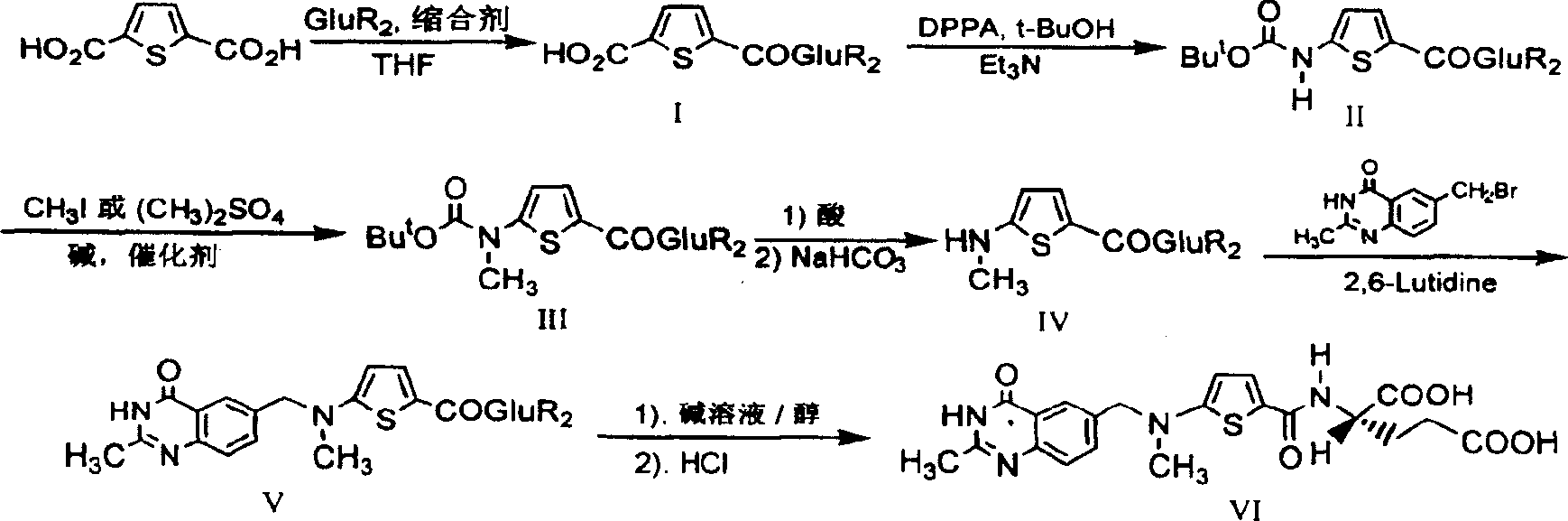
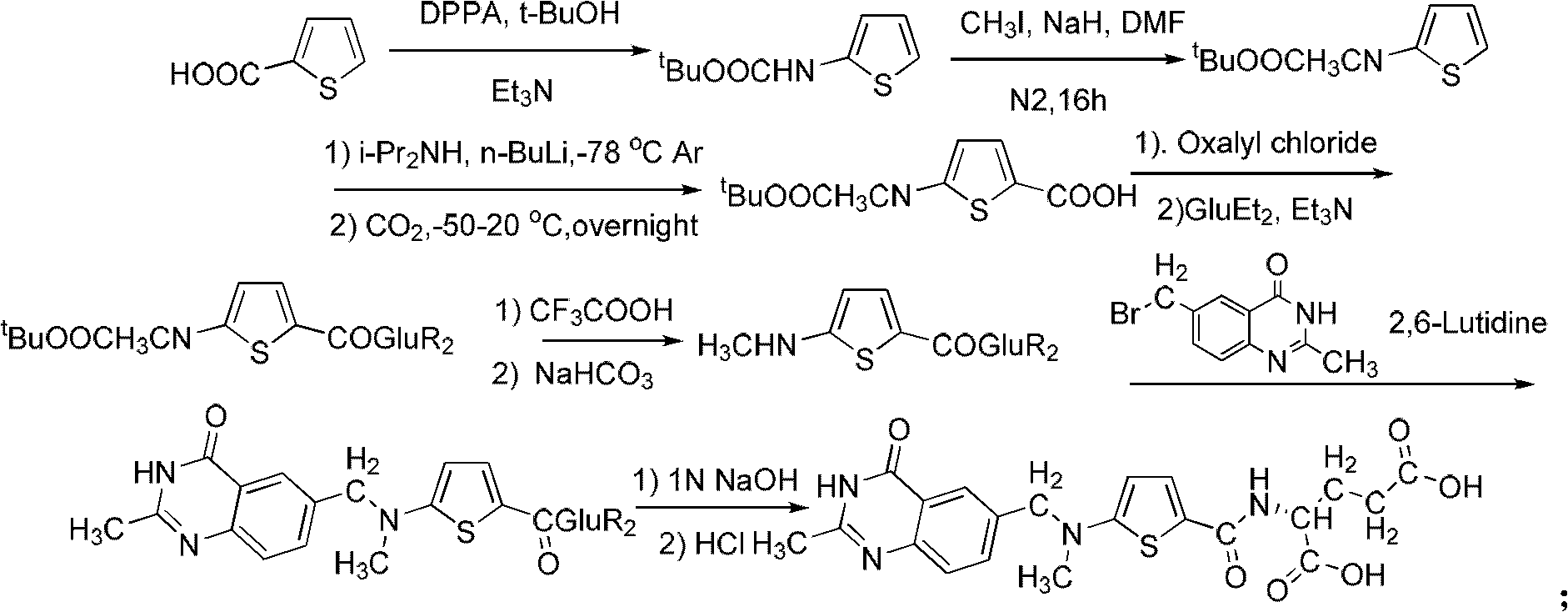
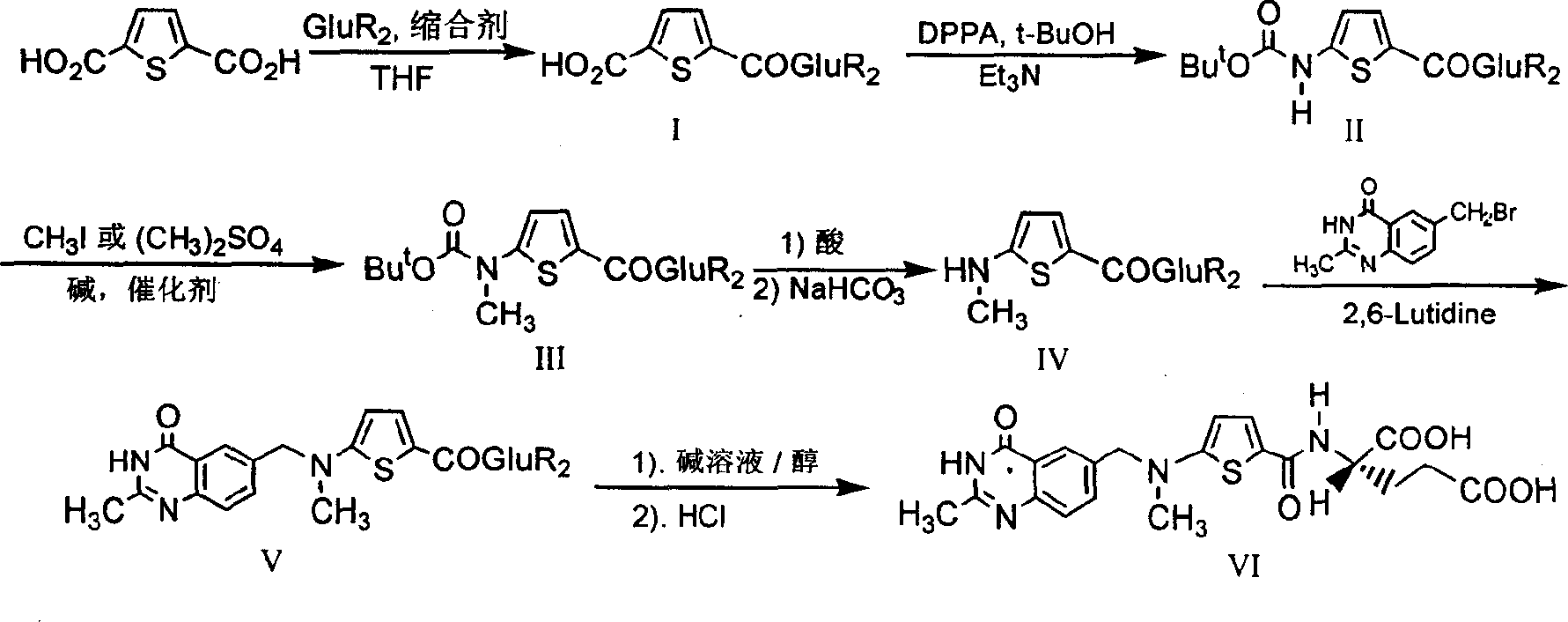
The invention relates to a new synthesis technology of anti-cancer drug Raltitrexed. The technology comprises the following steps: 1) using L-glutamic acid as raw material to perform esterification with alcohol under the action of halogenating agent and obtain L-glutamic acid diester hydrochloride; 2) using 2-amino-5-methyl-benzoic acid as raw material to prepare 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline through cyclization, amination and bromination; 3) using 2-thienyl-propanedioic acid as raw material to prepare N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester through nitrification, esterification, reduction, amino protection, N-methylation and device-esterification; 4) using L-glutamic acid diester hydrochloride and N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester to prepare N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester through dehydrant condensation and deamination protection; and 5) using N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester and 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline to perform condensation under the catalysis of alkali, recycling preparative chromatography, purifying, and performing de-esterification to obtain Raltitrexed.
Owner:深圳市普迈达科技有限公司
Synthesis of anticancer medicine Raltiprexed
InactiveCN1486985AShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
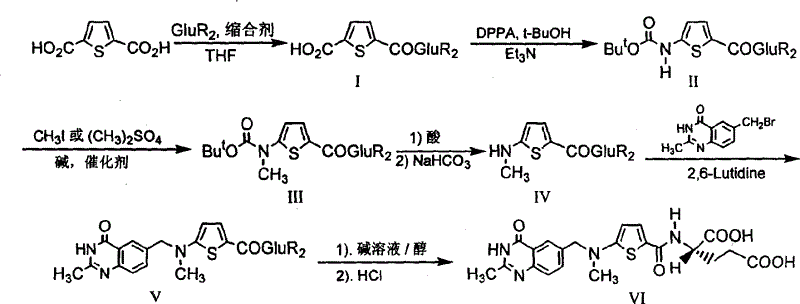
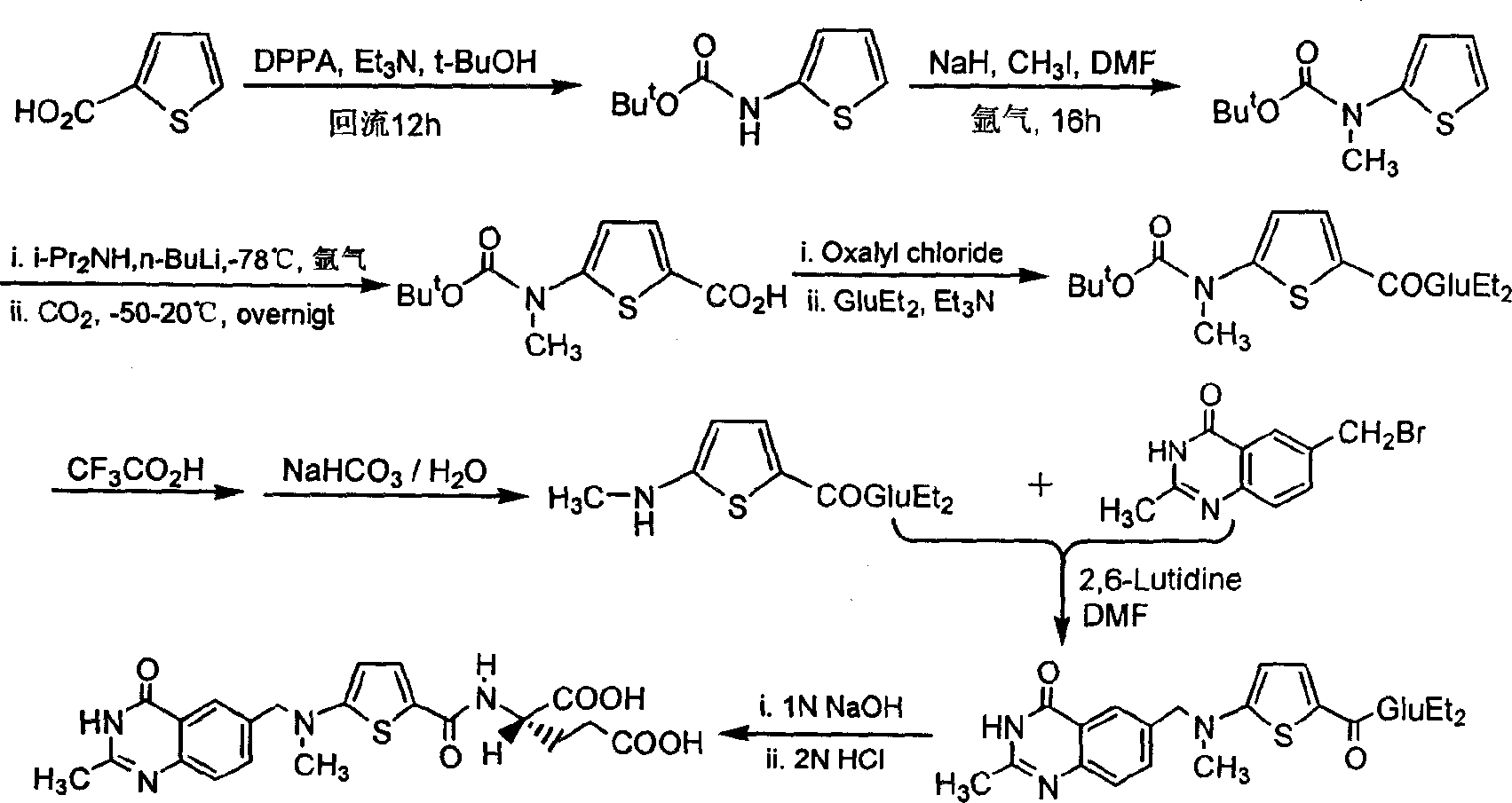
The synthesis of anticancer medicine Raltitrexed with 2, 5-thienyl diformic acid and diethyl glutamate as initial material and through six reaction steps of monocondensation, rearrangement, N-methylation, e;limination of tert-butoxy carbonyl group, condensation with 6-bromomethyl-2-methyl-4-quinbolone and saponification. The present invention has total yield of 18.1%, higher than that of available synthesis line, less reaction steps, mild condition and simple operation, and is suitable for mass production.
