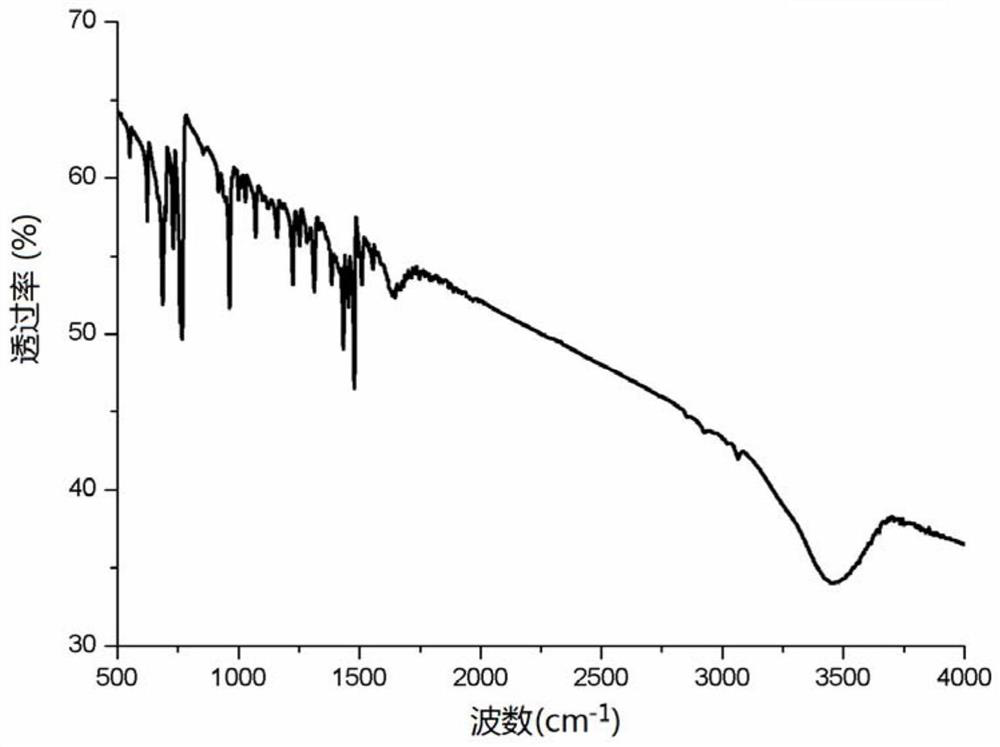
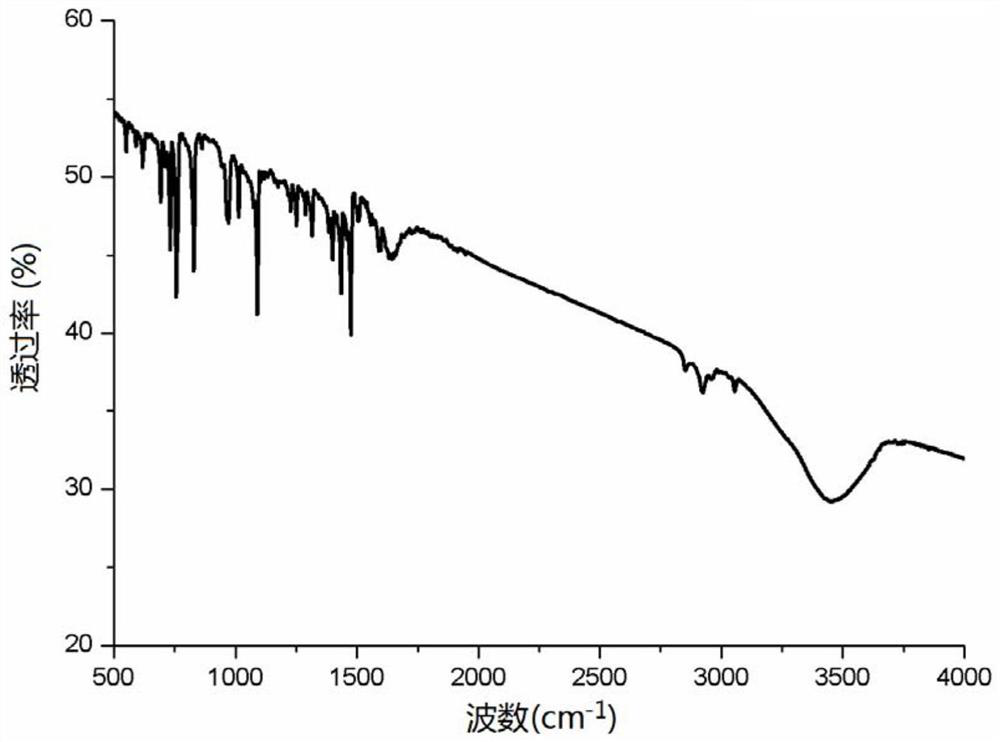
Patents
Literature
39results about How to "The synthesis process is simple and green" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of injectable natural polysaccharide self-healing hydrogel
ActiveCN103910894AGood biocompatibilityThe synthesis process is simple and greenSurgeryPharmaceutical non-active ingredientsSelf-healingPhosphate
The invention relates to a preparation method of injectable natural polysaccharide self-healing hydrogel. The preparation method of the injectable natural polysaccharide self-healing hydrogel comprises the following steps: firstly synthesizing water soluble N-carboxyethyl chitosan by carrying out a Michael addition reaction on acrylic acid and chitosan, carrying out an oxidization reaction on sodium periodate and sodium alginate to obtain oxidized sodium alginate, then carrying out a dehydration condensation reaction on N-carboxyethyl chitosan and oxidized sodium alginate and on adipic dihydrazide and oxidized sodium alginate in a phosphate buffer liquid at the temperature of 37 DEG C, so as to respectively generate invertible imine bond and acylhydrazone bond, and finally preparing injectable natural polysaccharide macromolecular hydrogel with self-healing property. The preparation method of the injectable natural polysaccharide self-healing hydrogel has the advantages that a synthetic process of the injectable self-healing hydrogel is simple and green, reaction conditions are mild, the injectable self-healing hydrogel can form gel in a physiological environment and has self-healing capability, and the injectable self-healing hydrogel has a good application prospect in the fields of drug sustained release and the like.