Owner:CAPITAL NORMAL UNIVERSITY
Preparation method of arbidol hydrochloride
InactiveCN102351778AHigh reaction yieldRaw materials are easy to getOrganic chemistryN methylationSolvent
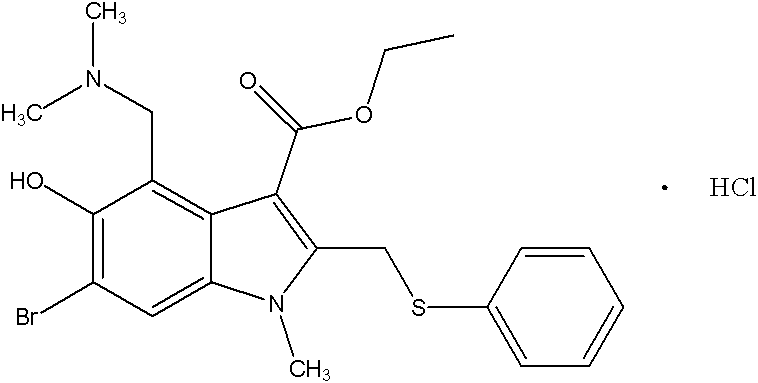
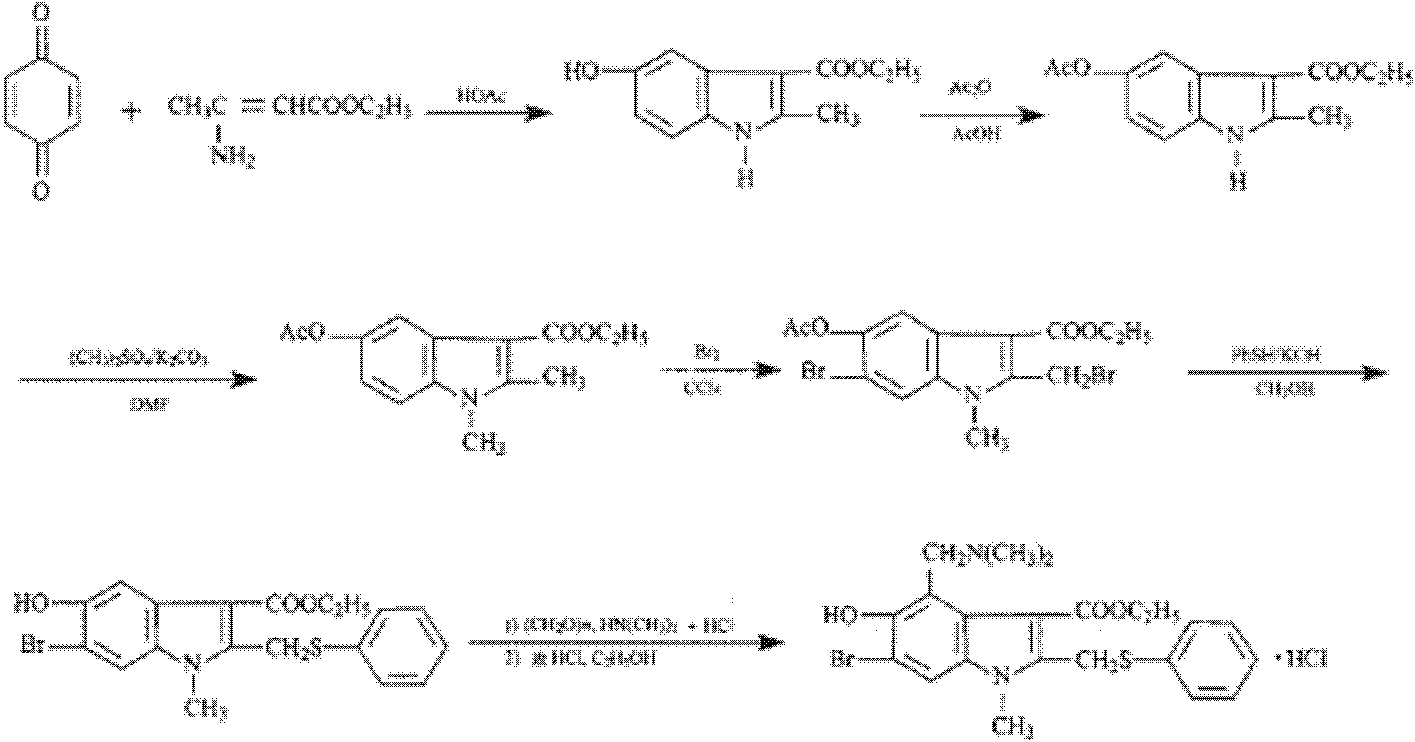
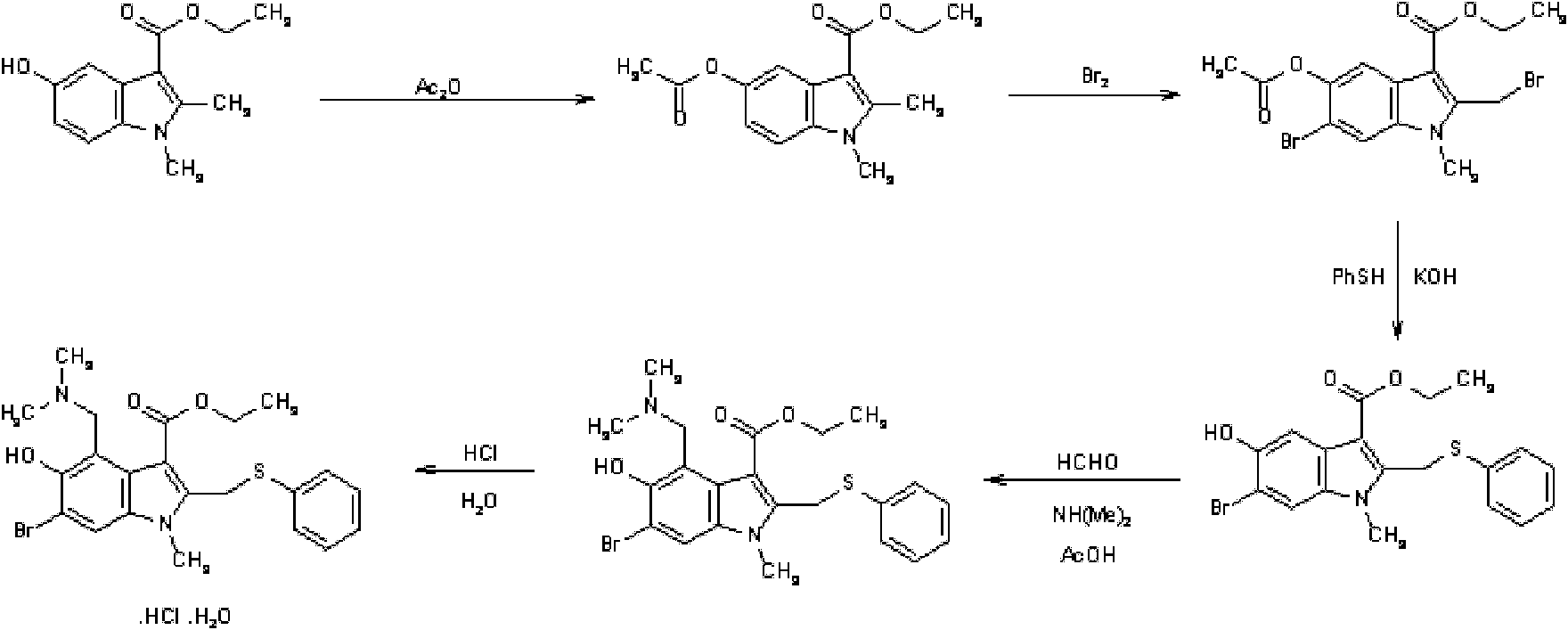
The invention discloses a preparation method of arbidol hydrochloride. The preparation method comprises the following steps of: (1) performing hydroxylation reaction on 3-iodo-4-nitrophenol serving as a raw material by using acetyl chloride to obtain a compound I; (2) performing substitution reaction on the compound I and ethyl acetoacetate under the action of alkali to obtain a compound II; (3) performing reduction-condensation concerted reaction on the compound II under the conditions of acetic acid and iron powder to synthesize an indole ring, and obtaining a compound III; (4) performing N-methylation reaction on the compound III by using dimethyl sulfate to obtain a compound IV; (5) performing di-bromination reaction on the compound IV in carbon tetrachloride by using bromine to obtain a compound V; (6) reacting the compound V and thiophenol under the alkali condition, and obtaining a compound VI after acidifying; (7) preparing a compound VII from the compound VI in the presence of a reaction solvent, namely aqueous solution of dimethylamine and formaldehyde; and (8) acidifying the compound VII in hot acetone to obtain the arbidol hydrochloride. The preparation method is facile in raw materials, mild in reaction conditions, high in yield, easy in separation and purification, low in cost and suitable for industrialized production.
Owner:湖北华龙生物制药有限公司
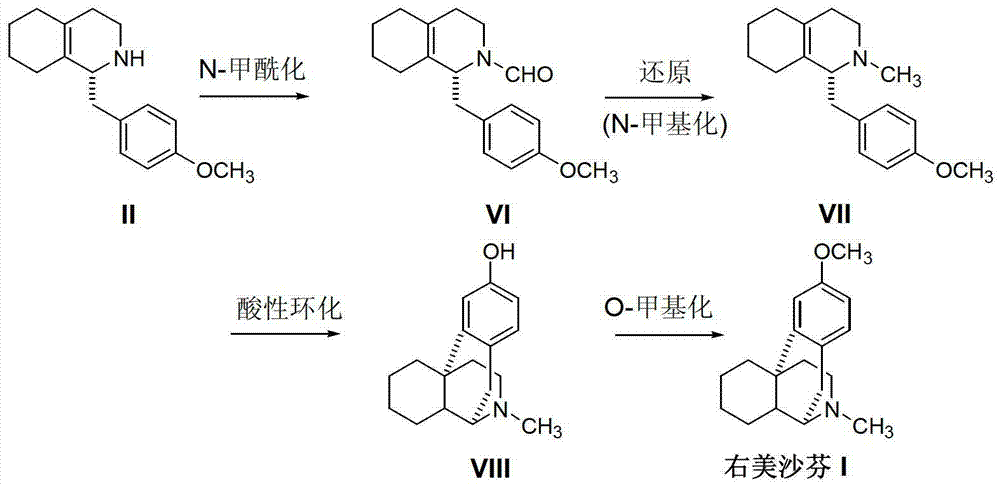
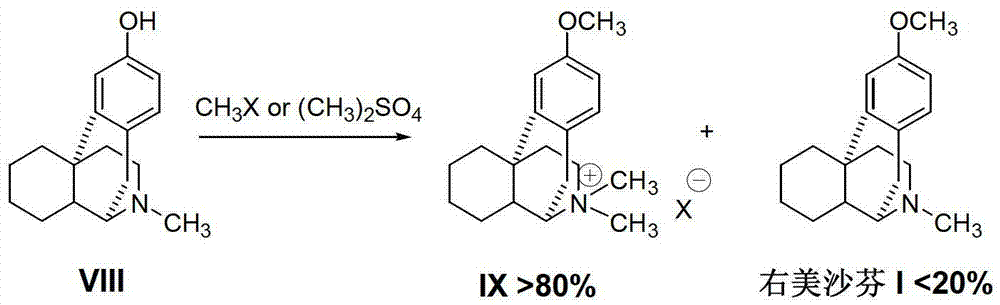
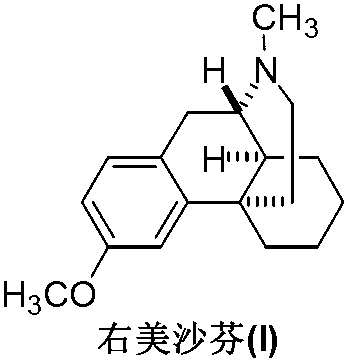
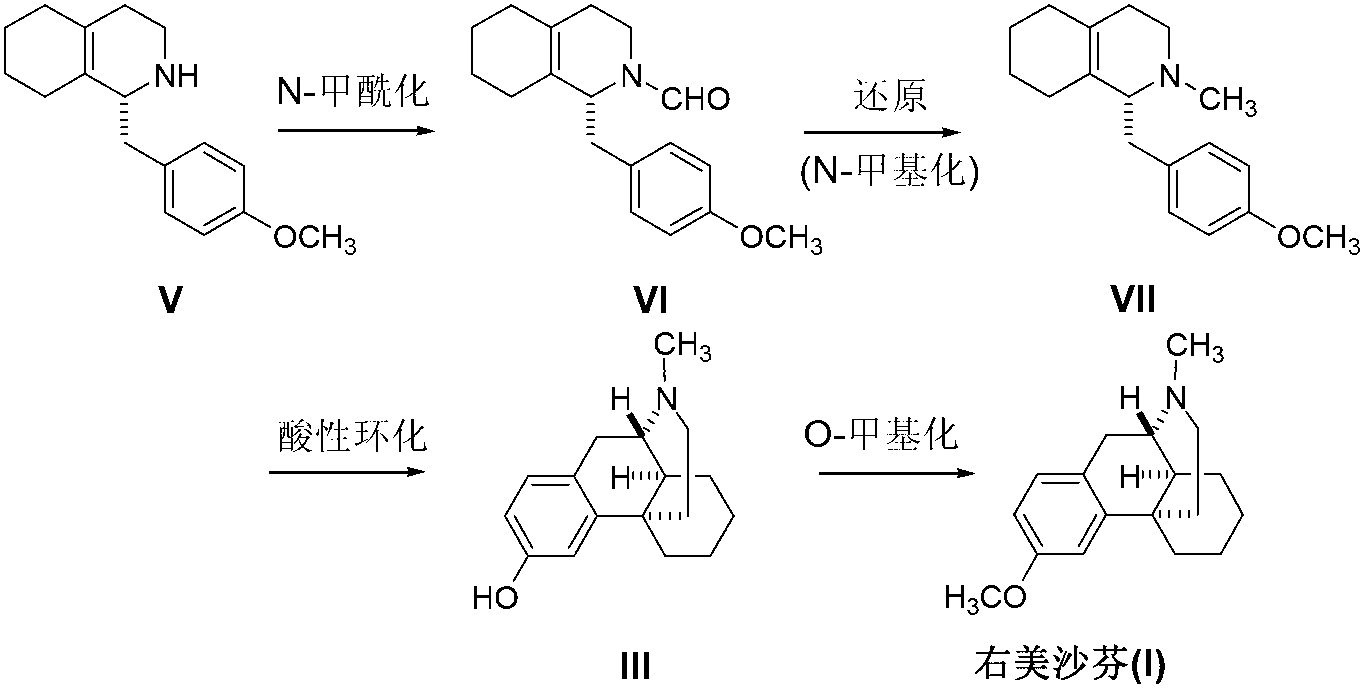
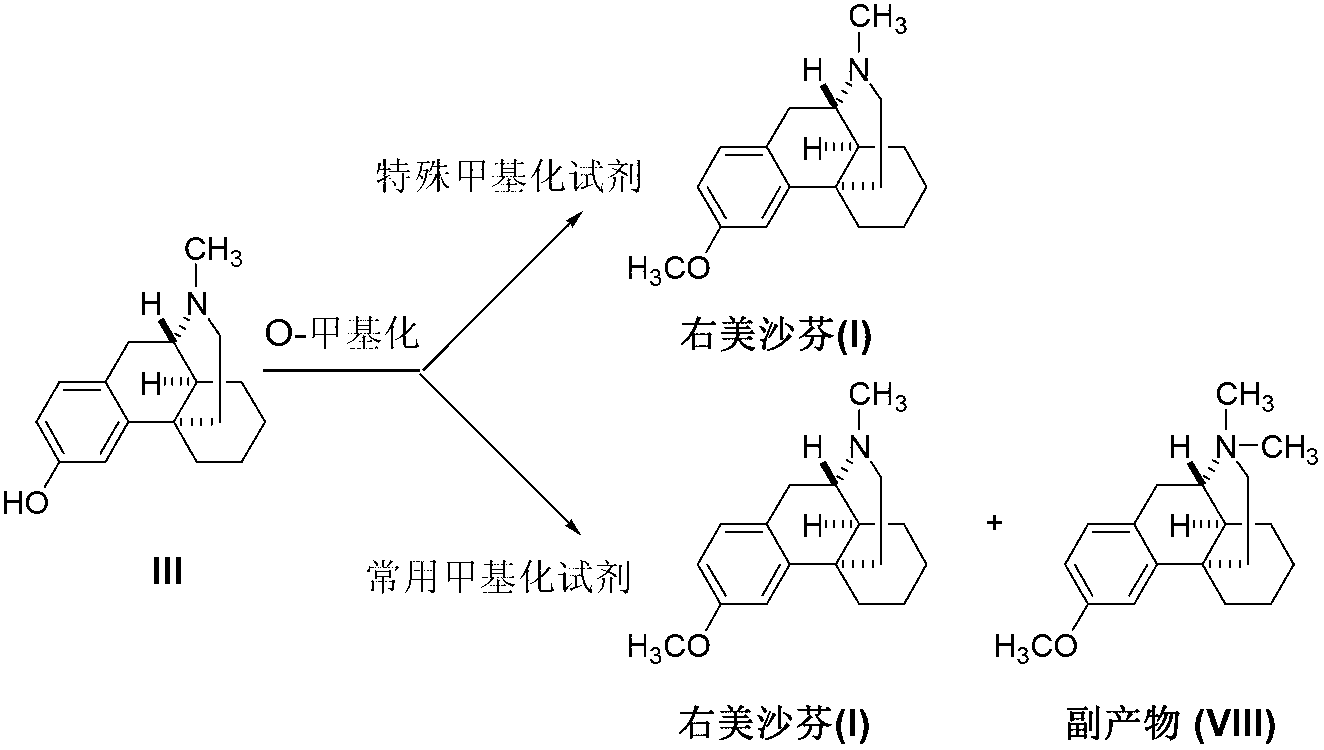
Preparation method of dextromethorphan
ActiveCN103044327AReduce manufacturing costPromote the development of economy and technologyOrganic chemistryIsoquinolineN methylation
The invention discloses a preparation method of dextromethorphan ((+)-3-methoxy-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane, I). The method comprises the following steps that a dextromethorphan intermediate (+)-1-(4-methoxy) benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (II) conducts N-benzylation reaction with a benzylation reagent under alkaline conditions to form (+)-1-(4-methoxy) benzyl-N-benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (III); the intermediate (III) is subjected to acid cyclization reaction to form (+)-3-hydroxy-17-benzyl-(9 alpha,13 alpha,14 alpha)-levorphane, (IV); the intermediate (IV) reacts with dimethyl sulfate or methine halide, and is subjected to O-methylation and N-methylation reaction to form (+)-3-methoxy group-17-benzyl-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane quaternary ammonium salt (V); (V) is subjected to catalytic hydrogenation reaction; benzyl is removed; and dextromethorphan (I) is obtained. Therefore, the preparation method can use a common and cheap methylation reagent to substitute an unusual methylation reagent such as phenyltrimethylammonium hydroxide, and the yield of reaction can be increased.
Owner:SUZHOU LIXIN PHARMA
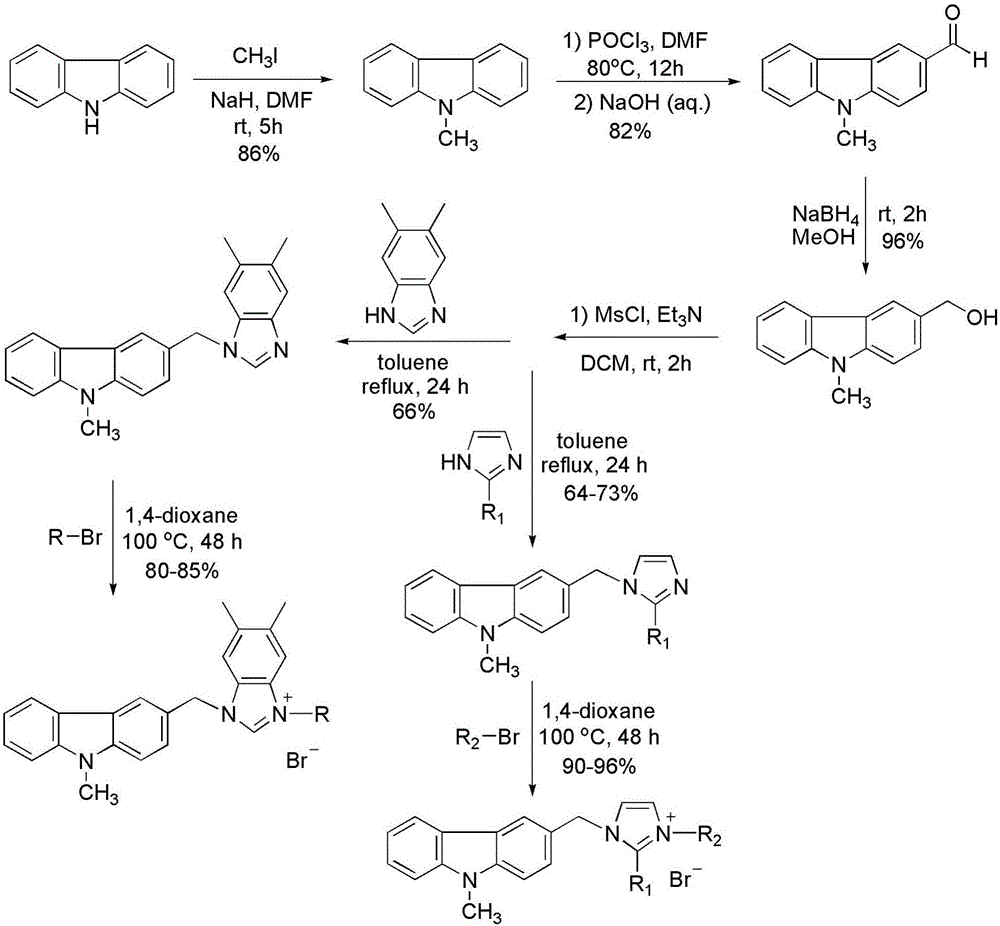
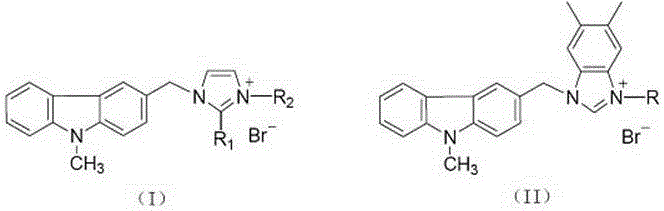
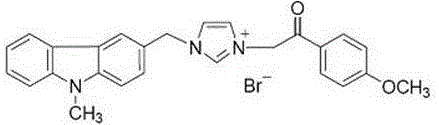
Substituted carbazole-imidazolate or benzimidazolium salt compounds, and preparation method thereof
The invention discloses a series of substituted carbazole-imidazolate or benzimidazolium salt compounds with a general structure represented by formula I or II, and a preparation method thereof. According to the preparation method, carbazole is taken as a raw material, and the target compound is obtained via five steps including N-methylation, acylation, reduction, combination with imidazole, and salifying. It is shown by results of in vitro antitumor activity cytotoxicity test that the substituted carbazole-imidazolate or benzimidazolium salt compounds possess excellent cytotoxic activity.
Owner:YUNNAN UNIV
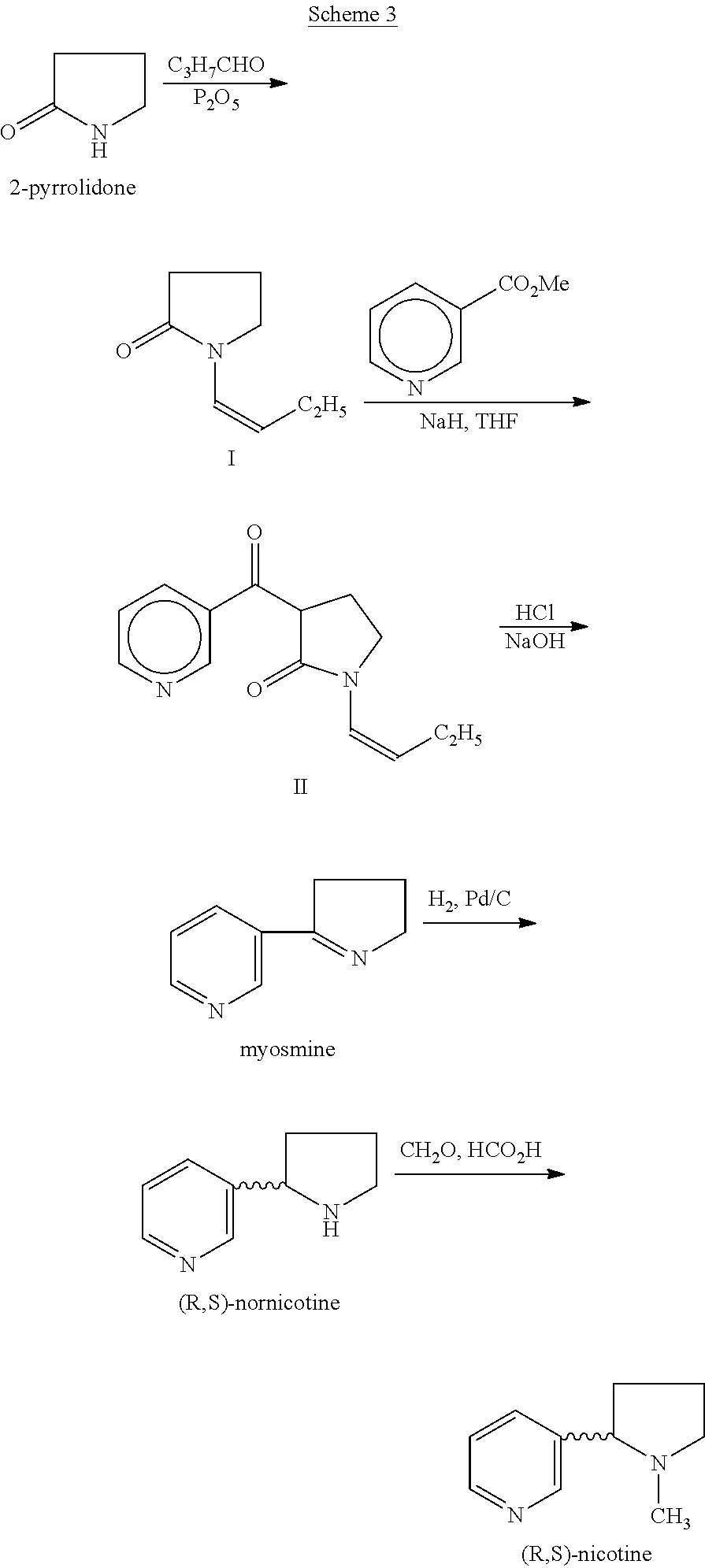
Process for the preparation of (r,s)-nicotine
Owner:DIVI S LAB LTD
Preparation method of N-methylamine compound
ActiveCN106316866AHigh catalytic activityImprove catalytic selectivityOrganic compound preparationAmino compound preparationReaction rateN methylation
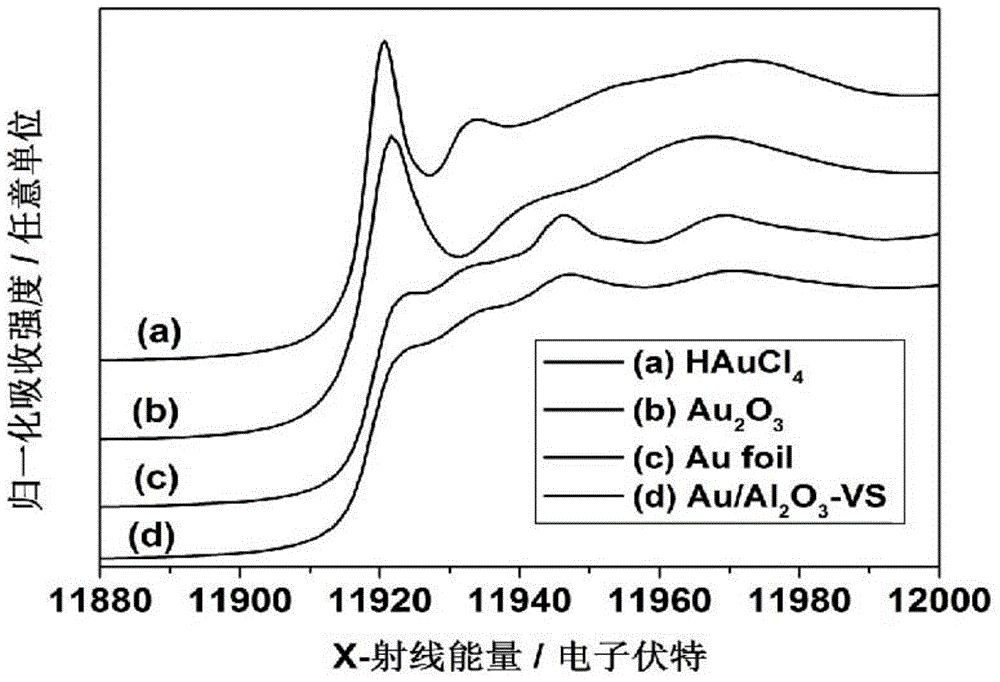
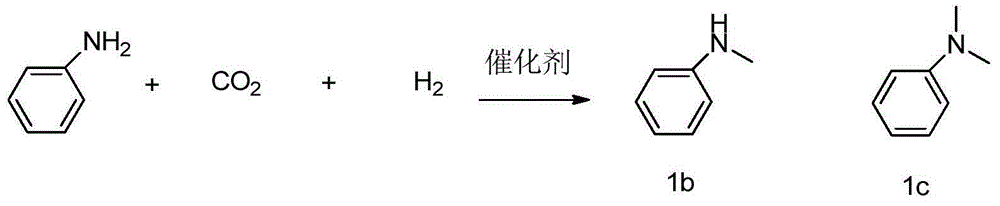
The invention discloses a preparation method of a N-methylamine compound. The preparation method comprises the following steps: under an inertia organic solvent or solvent-free condition and under a support-type nano-sized gold catalyst effect, a primary amine compound or a secondary amine compound is subjected to a N-methylation reaction with carbon dioxide and hydrogen to obtain the product. The preparation method takes CO2 as a methyl source, takes hydrogen as a reducing agent, and takes the support-type nano-gold as a catalyst, and has the advantages that process is simple, catalyst activity is high, reaction rate is fast, the catalyst recovery and utilization are convenient, the application scope of a substrate is wide, the production cost is low, the benifit is high, the post-treatment is simple, repeatability is good, safe performance is high, and environmental protection is achieved, and the method is adapted to industrial production.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
Method for catalysis of N-methylation of amines by using iridium complex with methanol as carbon source
InactiveCN110372517AImprove affordabilityMeet the basic requirements of green chemistryOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumSynthesis methods
The invention provides a method for catalysis of N-methylation of amines by using an iridium complex with methanol as a carbon source, and belongs to the technical field of energy and homogeneous catalysis. The methanol is used as the carbon source, and under the catalysis of the iridium complex, the reaction of N-methylation of various amine substrates is achieved. The method has the advantages that the methanol is used as the carbon source, toxicity is low, the cost is low, obtaining is easy, and economic applicability is higher; an only by-product is water, and requirements of green chemistry in nowadays society are met; the used preparation method of an iridium complex catalyst is easy, the cost is low, and stability is good; N-methylation of various primary amines and secondary aminescan be high-selectively achieved, and the product yield is excellent. The invention provides a green synthetic method for N-methylation reaction of the amines.
Owner:DALIAN UNIV OF TECH
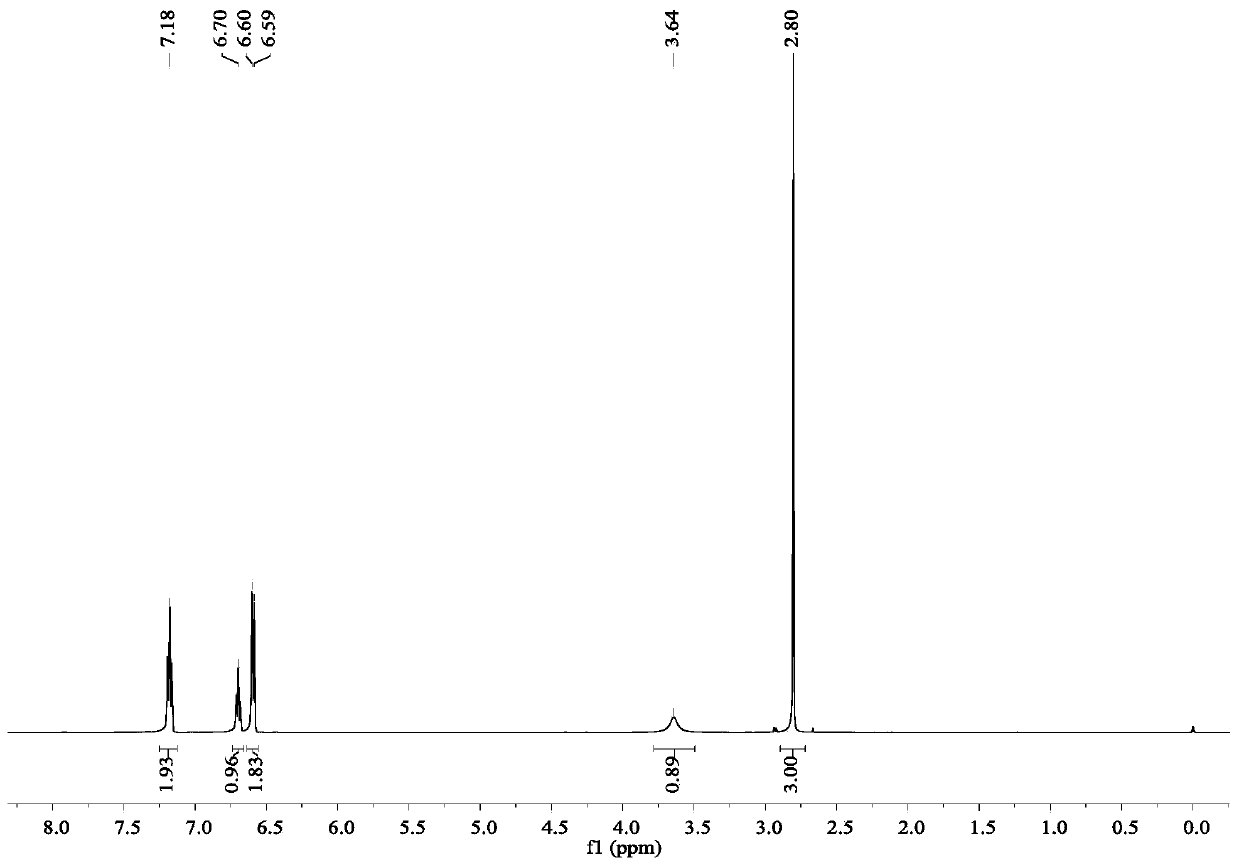
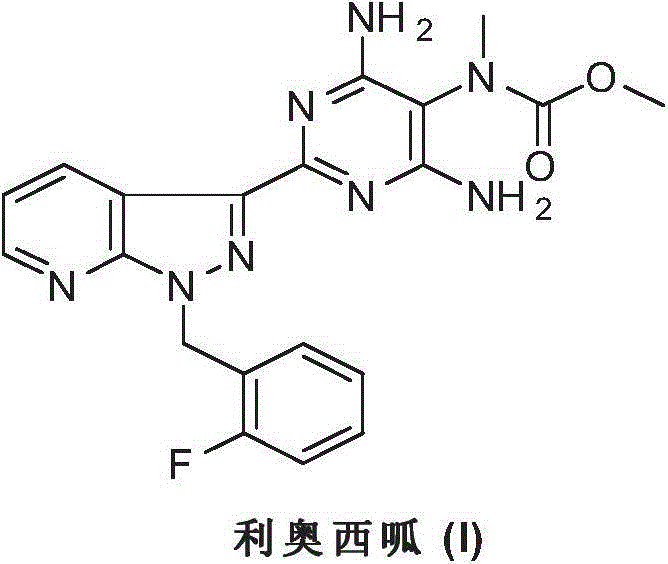
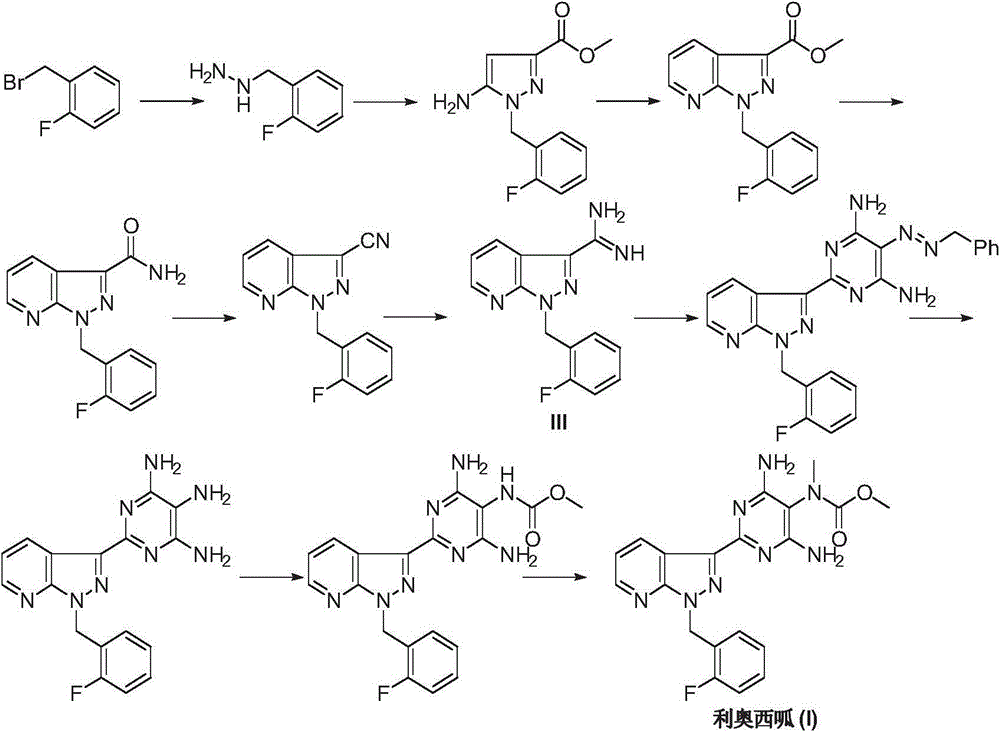
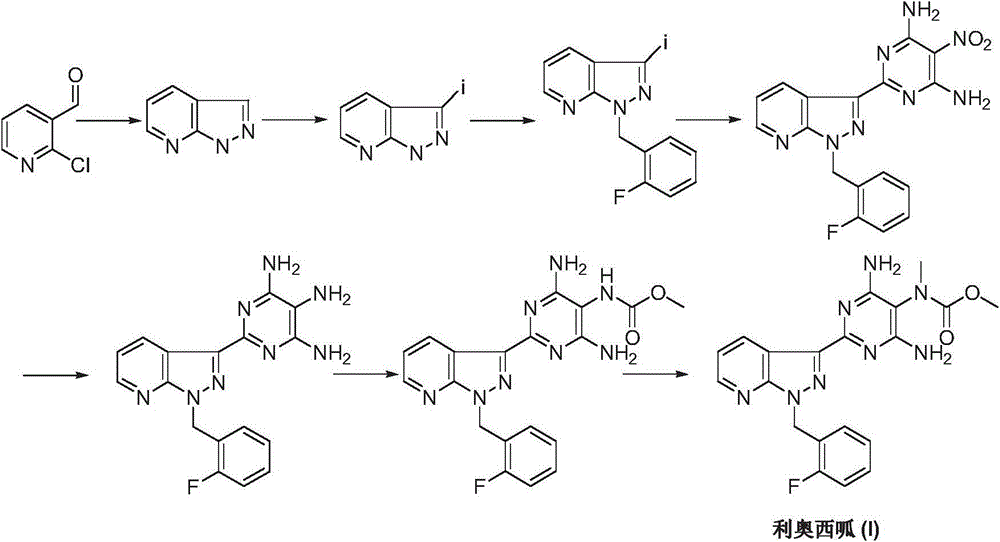
Riociguat intermediate and preparation method thereof
InactiveCN104892459AEase of industrial productionRaw materials are easy to getCarbamic acid derivatives preparationOrganic compound preparationAminomalononitrileN methylation
The invention discloses a Riociguat (I) intermediate, namely, N-methyl-N-methyl formate-2-amino malononitrile (II), and a preparation method thereof. The preparation method comprises preparation steps as follows: 2-amino malononitrile has an N-methylation reaction to prepare N-methyl-2-amino malononitrile (IV); N-methyl-2-amino malononitrile (IV) and methyl chloroformate or methyl bromoformate have an amidation reaction to prepare N-methyl-N-methyl formate-2-amino malononitrile (II). The intermediate II and 1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b] pyridine-3-formamidine (III) have a cyclization reaction to prepare Riociguat (I). According to the preparation method, the raw materials are easy to obtain, the process is concise, and the preparation method is economic and environment-friendly and suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Method for implementing amine compound N-methylation by photocatalysis
ActiveCN106986776ALow costLow toxicityOrganic compound preparationAmino compound preparationPhotocatalytic reactionN methylation
The invention belongs to the technical field of photocatalysis synthesis, and particularly relates to a method for implementing amine compound N-methylation by photocatalysis. The method includes the steps: 1) adding photocatalyst TiO2 and N-methylation carbon sources into a photocatalysis reactor to form a reaction system, and stirring the reaction system under ultraviolet irradiation; 2) adding reducing agents and amine compounds into the reaction system obtained in the step 1), and continually stirring mixture under ultraviolet irradiation to obtain N-methylation products. Compared with the prior art, the preparation method has the main advantages that selected raw materials are low in cost and toxicity, reaction conditions are mild, energy consumption is less, used catalysts are low in cost and easy to obtain, reaction operations are simple, and products are high in yield and good in selectivity.