Owner:XI AN JIAOTONG UNIV
Green method for increasing enzymolysis efficiency of cellulose in rice straws through stepwise pretreatment with two deep co-melting solvents
InactiveCN106755189AImprove enzymatic hydrolysis efficiencyHigh yieldFermentationCelluloseChemical industry
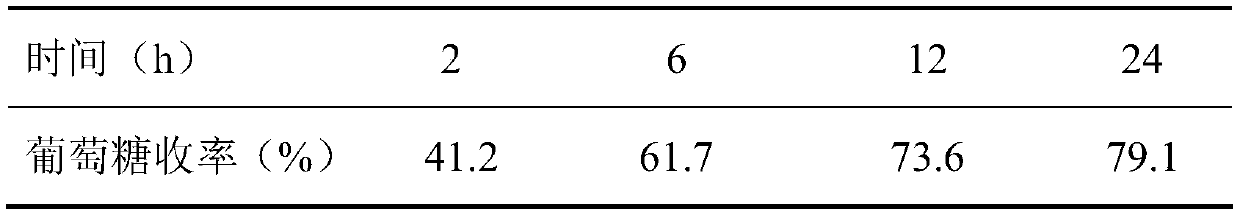
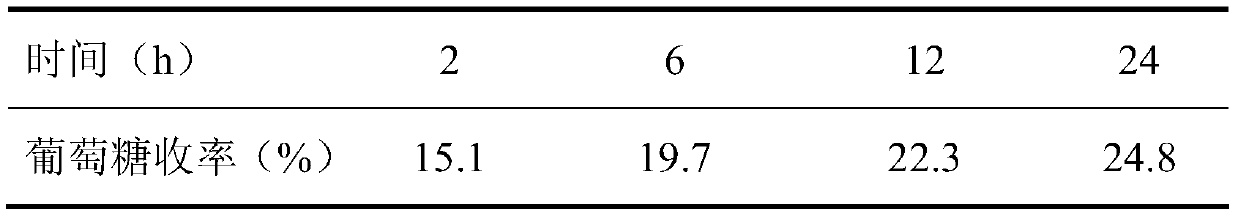
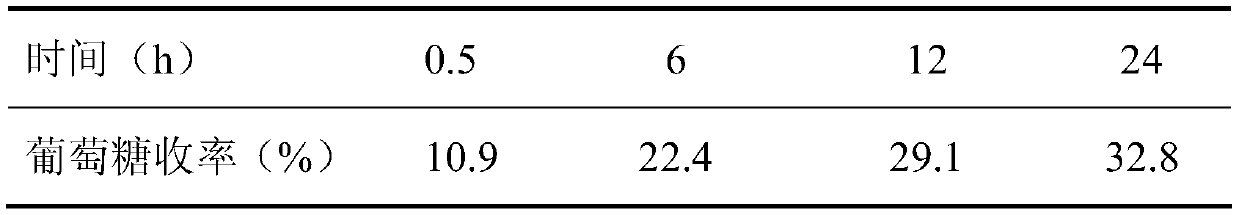
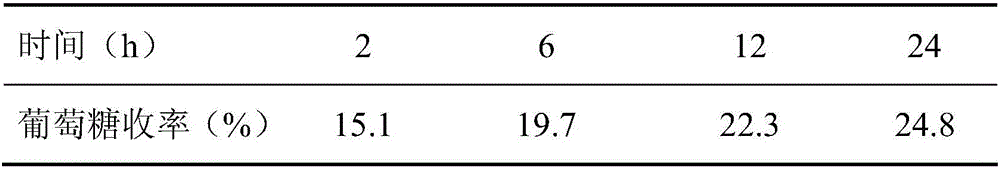
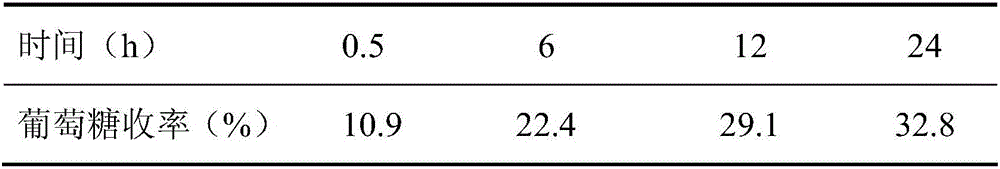
The invention belongs to the fields of utilization of lignocelluloses and energy and chemical industry application, and discloses a green method for increasing enzymolysis efficiency of cellulose in rice straws through stepwise pretreatment with two deep co-melting solvents. The method includes the steps of: with one deep co-melting substance as a solvent, pre-treating the rice straws, and performing filtration and separation to obtain a residue; drying the residue and pre-treating the residue with the other one deep co-melting solvent in the same way, thus finally obtaining pre-treated rice straws; enzymolyzing the rice straw residue, as a substrate, with cellulase to finally obtain a saccharide liquid mainly comprising glucose. The method increases the enzymolysis efficiency of the rice straws, increases yield of fermentable reduced saccharide (glucose), overcomes defects that an ionic liquid pretreatment process is high in cost and is not environment-friendly, and also solves a problem of poor pretreatment effect with single deep co-melting solvent. The method also expands application range and increases utilization rate of some green deep co-melting solvents.
Owner:GUANGDONG UNIV OF TECH
Green method for increasing cellulose enzymolysis efficiency in rice straws by utilizing lactic acid/guanidine hydrochloride to extract hemicellulose
InactiveCN106480128AImprove enzymatic hydrolysis efficiencyHigh yieldFermentationSolventIonic liquid
The invention belongs to the fields of lignocellulose utilization and energy and chemical application, and discloses a green method for increasing a cellulose enzymolysis efficiency in rice straws by utilizing lactic acid / guanidine hydrochloride to extract hemicellulose. The green method includes: taking the lactic acid / the guanidine hydrochloride as a solvent to preprocessing the rice straws, filtering and separating to obtain residues, and drying to obtain preprocessed rice straws; taking the rice straw residues as a base material, and subjecting the base material to enzymolysis by cellulose so as to obtain a liquid sugar which mainly comprises glucose. The green method has the advantages that the enzymolysis efficiency of the rice straws can be increased effectively, a yield rate of fermentable reducing sugar is increased, and the defects of high cost and environmental unfriendliness of an ionic liquid preprocessing process are overcome.