Owner:INST OF CHEM CHINESE ACAD OF SCI
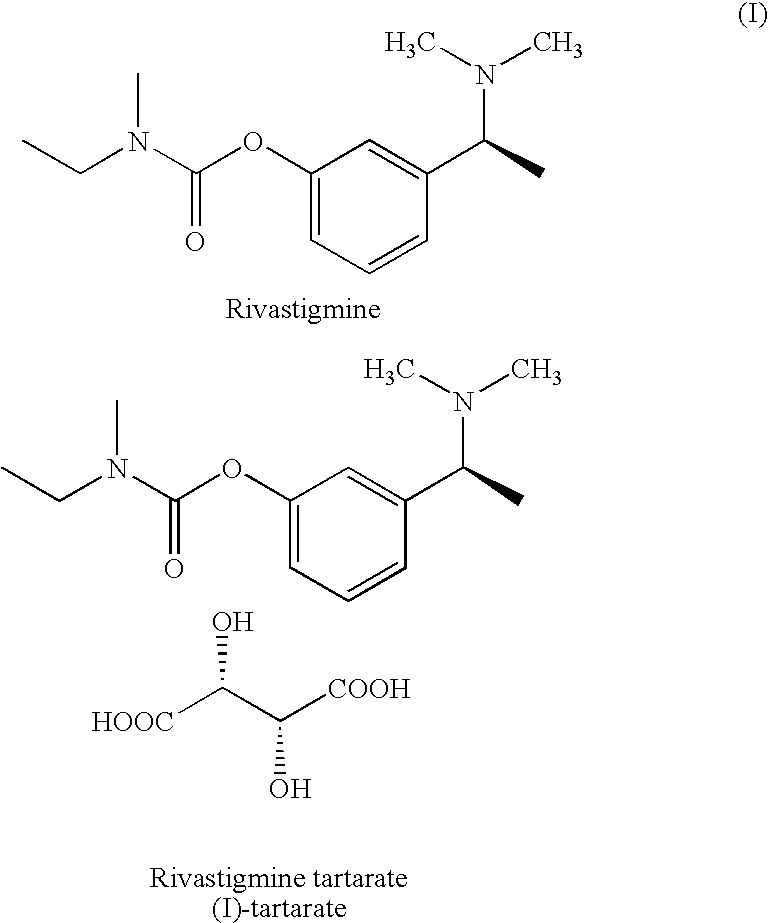
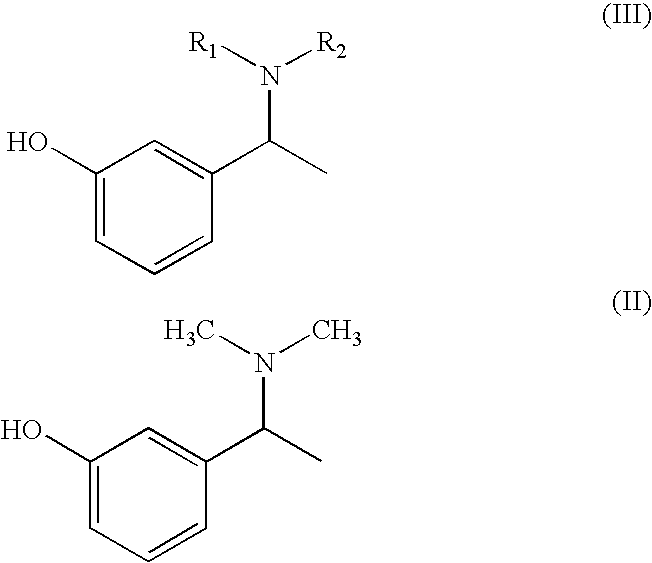
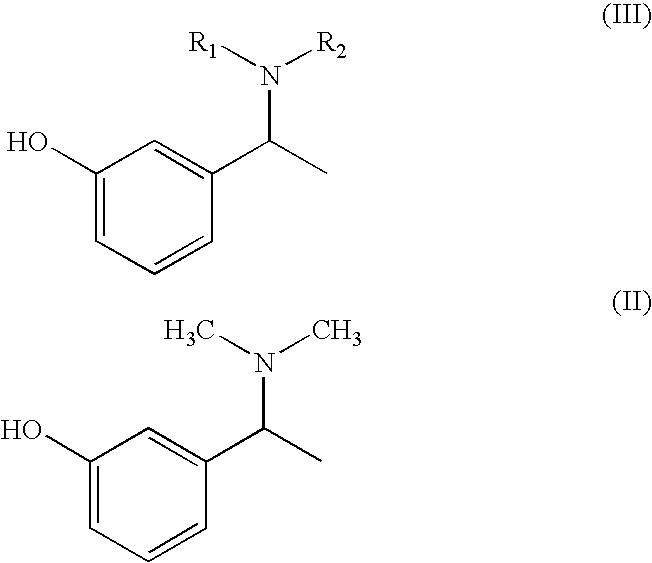
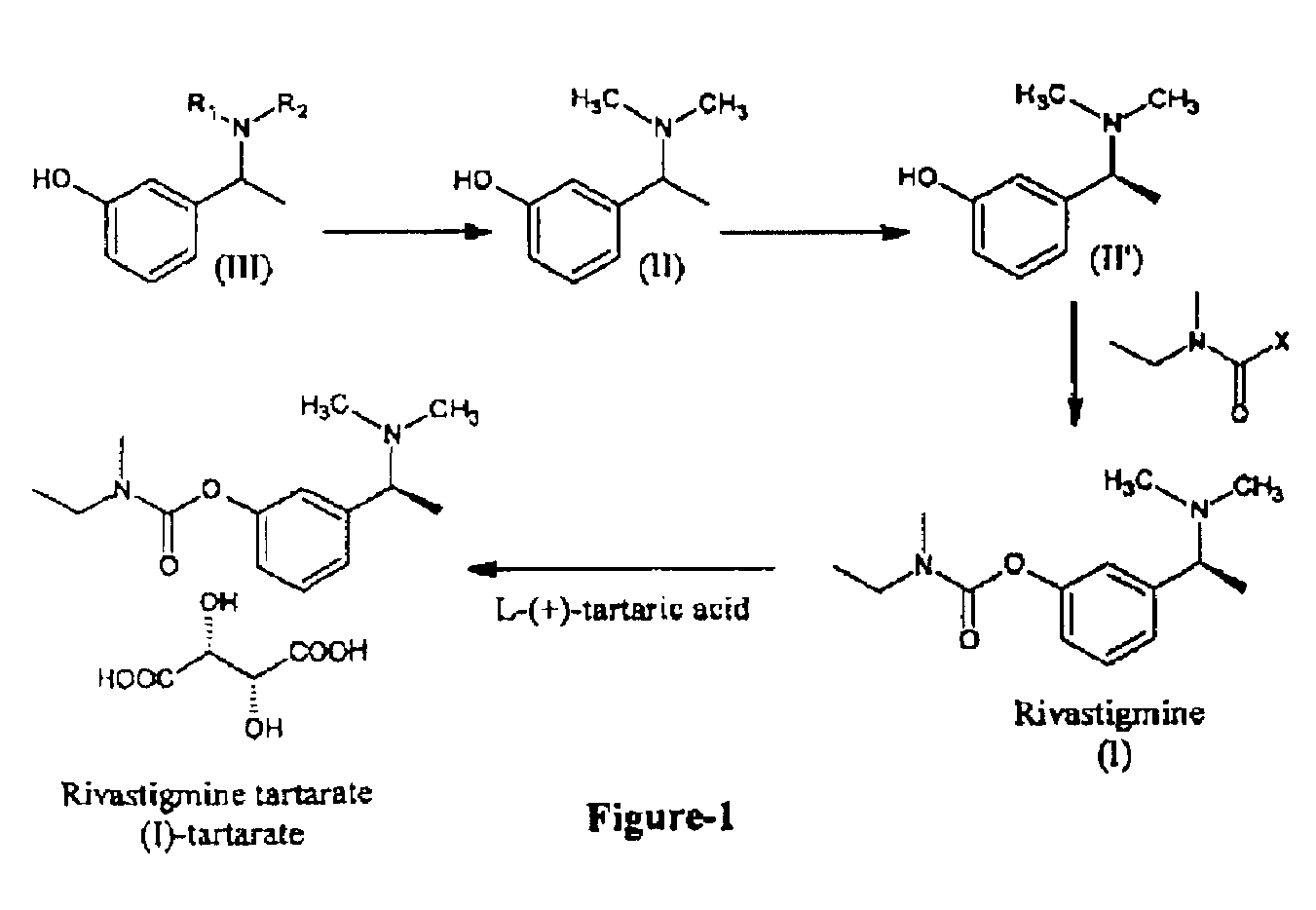
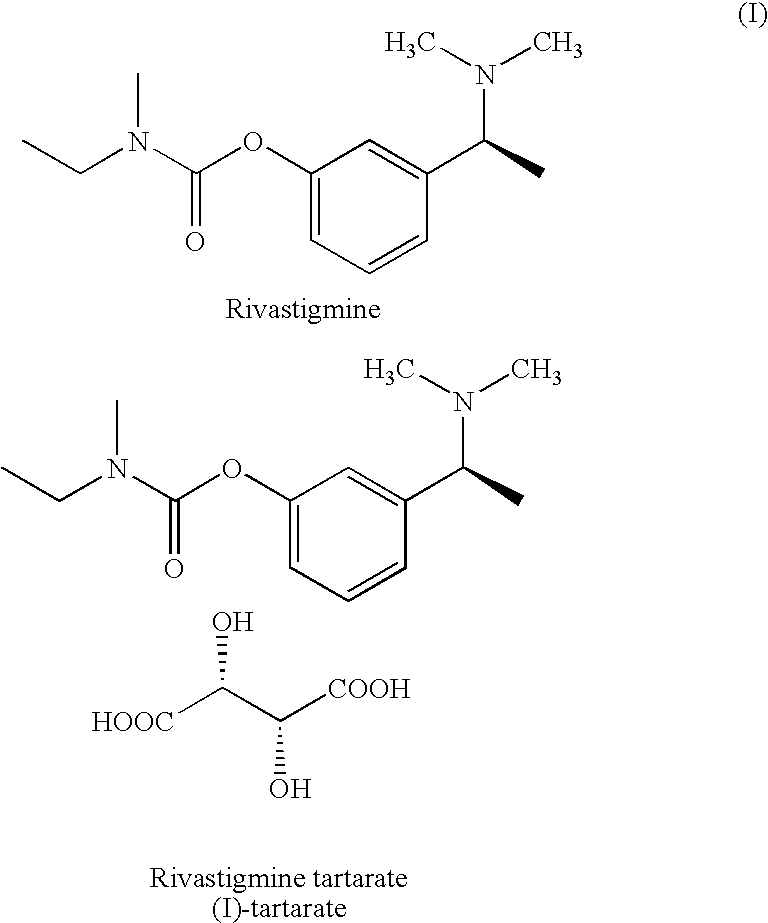
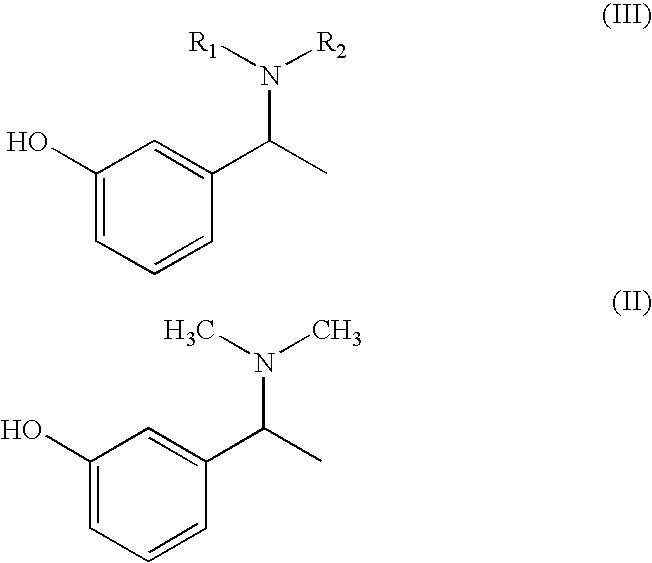
Process for the preparation of Rivastigmine
InactiveUS20080045743A1High yieldShort reaction timeCarbamic acid derivatives preparationOrganic compound preparationHydrogenN methylation
The present invention relates to an improved process for preparation of Rivastigmine of formula (I) or pharmaceutically acceptable salts thereof comprising a step of N-methylation of compound of formula (III), wherein R1=R2=H or R1=H and R2=CH3 or an acid addition salt thereof, using paraformaldehyde in the presence of Raney Nickel and hydrogen in a suitable solvent to obtain compound of formula (II).