Owner:GUANGDONG UNIV OF TECH
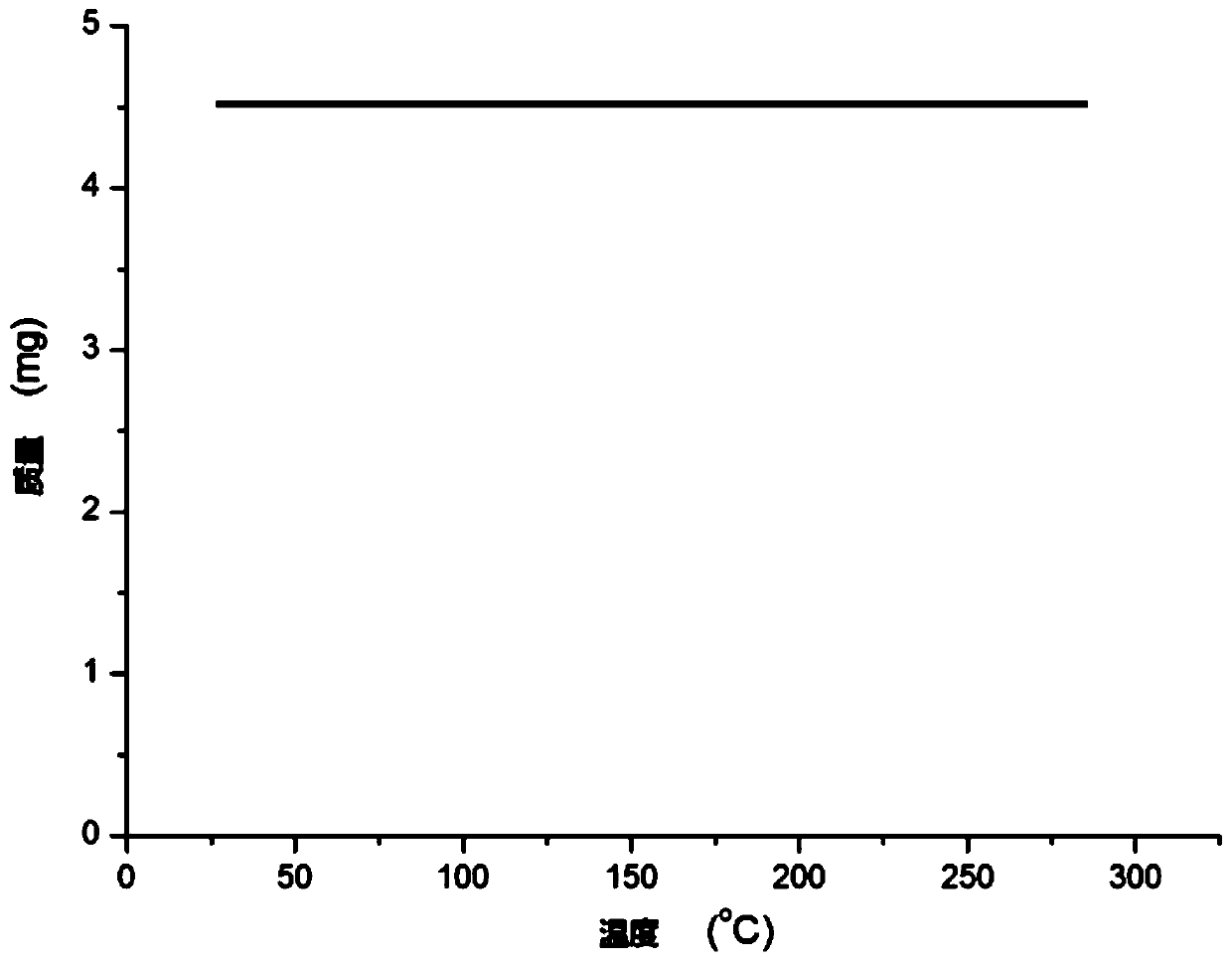
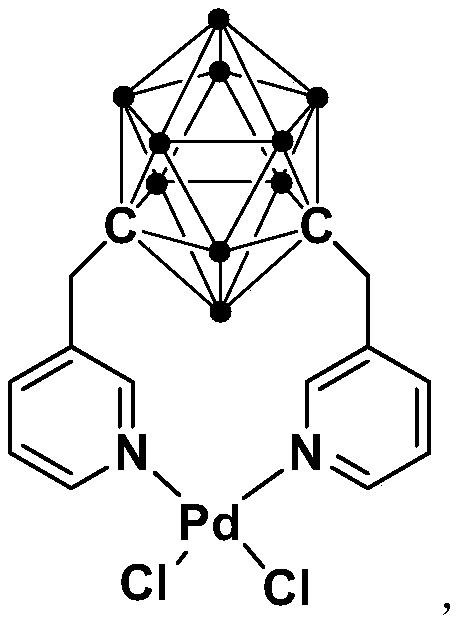
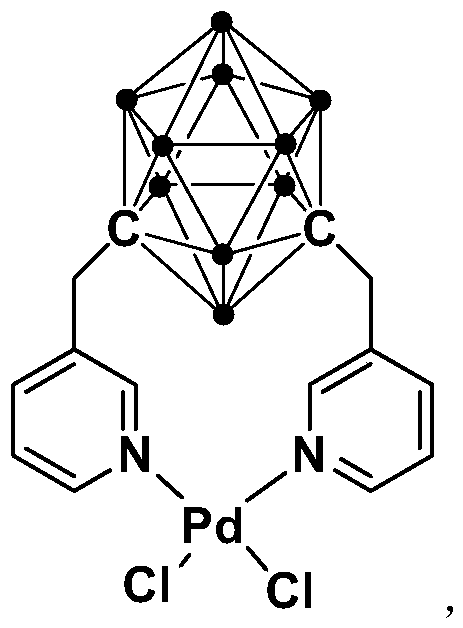
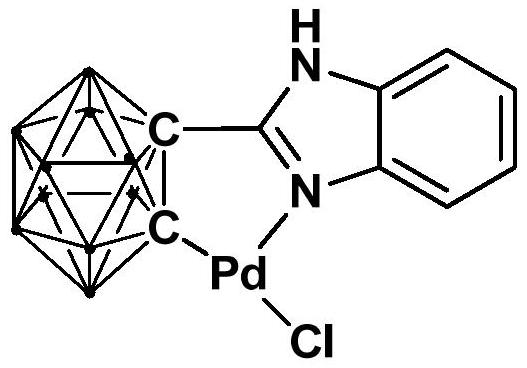
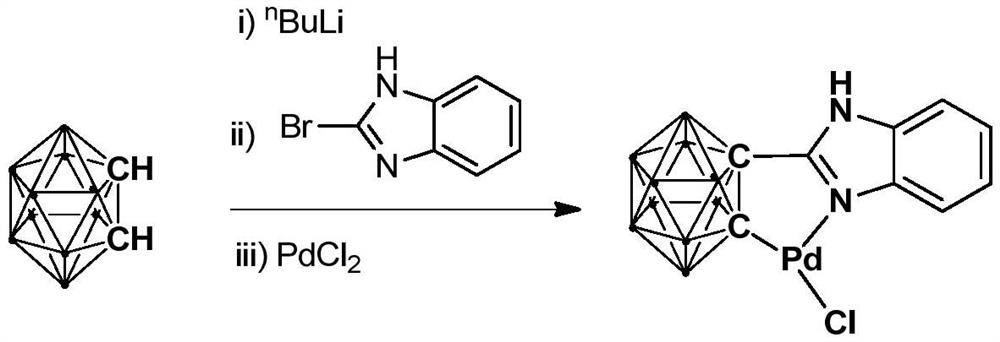
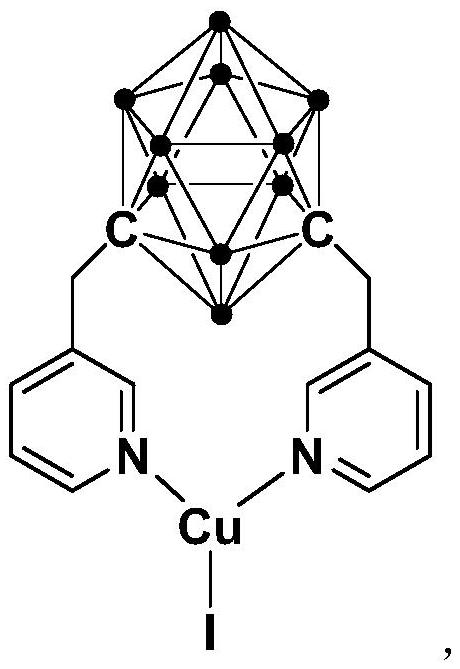
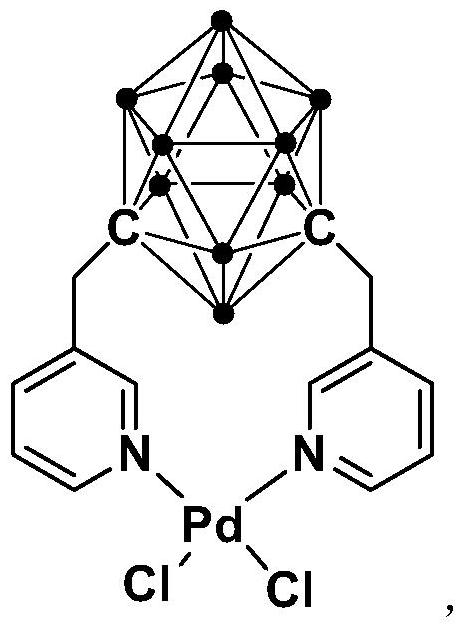
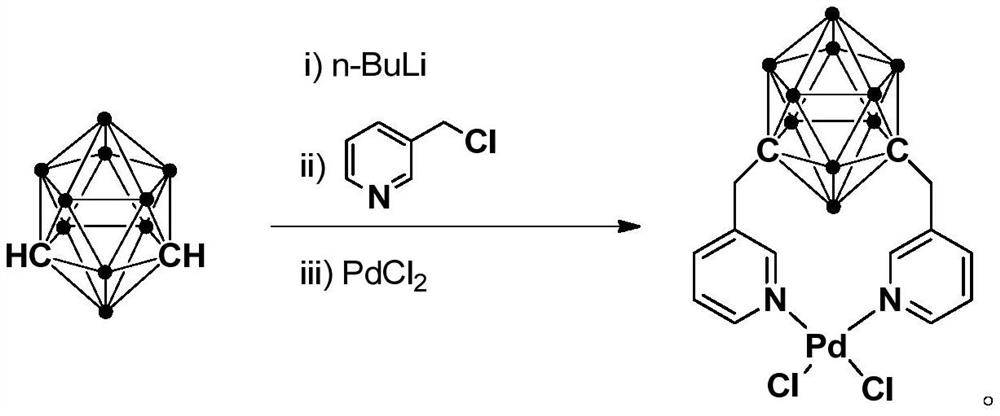
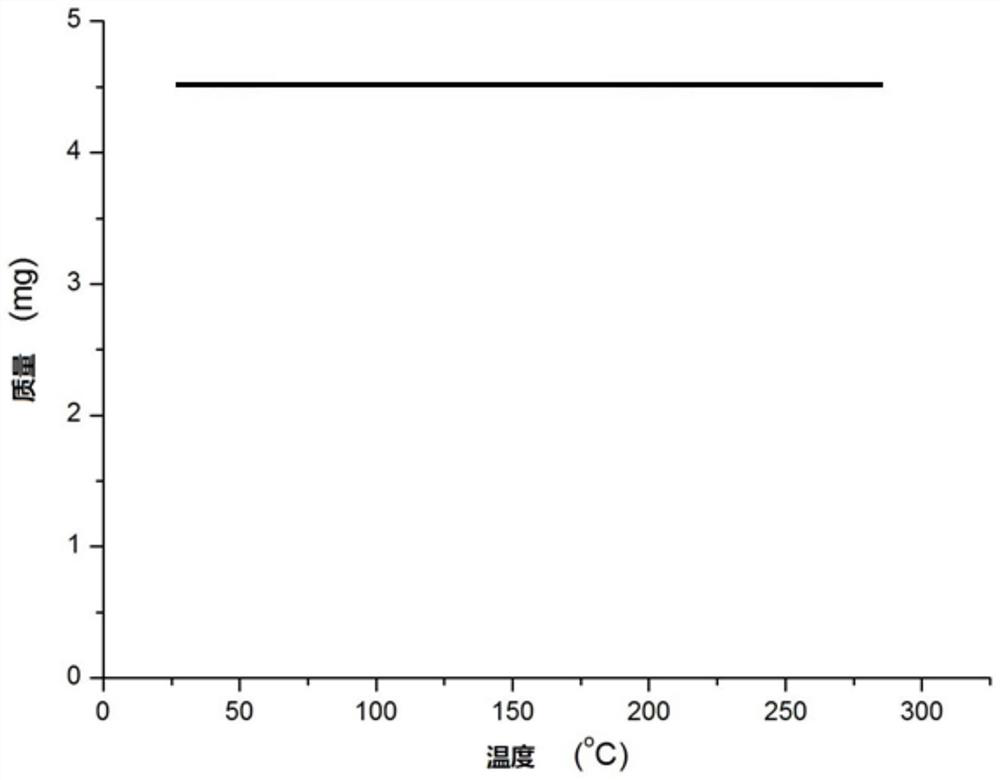
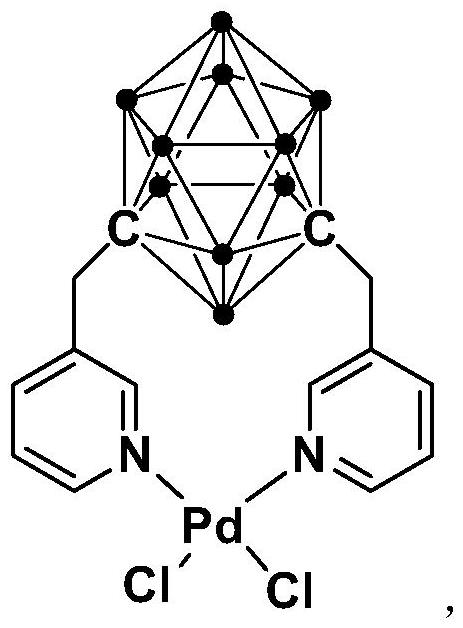
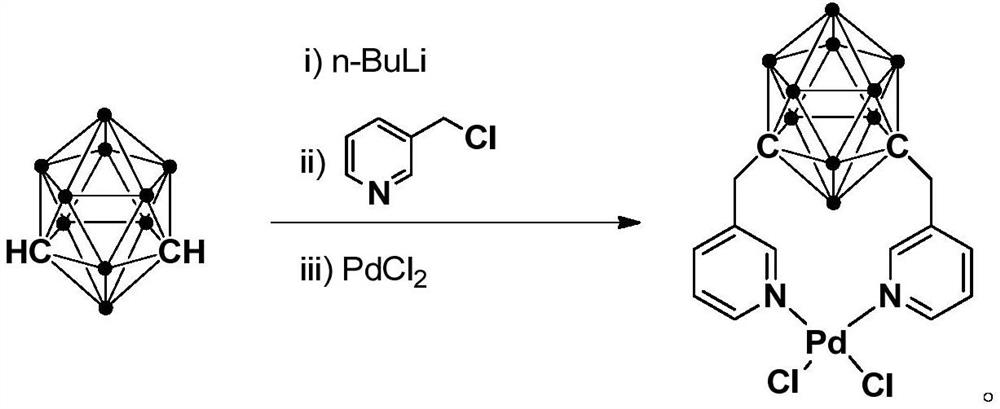
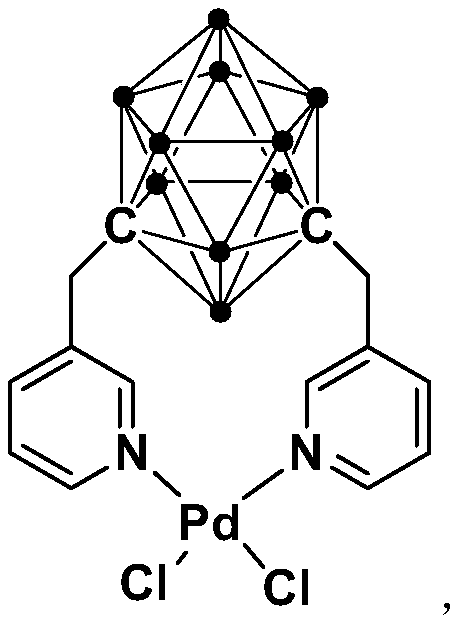
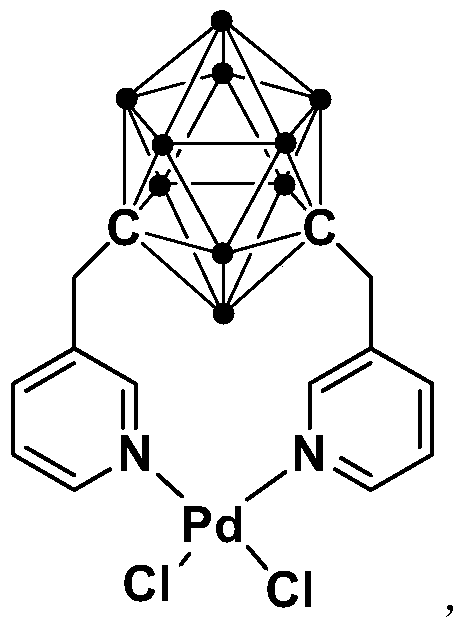
N,N-coordinated palladium complex with meta-carborane ligand and preparation and application of palladium complex
ActiveCN110372755AThe synthesis process is simple and greenGood choiceOrganic compound preparationGroup 8/9/10/18 element organic compoundsFormylation reactionCoordination complex
The invention relates to an N,N-coordinated palladium complex with a meta-carborane ligand and preparation and application of the palladium complex. A preparation method of the palladium complex comprises the following steps that (1) an n-BuLi solution is added into a meta-carborane solution and then a reaction is performed at a room temperature for 30-60 min; (2) 3-chloromethylpyridine is added and reacts at the room temperature for 3-5 h; (3) PdCl2 is added and reacts at the room temperature for 2-5 h, and the palladium complex is obtained through post-treatment; and the palladium complex isused for catalyzing an arylamine