Owner:ALEMBIC LTD
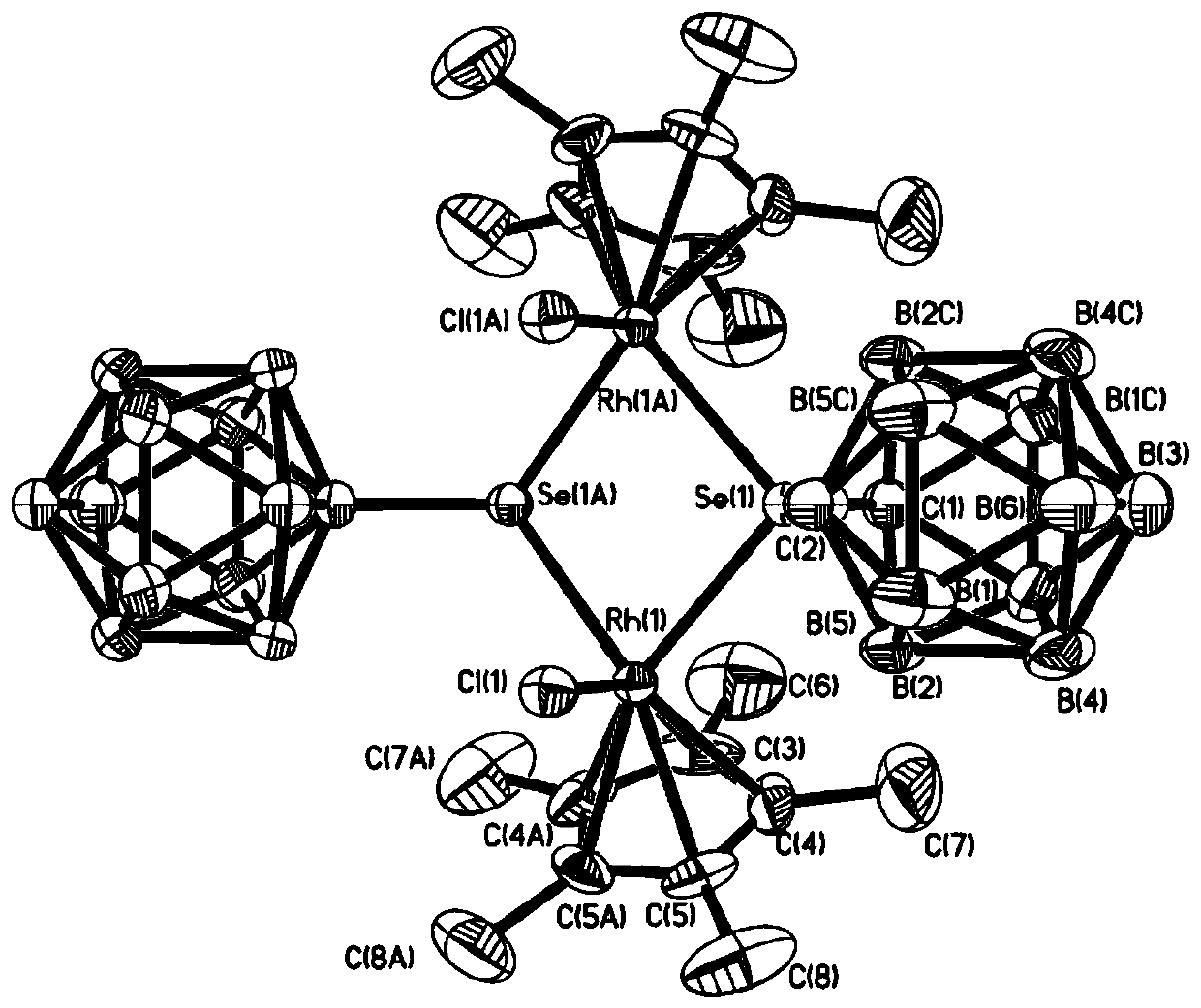
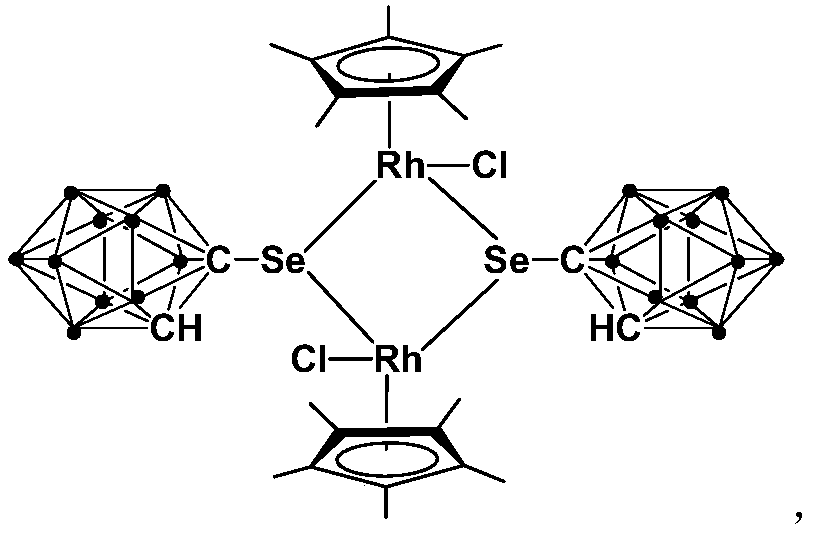
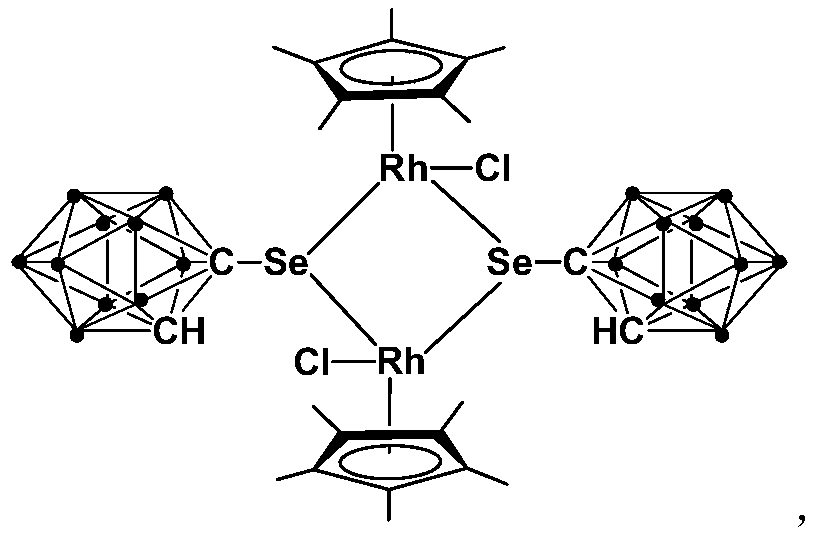
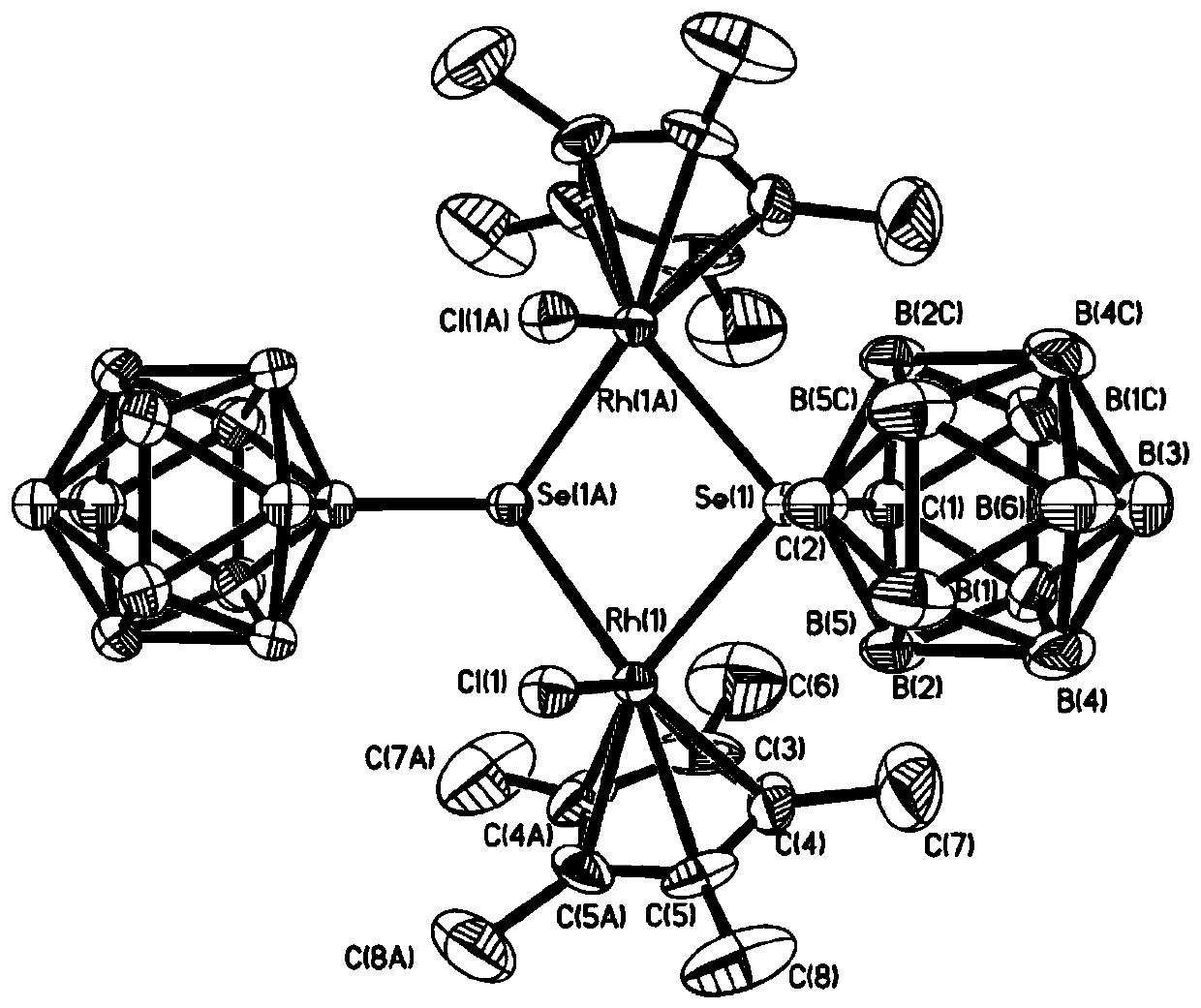
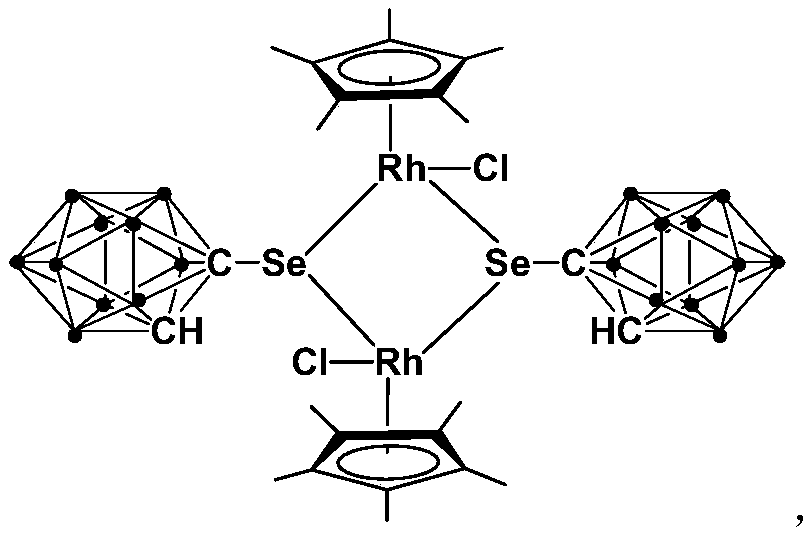
Binuclear rhodium complex containing ortho-carborane structure and preparation and application thereof
ActiveCN110240620AThe synthesis process is simple and greenGood choiceRhodium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsN methylationRoom temperature
The invention relates to a binuclear rhodium complex containing an ortho-carborane structure and preparation and application thereof. The preparation method of the rhodium complex comprises the following steps: 1) adding an n-BuLi solution into an ortho-carborane solution, and then reacting for 30-60 min at room temperature; 2) adding selenium and reacting for 1-2h at room temperature; 3) adding [Cp*RhCl2]2, reacting at room temperature for 3-6h, and performing post-treating to obtain the rhodium complex; and the rhodium complex is used to catalyze arylamine N-methylation reaction to prepare arylamine N-methylated derivatives. Compared with the prior art, the preparation method of the binuclear semi-sandwich rhodium complex containing the ortho-carborane structure is simple and green, has excellent selectivity and higher yield, and the prepared rhodium complex has higher catalytic activity at room temperature, can be used for catalyzing aromatic amine N-methylation reaction to prepare the aromatic amine N-methylation derivatives, has high catalytic reaction yield, and has wide industrial application prospect.
Owner:SHANGHAI INST OF TECH
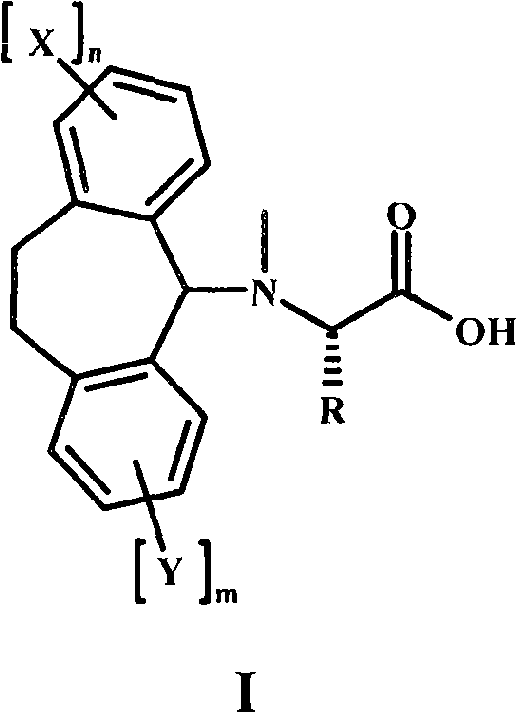
Alpha-n-methylation of amino acids
The present invention is directed to a method for alpha-N-methylation of amino acids suitable for on-resin methylation, compatible with Fmoc / tBu SPPS, and compatible with amino acids bearing protected or unprotected nucleophilic side-chains, notably Arg(Pbf), Met, Cys, and Trp. The present invention is further directed to a compound of Formula I wherein m, n, X and Y are defined as described herein.
Owner:F HOFFMANN LA ROCHE & CO AG
Process for the preparation of Rivastigmine
InactiveUS7683205B2High yieldShort reaction timeCarbamic acid derivatives preparationOrganic compound preparationHydrogenN methylation
Owner:ALEMBIC LTD
Method for preparing dextromethorphan
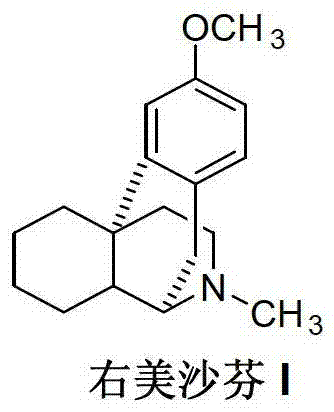
ActiveCN103073496AReduce manufacturing costPromote the development of economy and technologyOrganic chemistryN methylationDextromethorphan
The invention discloses a method for preparing dextromethorphan (I), which comprises the following steps: performing N-methylation by an intermediate ent-3-methoxymorphinan (II) to generate the dextromethorphan (I). The preparation method is simple and convenient, and favorable for reducing the production cost of the dextromethorphan and can improve the quality of the product.
Owner:SUZHOU LIXIN PHARMA
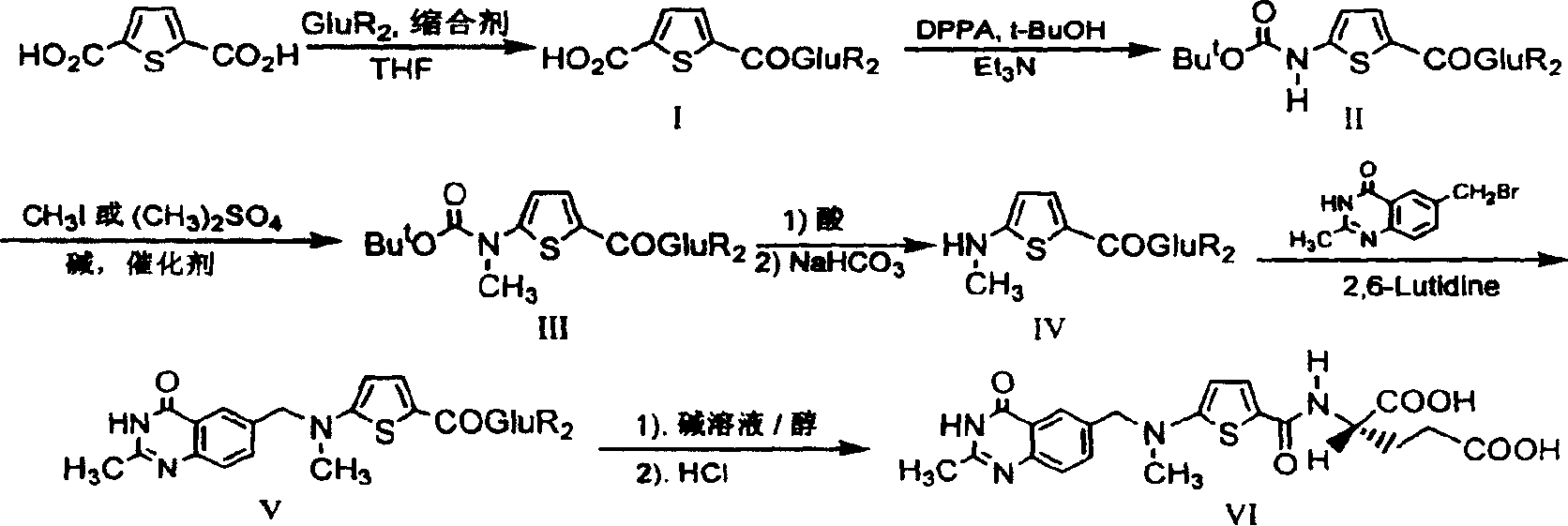
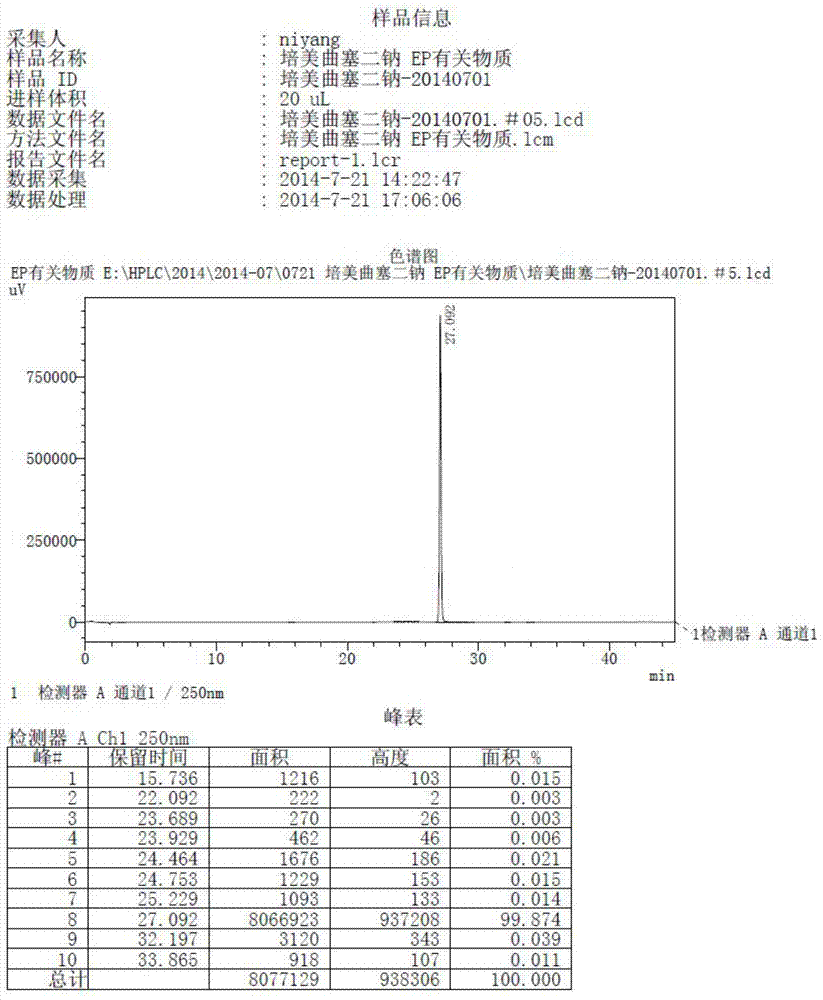
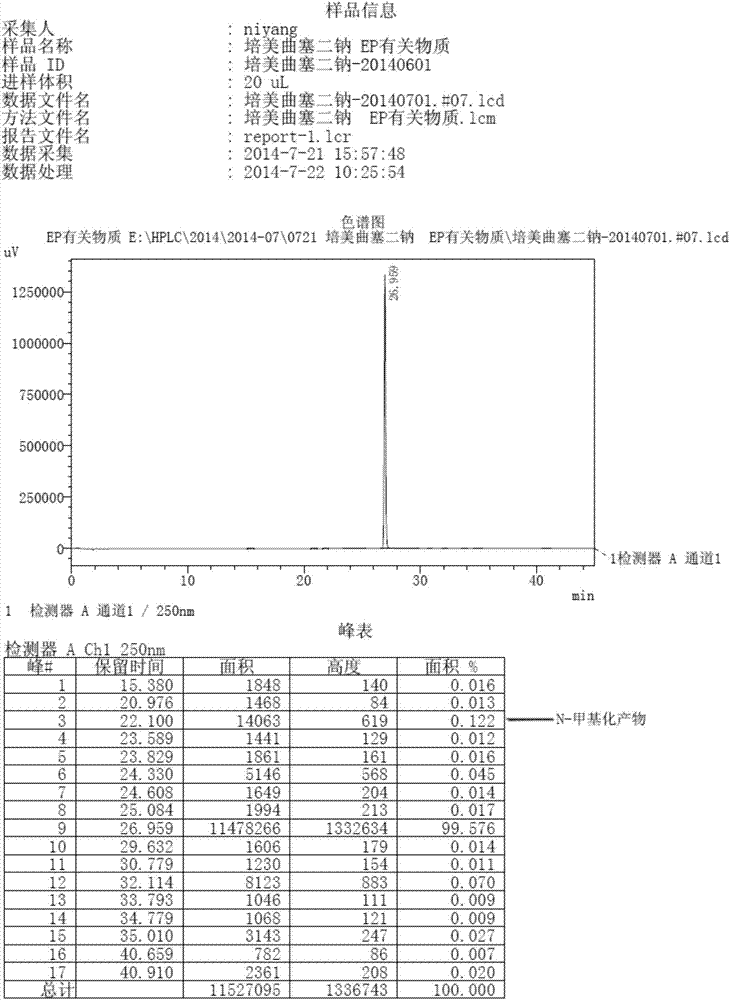
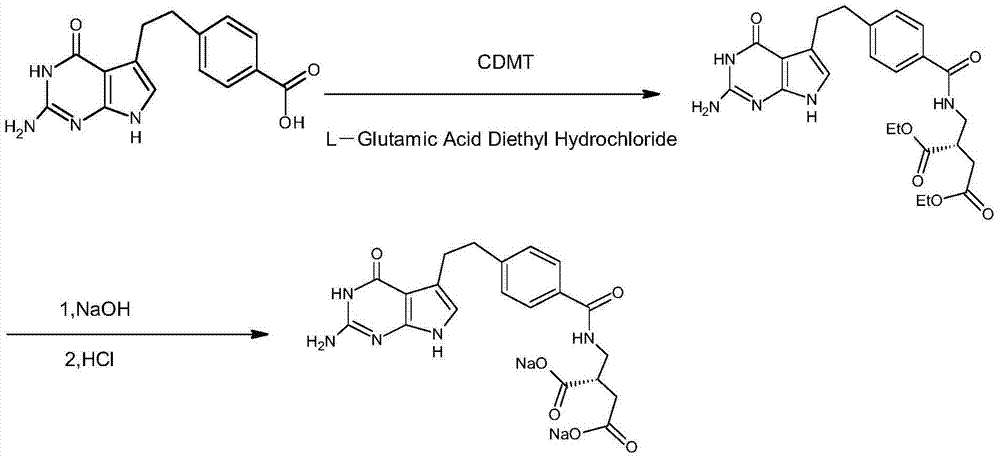
Preparation method of pemetrexed disodium
InactiveCN104119346AHigh yieldSimplify the manufacturing processOrganic chemistryTransient stateOrganic solvent
The invention discloses a preparation method of pemetrexed disodium, which can directly hydrolyzing into salt after an organic solvent is removed, rather than salifying together with p-toluenesulfonic acid and / or purifying a product by adopting a crystallization method during the preparation process, thus omitting the step of salifying together with p-toluenesulfonic acid and / or crystallization through ethanol, effectively improving the total yield of drugs to 68-75%; in the preparation process, a peptide condensing agent is firstly prepared and is fully reacted with pemedolac in the organic solvent into transient-state acid ester, so that the generation of N-methylation impurities can be effectively inhibited in the reaction together with L-glutamic acid diethyl ester hydrochloride, the defect that the content of N-methylation impurities during the one-pot preparation process in the prior art exceeds 0.1% can be avoided, the advantages for opening up Europe and America markets can be brought, the defect that the raw materials are not reacted thoroughly to cause high cost can also be overcome; the HPLC (High Performance Liquid Chromatograph) of the finished pemetrexed disodium obtained by adopting the method is more than or equal to 99.8%, and the content of single impurities is less than 0.1%.