formylation reaction to prepare an arylamine formamide compound. Compared with the prior art, the synthesis process of the palladium complex is simple and green, andhas excellent selectivity and a high yield; and the palladium complex has the characteristics such as stable physicochemical properties and thermal stability, and exhibits excellent catalytic activityin the arylamine formylation reaction.
Owner:SHANGHAI APPLIED TECHNOLOGIES COLLEGE
Composite photocatalyst doped in heterojunction interface and preparation method
InactiveCN107308978AEasy to useThe synthesis process is simple and greenHydrocarbon from carbon oxidesCatalyst activation/preparationHeterojunctionCharge carrier
The invention discloses a composite photocatalyst doped in a heterojunction interface and a preparation method. The prepared Bi12O17Cl2 / g-C3N4 composite photocatalyst doped in a heterojunction interface has extremely strong capability in converting carbon dioxide into methane under visible light. Since g-C3N4 and Bi12O17Cl2 nanosheets are adopted to be combined, a large-area heterojunction can be more easily formed, and thereby the separation of current carriers is promoted; by thermal diffusion, bismuth atoms on Bi12O17Cl2 at the heterojunction interface are successfully doped into a g-C3N4 crystal lattice, consequently, a super strong electric field at the heterojunction interface is induced, and super performance in reducing carbon dioxide under the visible light is achieved; the extremely large specific surface area and countless pores of porous g-C3N4 provide convenience for interface doping; the flow direction of current carriers is successfully controlled by matched band-gap structure and interface doping, selectively reducing carbon dioxide into methane is realized, and the recyclability of the photocatalyst is enhanced; material synthesis is simple and green, the scale is large, and the industrial application prospect is good.
Owner:HUAIBEI NORMAL UNIVERSITY
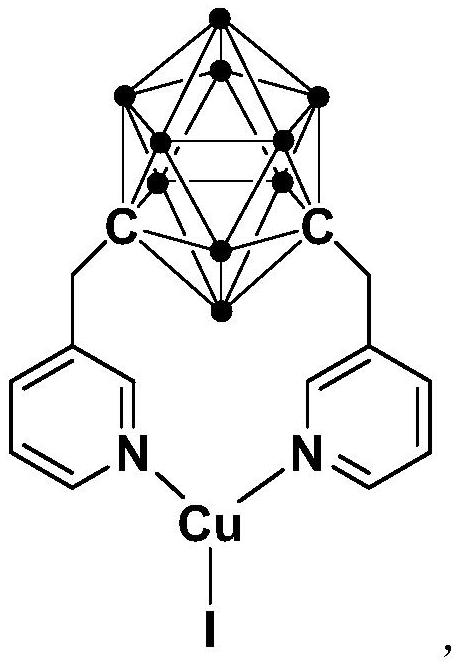
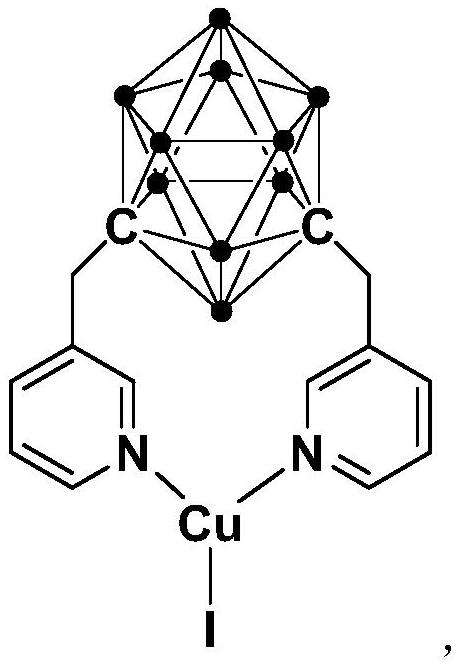
Synthesis method of pyrrole[1,2-a]quinoxaline derivative
ActiveCN112457339APreparation method simple greenImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsQuinoxalineReaction temperature
The invention relates to a synthesis method of a pyrrole [1,2a] quinoxaline derivative. The method comprises the steps: dissolving a cuprous complex, 2-bromoaniline, a pyrrole formaldehyde compound and an alkali in an organic solvent, carrying out a reaction, and carrying out separation and purification to obtain the pyrrole[1,2a] quinoxaline derivative, wherein the molar ratio of the cuprous complex to the 2-bromoaniline to the pyrrole formaldehyde compound to the alkali is (0.01-0.03): 1.0: 1.0: 1.5, the reaction temperature is 50-65 DEG C, and the reaction time is 6-8 hours. Compared with the prior art, the method has the advantages of mild reaction conditions, high yield, high substrate universality, less waste and the like.