Owner:NINGBO MENOVO PHARMA
N-methylation method of aromatic amine
InactiveCN103288660AHigh yieldEasy to operateAmino preparation from aminesOrganic compound preparationN methylationSolvent
The invention discloses an N-methylation method of an aromatic amine. The method comprises following steps: under protection of inert gas, taking dimethylsulfoxide as a solvent; subjecting the aromatic amine, formic acid and triethylamine to reaction with a mole ratio of 1:15-25:15-25; and then the N-methylated aromatic amine is obtained. Advantages are that: operation is simple, the method can be used for substrates of a wide range, the N-methylated aromatic amine can be obtained with high yield in the absence of catalysts, and the method is suitable for industrial production.
Owner:SHAANXI NORMAL UNIV
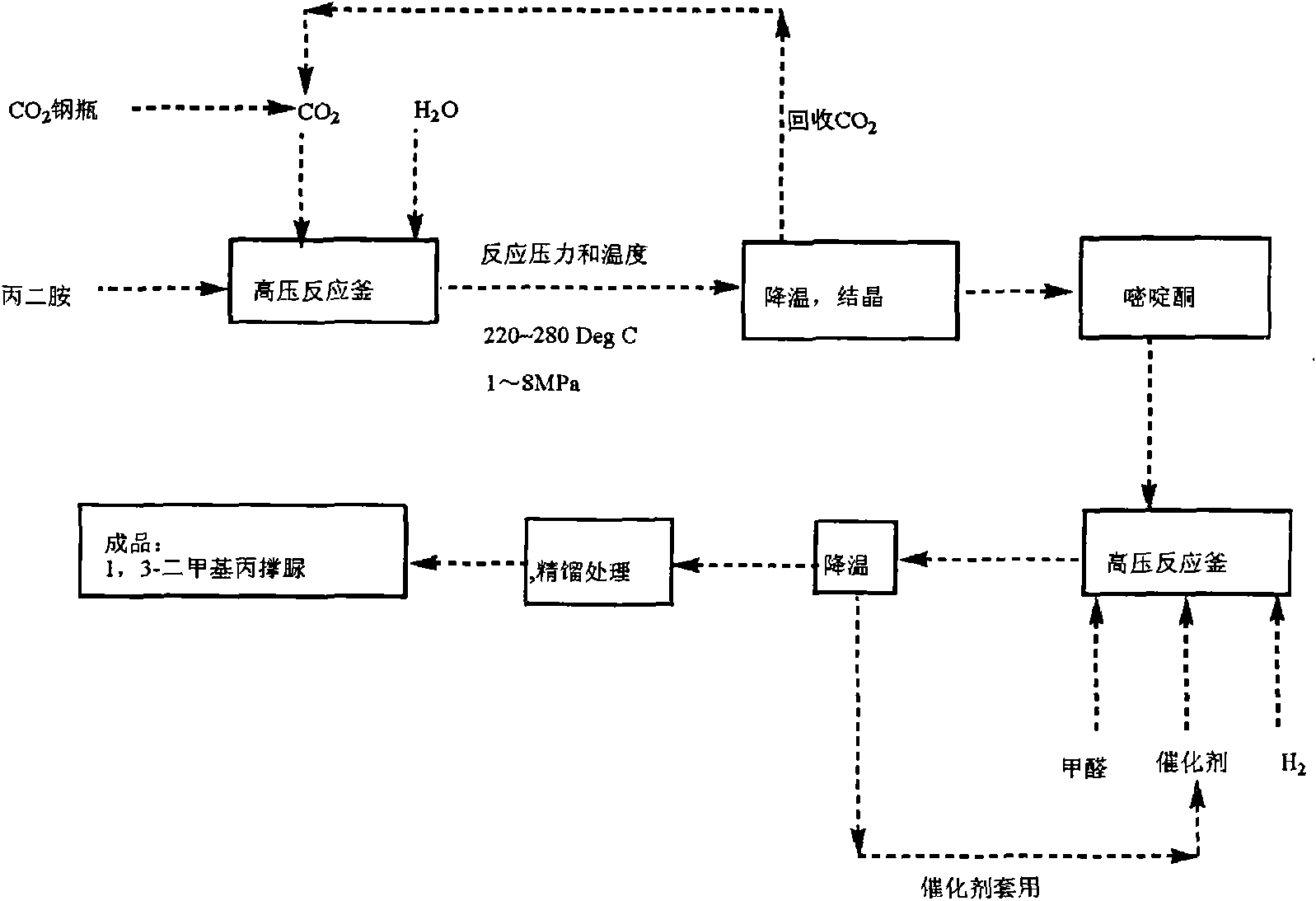
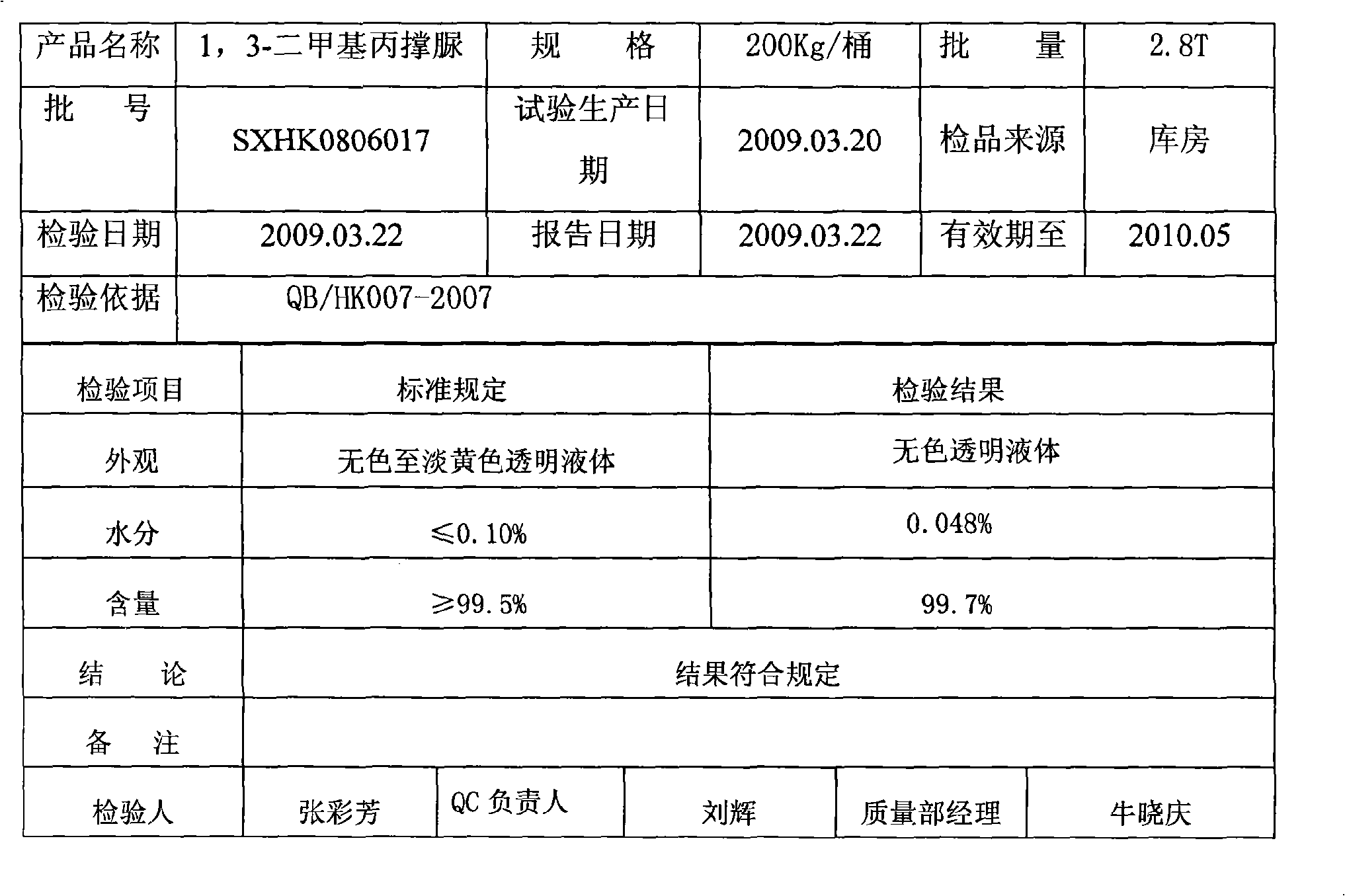
Improved production process of 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
InactiveCN101555233ASolve pollutionSolve production costOrganic chemistryChemical recyclingN methylationSolvent
The invention relates to an improved production process of 1, 3-Dimethyl-3, 4, 5, 6-tetrahydro-2(1H)-pyrimidinone, which is improved on the basis of a prior production process of 1, 3-Dimethyl-3, 4, 5, 6-tetrahydro-2(1H)-pyrimidinone. In the invention, in a step of synthesizing pyrimidone midbody, a carbon dioxide (or urea)high-temperature and high-pressure method is adopted so as to solve the problems of production process of pyrimidone midbody, environmental pollution of organic solvent and production cost; in a step of N-methylation, formaldehyde is used as a reactant to carry out hydrogenation to finish the N-methylation under the condition of an acidic catalyst, so that the problem of corrosion of methanoic acid and the problem of pollution of waste acid and waste water in the prior art are thoroughly solved. The production process is advanced, has no pollution and accords with green high-new fine chemical engineering manufacturing technique which is emphatically supported by the nation.
Owner:SHANXI HOOKIN PHARMA & CHEM
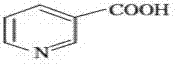
Preparation method of arecoline
The invention provides a preparation method of arecoline. On the presence of organic base, reaction raw materials such as nicotinic acid and the like have esterification and alkylation reactions with a compound capable of providing alkyl in the organic solvent, and arecoline can be prepared through a reduction reaction and aftertreatment. Two procedures including esterification and N-methylation are combined, and the process route of preparation of arecoline is shortened. According to the method, the operation conditions are simple, and the intermediate treatment is more convenient; expensive alkyl halide (such as methyl iodide) cannot be used as a reaction raw material, cheaper sulfolipid or phosphate ester compounds are selected, a good implementation effect is also obtained, and the product has a cost advantage.
Owner:QINGDAO KANGYUAN PHARMA
Method for preparing important chemicals by using lignin as methyl sources
InactiveCN109776302ACarboxylic acid nitrile preparationOrganic compound preparationAcetic acidCompound (substance)
The invention relates to preparation of important chemical products by using methoxyl groups in lignin. According to a method, methyl provided by the lignin reacts with carbon monoxide and water underthe catalysis effects of catalysts to obtain acetic acid; reaction is performed with aminated compounds to obtain N-methylation products. The methoxyl groups in the lignin are used for preparing important chemical products for the first time; an important and continuous production path is provided for the preparation of various important chemical products.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Azithromycin related substance and preparation method thereof
ActiveCN109293722AImprove medication safetyHigh puritySugar derivativesSugar derivatives preparationN methylationOrganic layer
The invention relates to an azithromycin related substance and a preparation method thereof, and belongs to the technical field of synthesis of heterocyclic compounds. The preparation method comprisesthe steps: with erythromycin imide ether as a raw material, dissolving, then adjusting the pH to 4-5, adding platinum carbon as a catalyst, introducing hydrogen gas, maintaining the pressure, and carrying out heat preservation reaction, wherein the platinum carbon is pre-treated before being used as the catalyst, wherein the pretreatment comprises the steps of stirring 5% platinum carbon in waterand adding metal ions and beating for half an hour; filtering the catalyst, recovering a material liquid thoroughly and adding acetone, adding formaldehyde formic acid, controlling the pH to 5-6, carrying out heat preservation and carrying out N methylation reaction; after the reaction is finished, extracting and separating to remove a saline water layer, adding water to an organic layer and crystallizing, to obtain a crude product; and purifying, and thus obtaining the finished product. The preparation method is applied in synthesis of azithromycin and the related substance thereof, and theazithromycin not only can be used as a reference substance applied for qualitative and quantitative analysis of azithromycin impurities, but also can be conducive to improvement of the pharmacy safetyof azithromycin.
Owner:ZHEJIANG GUOBANG PHARMA +1
5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method
InactiveCN103435549AReduce manufacturing costSimple preparation processOrganic chemistryPhenylboronic acidEthyl chloroformate
The invention relates to a 5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method, wherein the method takes bromoaniline and phenylboronic acid as raw materials, a Suzuki coupling reaction is conducted to get 2-aminobphenyl, amino is protected with ethyl chloroformate, then potassium tert-butoxide is used as an acid-binding agent, the 2-aminobphenyl is subjected to N-methylation with methyl iodide, deprotected with amino, reacted with chloracetyl, and subjected to a Friedel Craft cyclizing hydrocarbylation to get the target product. The 5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method has the advantages of simple process, high overall yield and low production cost, and is applicable to industrial mass production.
Owner:HUIFEICHEM WUXI PHARMATECH CO LTD
Production method for 1,2-N,N'dimethylcyclohexanediamine
InactiveCN102731316AEasy to operateGood choiceAmino preparation from aminesSulfonyl chlorideN methylation
The present invention discloses a production method for 1,2-N,N'dimethylcyclohexanediamine. According to the method, N single protection is performed on o-phenylene diamine and aromatic benzene sulfonyl chloride; methyl iodide or dimethyl sulphate is adopted to carry out a N methylation reaction; N single protection is performed on 1,2-diaminocyclohexane and aromatic benzene sulfonyl chloride, then methyl iodide or dimethyl sulphate is adopted to carry out a N methylation reaction, a hydrolysis reaction is performed in the presence of sulphuric acid to remove aromatic benzenesulfonyl to obtain the target product 1,2-N,N'dimethylcyclohexanediamine. The process of the present invention is simple and easy to operate, and the high purity target product can be obtained with the method of the present invention.
Owner:姜树林
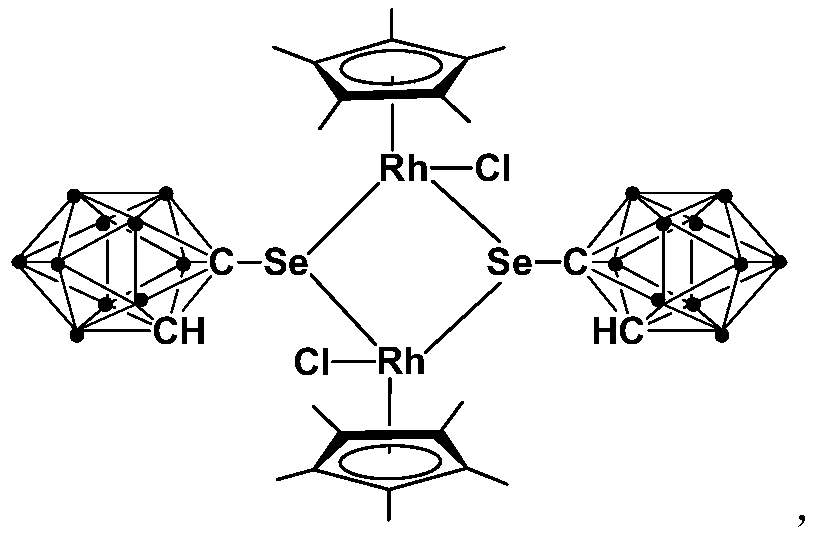
Application of dinuclear rhodium complex in fatty amine N-methylation reaction
ActiveCN110201720AThe synthesis process is simple and greenGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsAmino preparation by functional substitutionOrganic solventFatty amine
The invention relates to application of a dinuclear rhodium complex in a fatty amine N-methylation reaction. The dinuclear rhodium complex is taken as a catalyst, CH3I is taken as a methylation reagent, and the fatty amine N-methylation reaction is catalyzed in an organic solvent for preparing a fatty amine N-methylation derivative. Compared with the prior art, according to the application of thedinuclear rhodium complex, the dinuclear rhodium complex has high catalytic activity at the indoor temperature and can be used for catalyzing the fatty amine N-methylation reaction for preparing the fatty amine N-methylation derivative, according to the catalytic reaction, the yield (90-97%) is high, and the reaction conditions are mild; participation of strong alkali is not needed, all the reagents have stable performance for air and water, the requirements for reaction equipment are low, and the dinuclear rhodium complex has a wide industrial application prospect.