Owner:SHANGHAI INST OF TECH
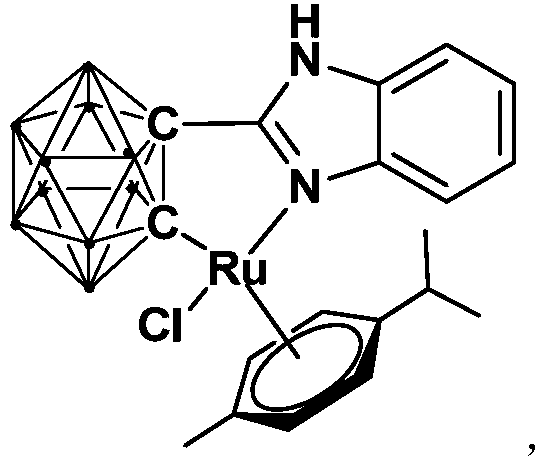
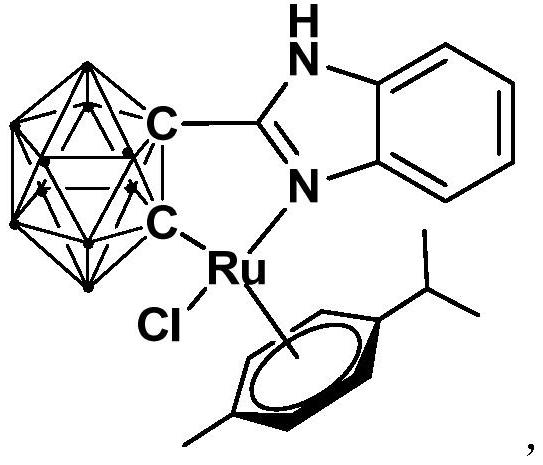
Ruthenium complex containing carboranyl benzimidazole structure as well as preparation method and application of ruthenium complex
ActiveCN110016061AHigh catalytic activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsMetallocenesCoordination complexBenzimidazole
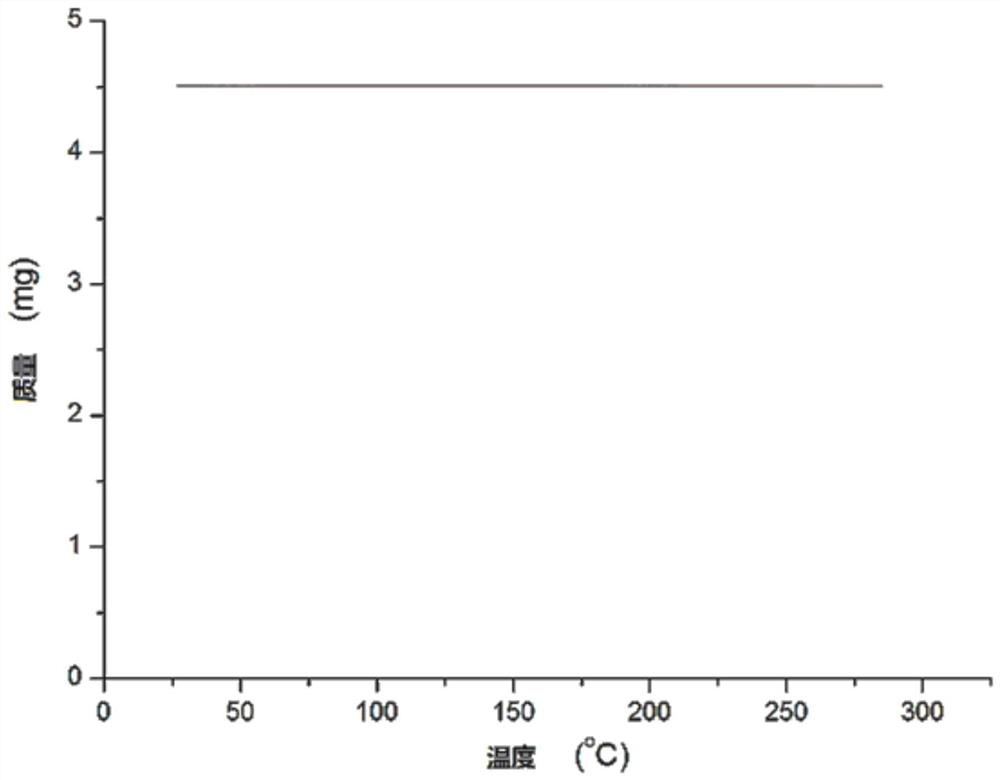
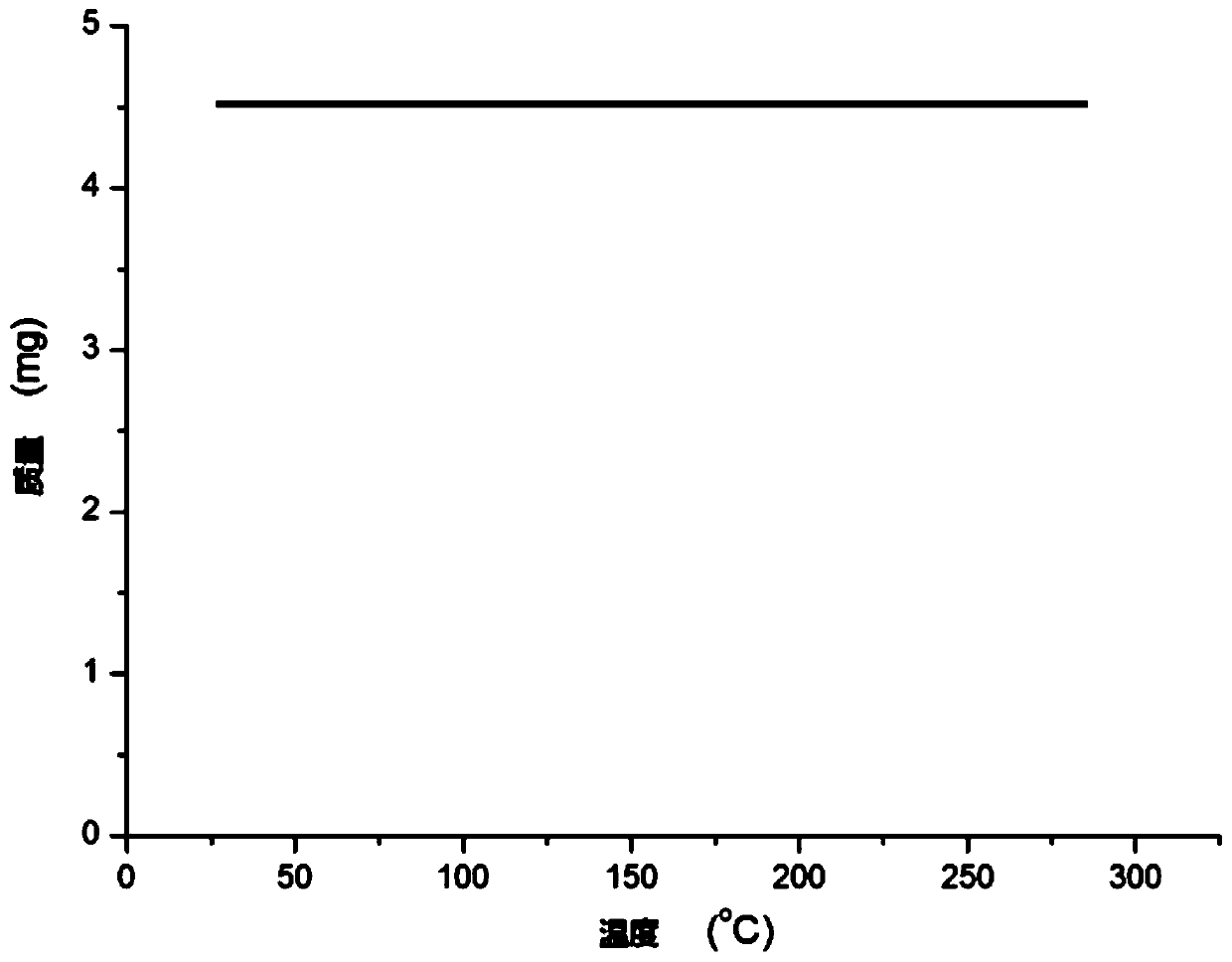
The invention relates to a ruthenium complex containing a carboranyl benzimidazole structure as well as a preparation method and application of the ruthenium complex. The preparation method of the ruthenium complex comprises the following steps: 1) adding a n-BuLi solution into a carborane solution, and performing a reaction at room temperature for 30-60 min; 2) adding bromobenzimidazole, and performing a reaction at room temperature for 6-8 h; and 3) adding [(p-cymene)RuCl2]2, performing a reaction at room temperature for 3-5 h, and performing post treatment to obtain the ruthenium complex. The ruthenium complex is used to catalyze self-oxidative coupling of primary amines to prepare imine compounds. Compared with the prior art, the method provided by the invention uses the dinuclear ruthenium compound [(p-cymene)RuCl2]2 as a raw material, and the [(p-cymene)RuCl2]2 is reacted with the n-BuLi and o-carbonboryl benzimidazole to obtain the bivalent half-sandwich ruthenium complex containing the o-carbonboryl benzimidazole structure; and the ruthenium complex provided by the invention has stable physicochemical properties and thermal stability, is still stable at high temperature of300 DEG C, has a simple and green synthetic process, and exhibits excellent activity in the reaction of catalyzing the self-oxidative coupling of primary amines to prepare the imine compounds.
Owner:SHANGHAI INST OF TECH
Nuclear magnetic imaging and photodynamic/chemotherapy degradable silicon-based nano diagnosis agent and preparation method thereof
InactiveCN107929736ARealize internal loadGuaranteed sustained release effectAntipyreticPhotodynamic therapyProtonationComposite nanoparticles
The invention belongs to the field of nano material preparation and biological medical application and particularly relates to a nuclear magnetic imaging and photodynamic / chemotherapy degradable silicon-based nano diagnosis agent and a preparation method thereof. The nano diagnosis agent disclosed by the invention is a SiO2-TA-Mn<+>-medicine-PEI compound nano particle; under a low pH and high GSHenvironment stimulation, a TA-Mn<+> structure in the compound nano particle can perform protonation and reduction reaction, a net structure is deconstructed, and medicine and metal ions for nuclear magnetic imaging are released out. Therefore, the nuclear magnetic imaging and photodynamic / chemotherapy degradable silicon-based nano particle nano diagnosis agent disclosed by the invention has the characteristics that the SiO2-TA-Mn<+>-medicine-PEI compound nano particle nano diagnosis agent has tumor position degradation response, magnetic resonance imaging, medicine response control release andthe like, and photodynamic therapy and chemotherapy combined synergistic oncotherapy under nuclear magnetic resonance imaging guidance is achieved.
Owner:FUZHOU UNIV
Application of palladium complex to fatty amine formylation reaction