Owner:SHANGHAI INST OF TECH
Synthesis of anticancer medicine Raltiprexed
InactiveCN1216883CShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
Owner:CAPITAL NORMAL UNIVERSITY
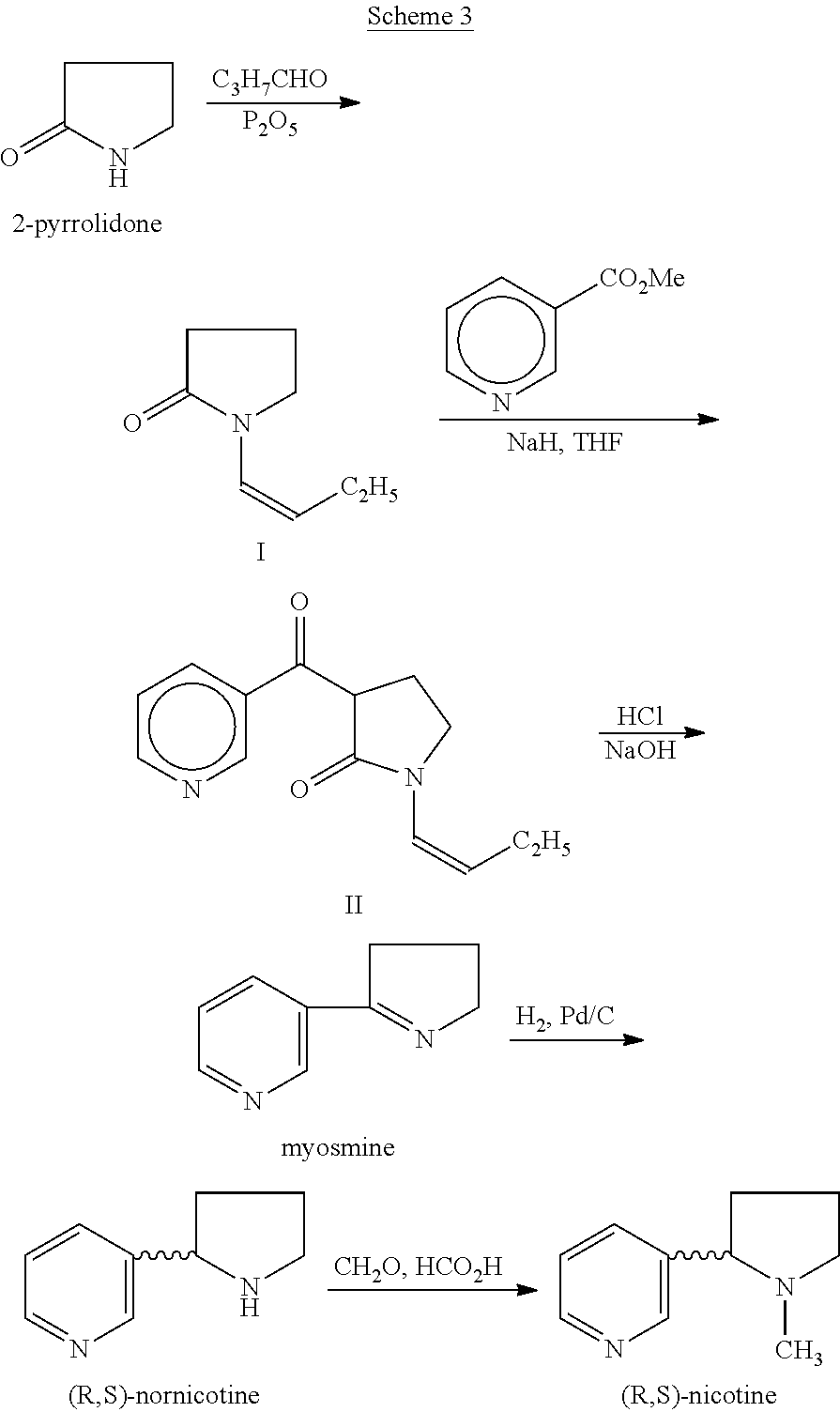
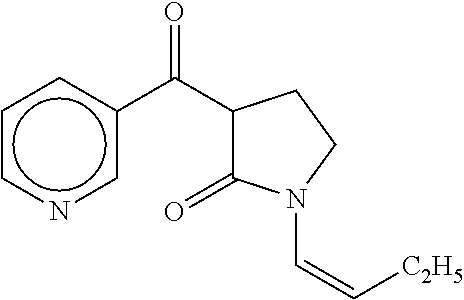
Process for the preparation of (r,s)-nicotine
A process for (R,S)-nicotine is described. Condensation of 1-(but-1-enyl) pyrrolidin-2-one with nicotinic acid ester gave 1-(but-1-enyl)-3-nicotinoylpynolidin-2-one which on treatment with an acid and a base gave myosmine. Myosmine was converted to (R,S)-nicotine by reduction followed by N-methylation.
Owner:DIVI S LAB LTD
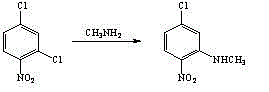
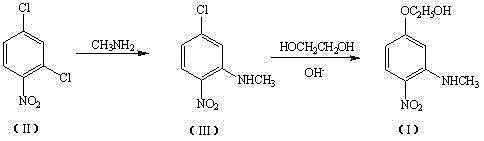
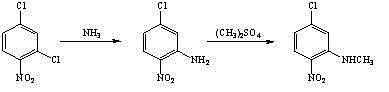
Method for preparing 3-methylamino-4-nitrophenoxyethanol
ActiveCN104130143ALow toxicityImprove adaptabilityOrganic compound preparationAmino compound preparationN methylationHigh pressure
The invention relates to a method for preparing 3-methylamino-4-nitrophenoxyethanol. The method comprises the steps of performing methylamination reaction by using 2,4-dichloronitrobenzene as a raw material and using methanol as a solvent to obtain 3-methylamino-4-nitrochlorobenzene, performing nucleophilic substitution with a sodium hydroxide (or other alkaline matter) solution of ethylene glycol to substitute the remaining other chlorine group so as to obtain crude 3-methylamino-4-nitrophenoxyethanol, and performing re-crystallization to obtain high-purity 3-methylamino-4-nitrophenoxyethanol. The process of preparing the 3-methylamino-4-nitrochlorobenzene intermediate through a one-step method replaces a two-step method process of preparing the intermediate through high pressure ammonolysis of 2,4-dichloronitrobenzene and N-methylation reaction; in addition, methanol is adopted in the one-step method to replace a tetrahydrofuran solution, so that the purposes of reducing the recovery cost and reducing the cost of the solvent are achieved; the method has the characteristics of conventional equipment, simplicity in operation, cheap and readily available raw materials, low cost, high yield and the like.
Owner:ZHEJIANG DINGLONG TECH
Method for synthesizing 1,2-dimethylimidazole and used supported catalyst
InactiveCN104549323BHigh activityGood choiceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsDistillationN methylation
Owner:ZHEJIANG UNIV
Green preparation method of pazopanib hydrochloride
The invention belongs to the technical field of pharmaceutical chemistry synthesis and particularly relates to a green preparation method of pazopanib hydrochloride. The method comprises the steps ofallowing o-toluidine and N-chlorosuccinimide to give a chlorination reaction to form 2-methyl-5-chlorine-aniline, allowing 2-methyl-5-chlorine-aniline to react with a nitrous acid compound to form 6-chlorine-2H-indole hydrochloride, performing N