ActiveCN110368989AThe synthesis process is simple and greenGood choiceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFormylation reactionFatty amine
The invention relates to application of a palladium complex to a fatty amine formylation reaction. A N,N-coordinated palladium complex containing a meta-carbobane ligand is used as a catalyst, N,N-dimethyl formamide is used as a formylating reagent and a solvent to catalyze the fatty amine formylation reaction to prepare a fatty amine carboxamide compound. Compared with the prior art, the N,N-coordinated divalent palladium complex containing the metacarbanyl ligand in the application is high in catalytic activity under mild conditions and can catalyze the formylation reaction of a fatty amine.DMF is further used as a formylating reagent and a solvent, and is inexpensive, low in toxicity, easy to separate and high in yield (88-96%).
Owner:SHANGHAI INST OF TECH
Application of dinuclear rhodium complex in fatty amine N-methylation reaction
ActiveCN110201720AThe synthesis process is simple and greenGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsAmino preparation by functional substitutionOrganic solventFatty amine
The invention relates to application of a dinuclear rhodium complex in a fatty amine N-methylation reaction. The dinuclear rhodium complex is taken as a catalyst, CH3I is taken as a methylation reagent, and the fatty amine N-methylation reaction is catalyzed in an organic solvent for preparing a fatty amine N-methylation derivative. Compared with the prior art, according to the application of thedinuclear rhodium complex, the dinuclear rhodium complex has high catalytic activity at the indoor temperature and can be used for catalyzing the fatty amine N-methylation reaction for preparing the fatty amine N-methylation derivative, according to the catalytic reaction, the yield (90-97%) is high, and the reaction conditions are mild; participation of strong alkali is not needed, all the reagents have stable performance for air and water, the requirements for reaction equipment are low, and the dinuclear rhodium complex has a wide industrial application prospect.
Owner:SHANGHAI INST OF TECH
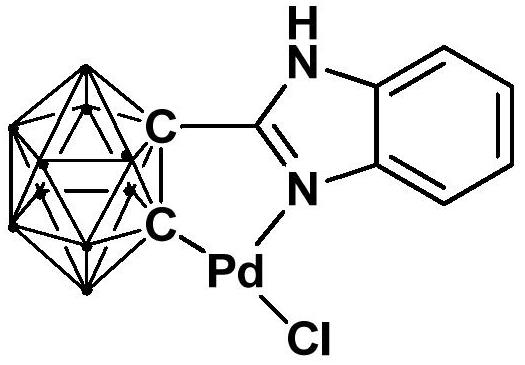
Palladium complex containing ortho-position carborane based benzimidazole structure, preparation method and application thereof
ActiveCN111635437AImprove thermal stabilityThe synthesis process is simple and greenOrganic-compounds/hydrides/coordination-complexes catalystsPalladium organic compoundsOrtho positionCarboxylic acid
The invention relates to a palladium complex containing an ortho-position carborane based benzimidazole structure, a preparation method and application thereof. The palladium complex is prepared by the following method of: dropwise adding an n-BuLi solution into a carborane solution and carrying out stirring reaction, then adding bromo-benzimidazole for continuous reaction, then adding PdCl2 intoa reaction system for continuous reaction, performing separation at the end of the reaction to obtain a palladium complex containing an ortho-position carborane based benzimidazole structure, and applying the complex to catalyzing the reaction of alkyne, carboxylic acid and olefin to prepare the alpha-methylene-gamma-butyrolactone compounds by a one-pot method. Compared with the prior art, the palladium complex disclosed by the invention is high in catalytic efficiency, and the lactone compound can be synthesized by utilizing simple and cheap raw materials under mild conditions.