methylation reaction to form N-methyl-6-chlorine-2H-indole, performing 3-delta carbon alkylation reaction in the presence of dimethyl sulfoxide to form 2,3-dimethyl-6-chlorine-2H-indazole, allowing 2,3-dimethyl-6-chlorine-2H-indazole to react with 2-chlorine-4-amino-pyrimidine and methyl iodide to form N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine, and at last, allowing N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine to react with 3-sulfamate-4-methyl-aniline to form pazopanib hydrochloride. The method is lowin raw material price, simple to operate and low in operational risk, and avoids generation of waste acid; and a reaction yield and purity are high.
Owner:JINAN ASIA PHARMA TECH
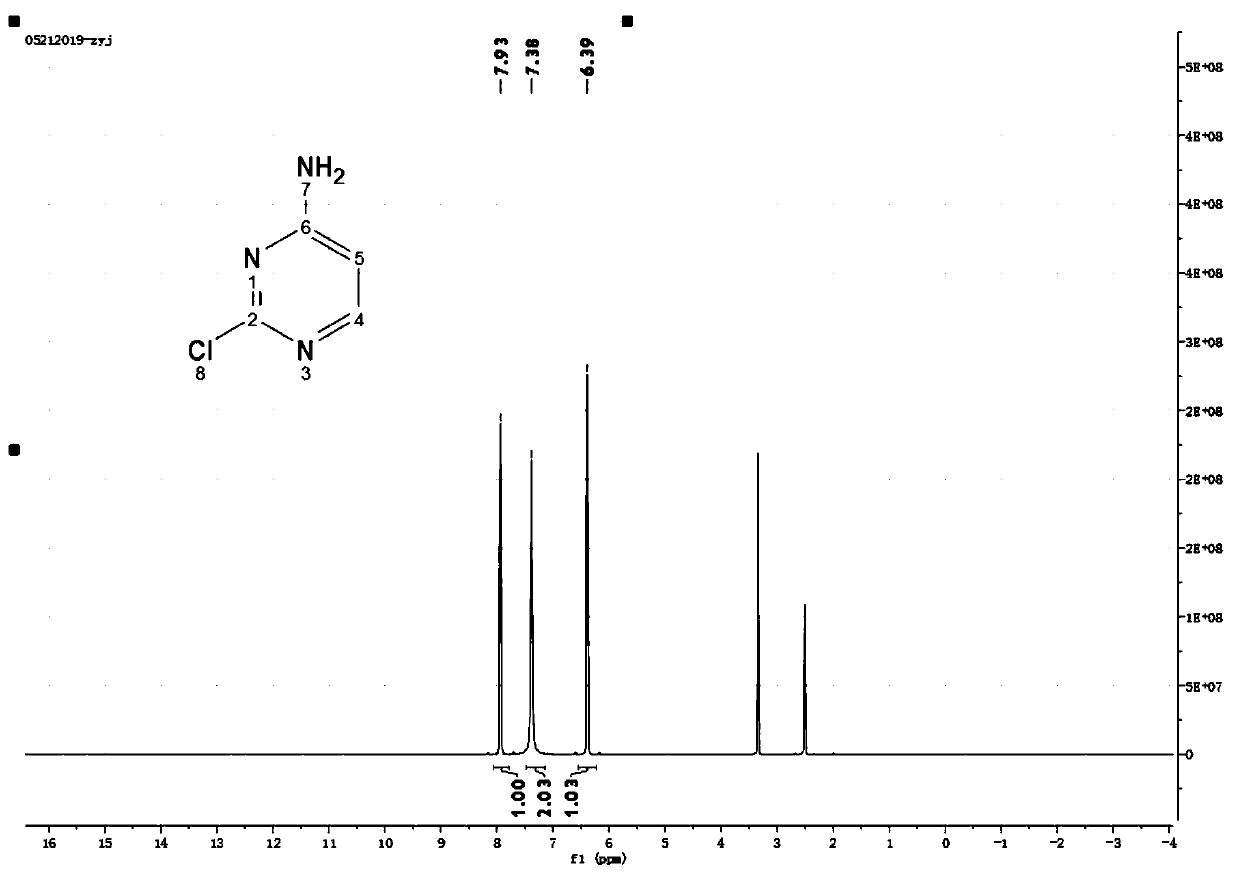
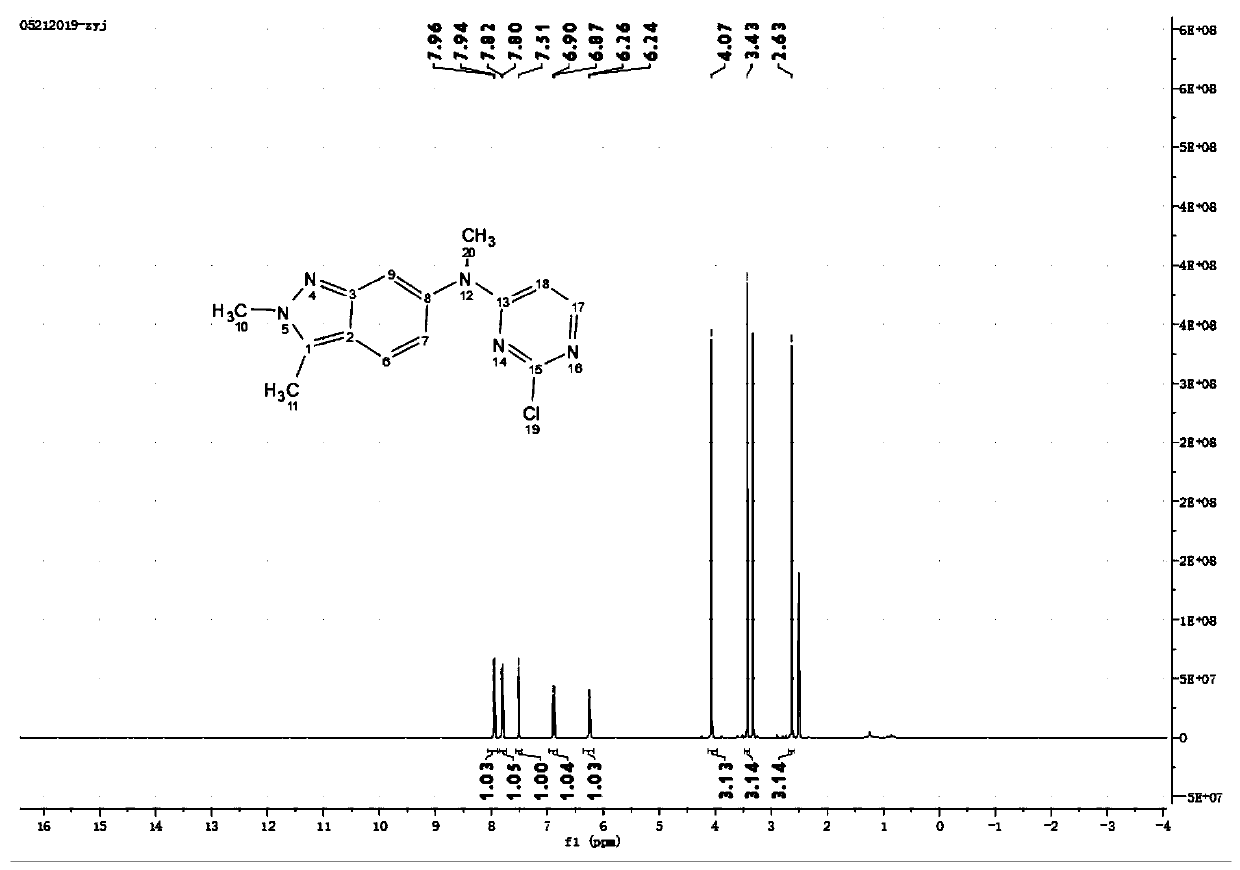
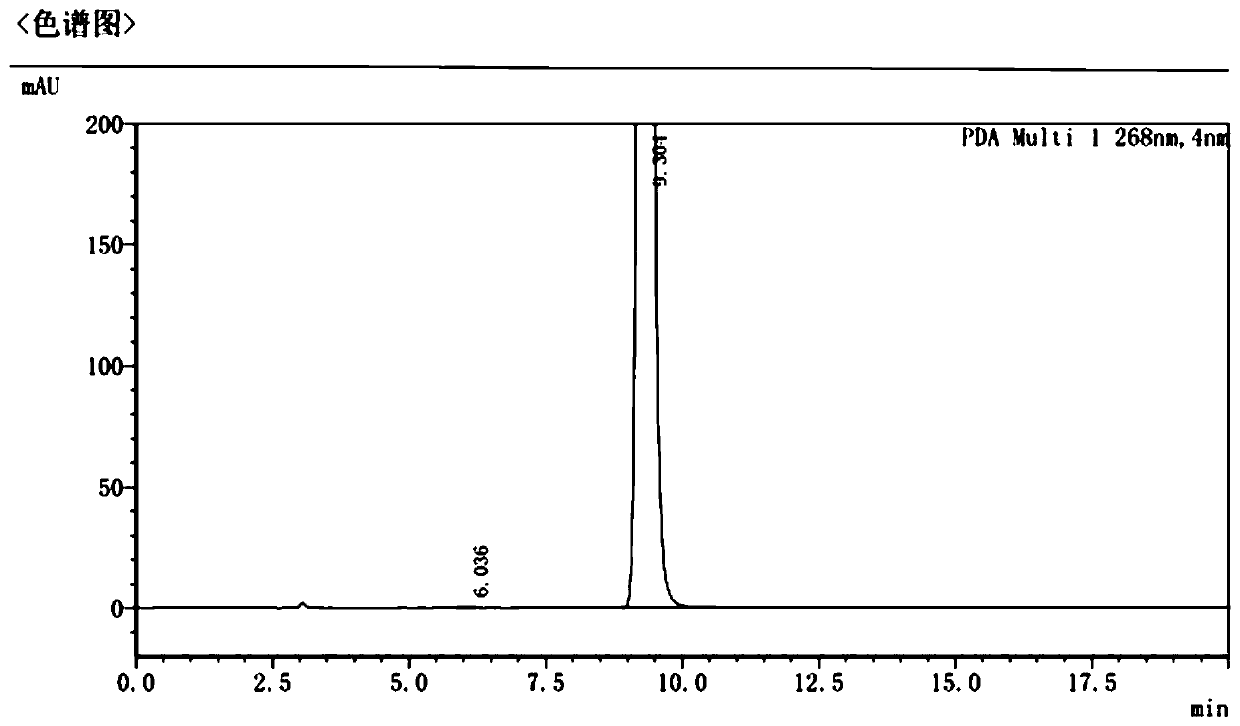
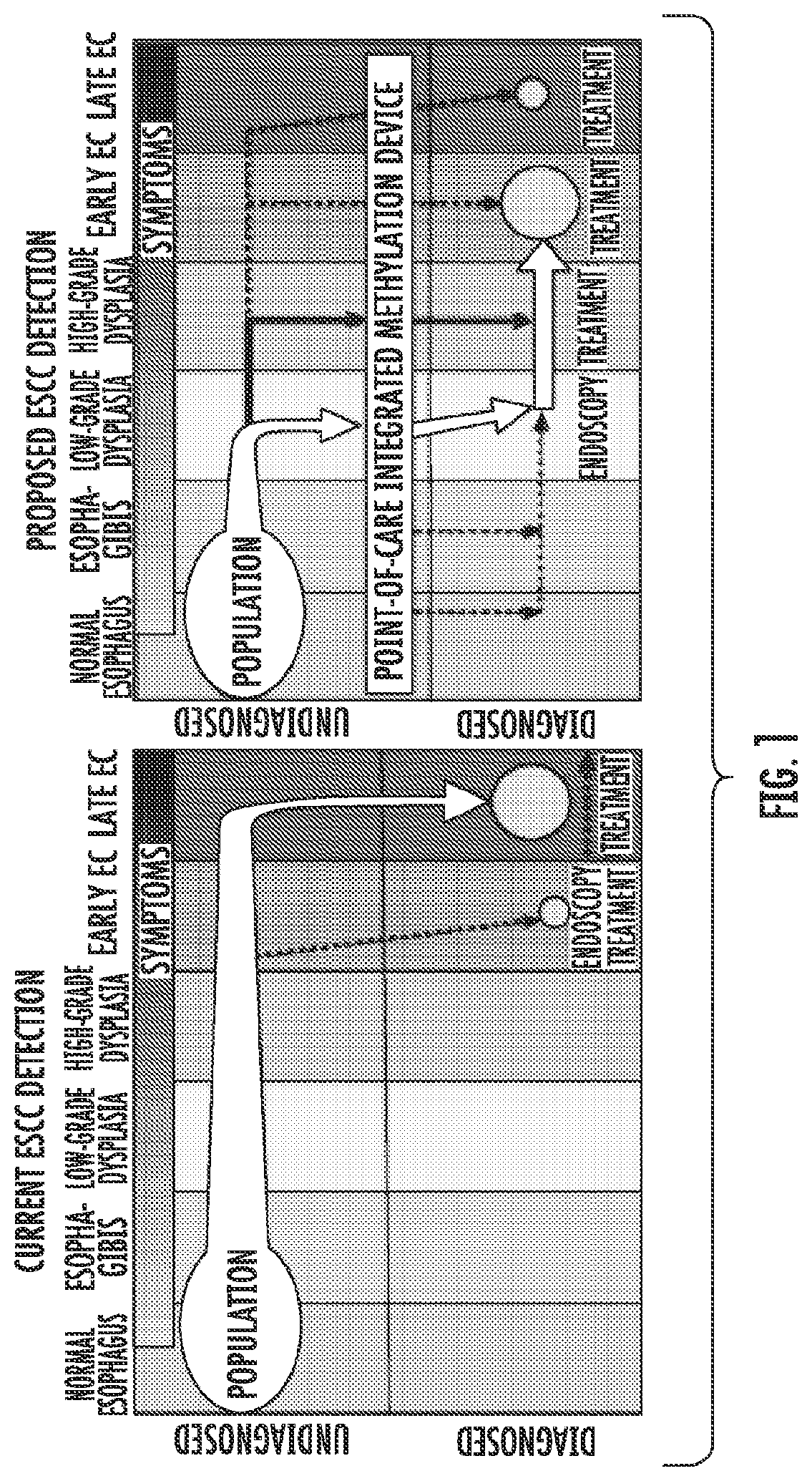
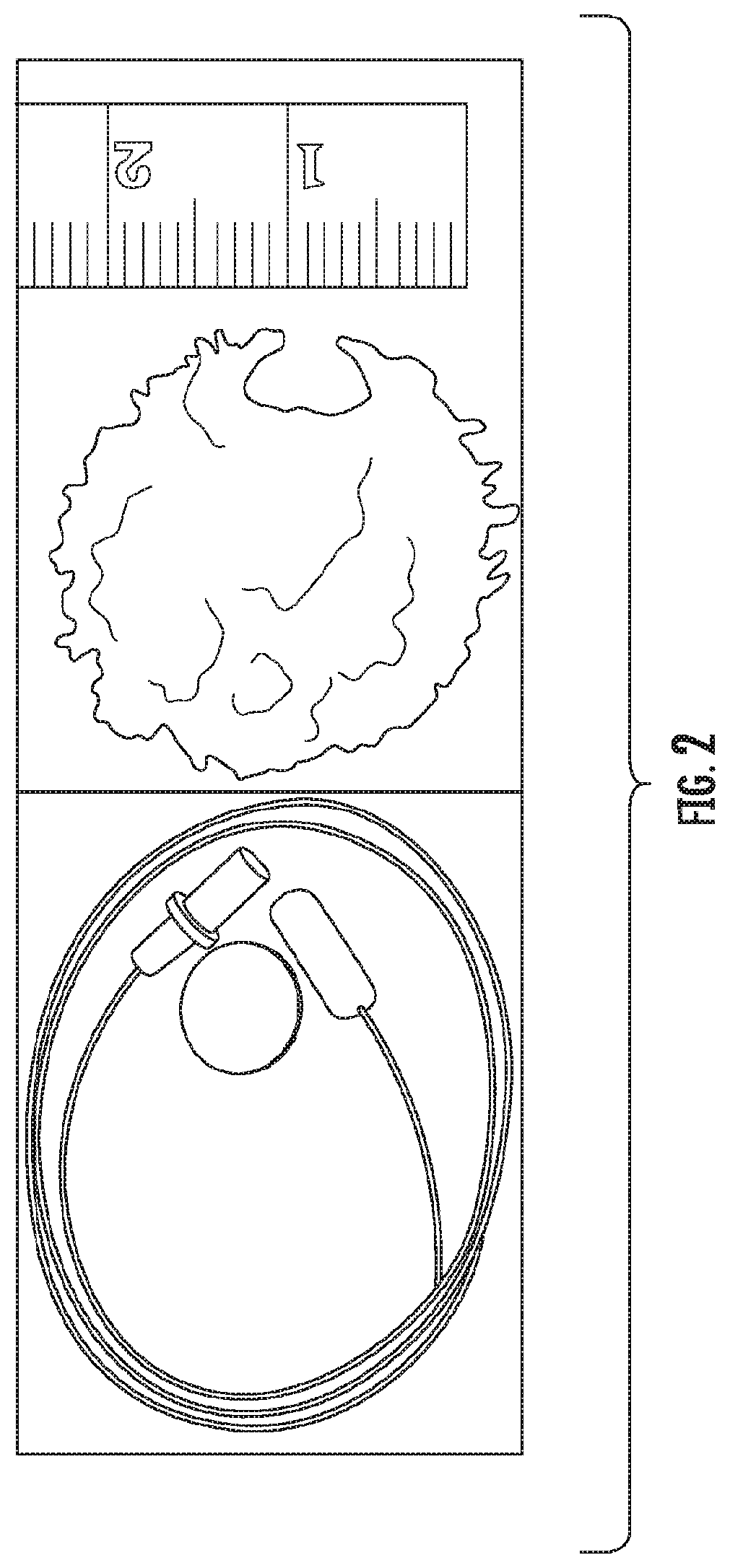
Methods for Detecting and Treating Esophageal Cancer
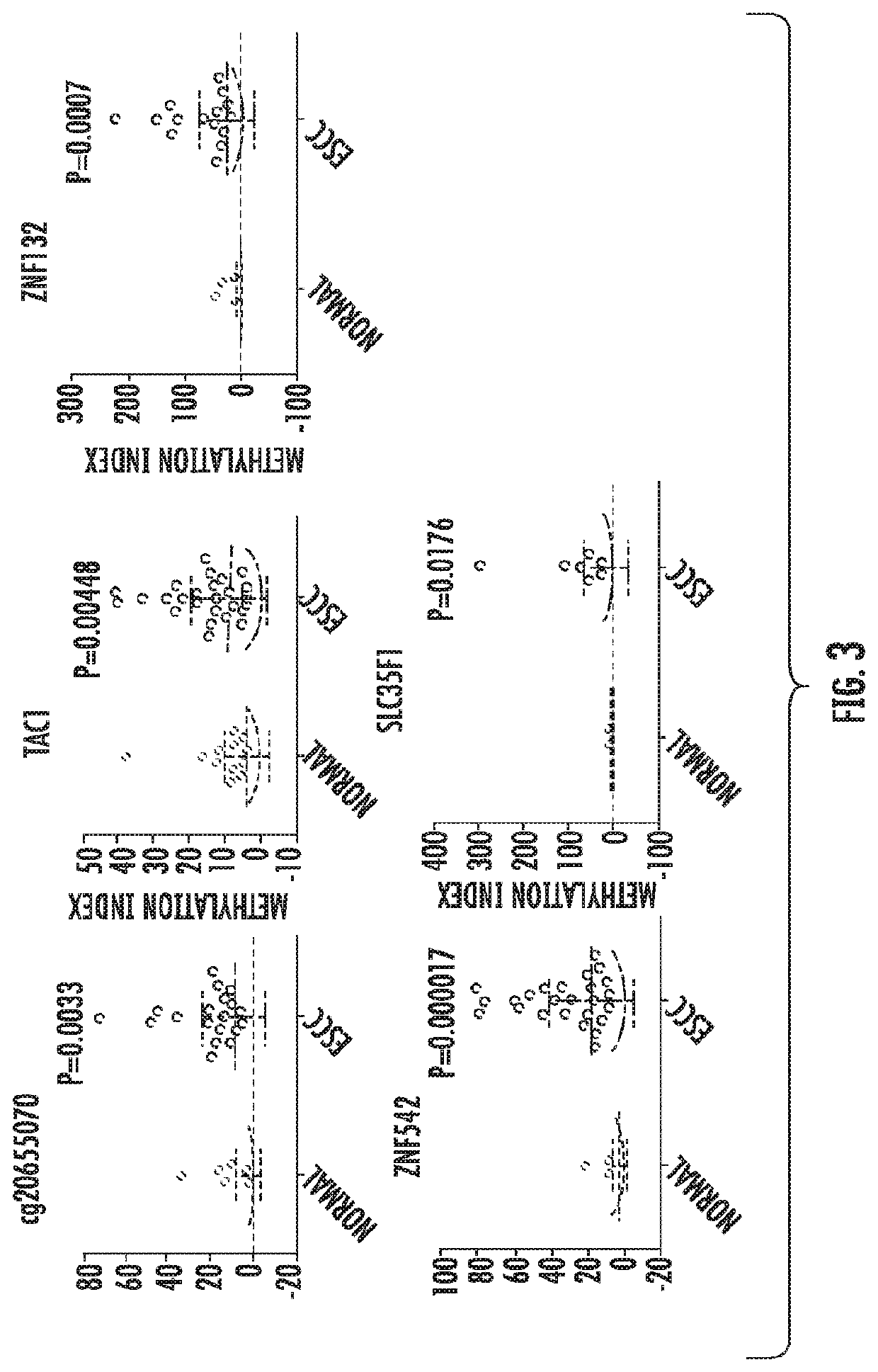
The present invention relates to the field of cancer. More specifically, the present invention provides compositionsand methods useful for detecting and treating esophageal cancer. In a specific embodiment, a method for identifying a subject having esophageal squamous cell carcinoma (ESCC) comprises (a) extracting genomic DNA from a sample obtained from the subject; (b)performing a conversion reaction on the genomic DNA in vitro to convert unmethylated cytosine to uracil by deamination; and (c)detecting nucleic acid methylation of one or more genes in the converted genomic DNA, wherein detecting nucleic acid methylation e identifies the subject as having ESCC. The one or more genes can comprise ZNF542, ZNF132, cg20655070, TAC1 and SLC35F1. In amore specific embodiment, the one or more genes comprise ZNF542 and ZNF132 and can further comprise detecting the nucleic acid N methylation of one or more of cg20655070, TAC1 and SLC35F1.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
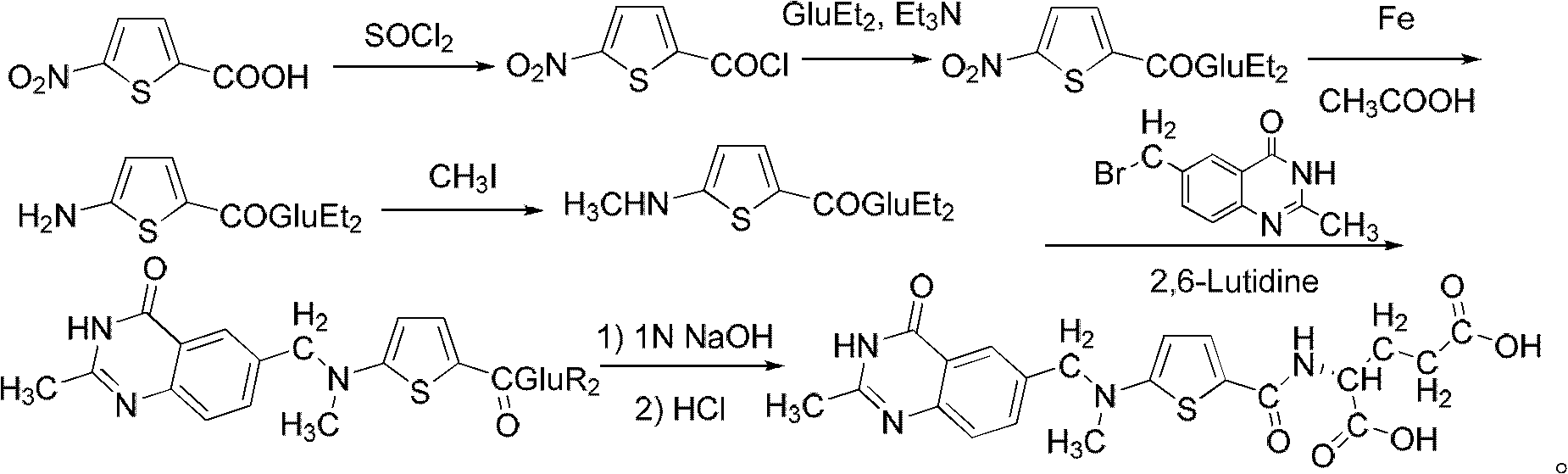
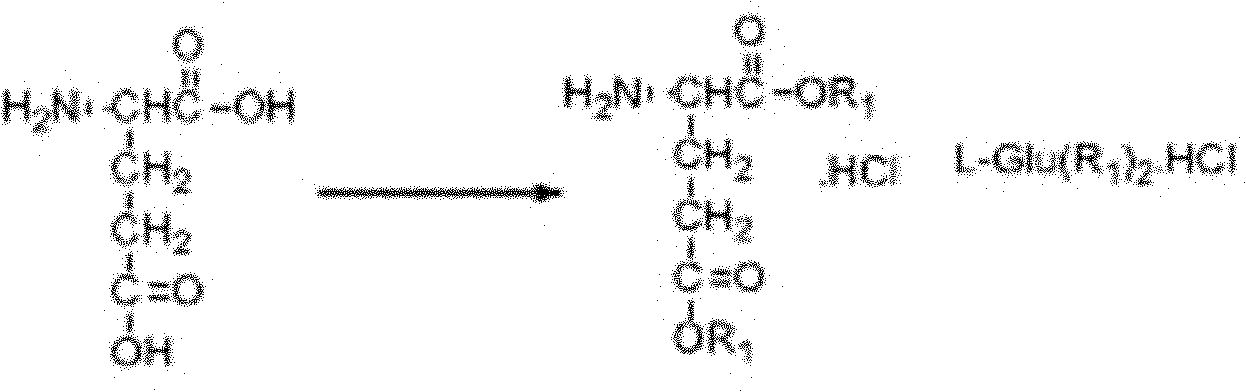
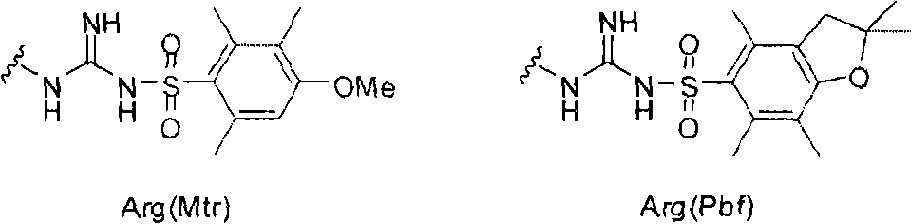
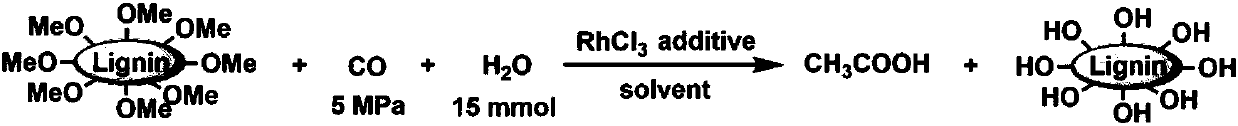
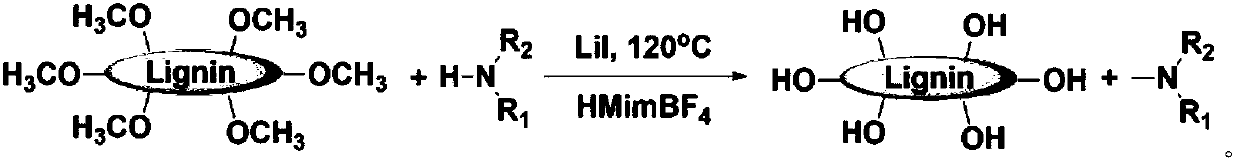
![5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method 5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method](https://images-eureka.patsnap.com/patent_img/2df62c64-dafb-4446-93ad-76ac0c7808cd/HDA00003667275700011.PNG)
![5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method 5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method](https://images-eureka.patsnap.com/patent_img/2df62c64-dafb-4446-93ad-76ac0c7808cd/BDA00003667275600011.PNG)
![5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method 5-methyl-7-amino-5H,7H-dibenzo[b,d]azepin-6-ketone preparation method](https://images-eureka.patsnap.com/patent_img/2df62c64-dafb-4446-93ad-76ac0c7808cd/BDA00003667275600021.PNG)