Owner:SHANGHAI INST OF TECH
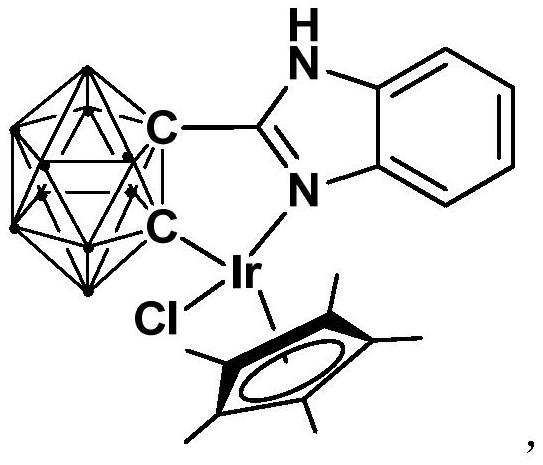
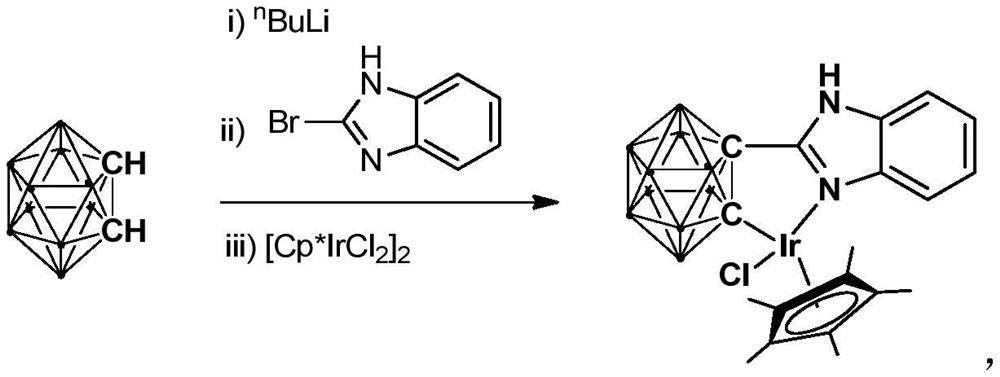
Iridium complex containing carboryl benzimidazole structure and its preparation method and application
ActiveCN110105403BHigh catalytic activityImprove stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholOrtho position
The invention relates to an iridium complex containing a carboryl benzimidazole structure and a preparation method and application thereof. The preparation method of the iridium complex comprises the following steps: 1) adding the n-BuLi solution to the carborane solution, and then React at room temperature for 30-60min; 2) Add bromobenzimidazole and react at room temperature for 6-8h; 3) Add [Cp*IrCl 2 ] 2 , and react at room temperature for 3‑5h, after post-treatment to obtain the iridium complex; the iridium complex is used to catalyze the asymmetric reduction of aryl ketones to prepare chiral alcohols. Compared with prior art, the present invention uses binuclear iridium compound [Cp*IrCl 2 ] 2 As a raw material, it is reacted with n-BuLi and ortho-carboryl benzimidazole to obtain a trivalent iridium complex containing an ortho-carboryl benzimidazole structure. The iridium complex has stable physical and chemical properties and Thermal stability, and the synthesis process is simple and green. It catalyzes the asymmetric reduction reaction of aryl ketones to synthesize chiral alcohols under mild conditions. It has excellent catalytic activity, enantioselectivity and high yield.
Owner:SHANGHAI INST OF TECH
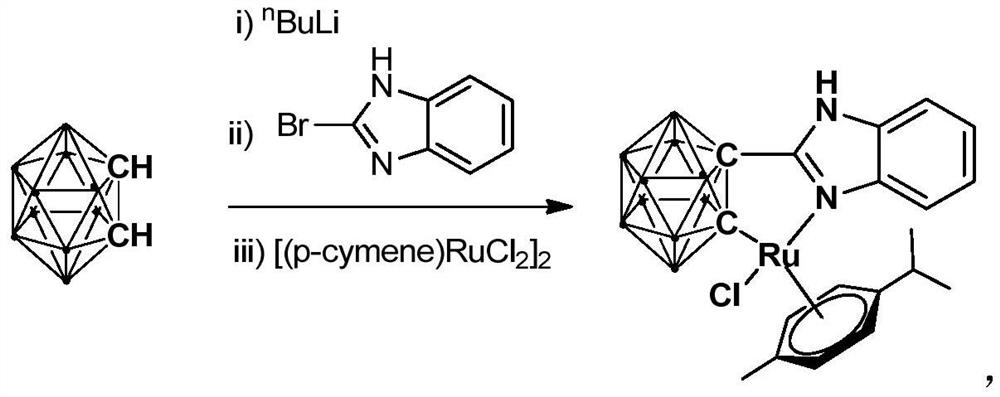
Ruthenium complex containing carboryl benzimidazole structure and its preparation method and application
ActiveCN110016061BHigh catalytic activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsMetallocenesOrtho positionCombinatorial chemistry
The invention relates to a ruthenium complex containing a carboranyl benzimidazole structure as well as a preparation method and application of the ruthenium complex. The preparation method of the ruthenium complex comprises the following steps: 1) adding a n-BuLi solution into a carborane solution, and performing a reaction at room temperature for 30-60 min; 2) adding bromobenzimidazole, and performing a reaction at room temperature for 6-8 h; and 3) adding [(p-cymene)RuCl2]2, performing a reaction at room temperature for 3-5 h, and performing post treatment to obtain the ruthenium complex. The ruthenium complex is used to catalyze self-oxidative coupling of primary amines to prepare imine compounds. Compared with the prior art, the method provided by the invention uses the dinuclear ruthenium compound [(p-cymene)RuCl2]2 as a raw material, and the [(p-cymene)RuCl2]2 is reacted with the n-BuLi and o-carbonboryl benzimidazole to obtain the bivalent half-sandwich ruthenium complex containing the o-carbonboryl benzimidazole structure; and the ruthenium complex provided by the invention has stable physicochemical properties and thermal stability, is still stable at high temperature of300 DEG C, has a simple and green synthetic process, and exhibits excellent activity in the reaction of catalyzing the self-oxidative coupling of primary amines to prepare the imine compounds.
Owner:SHANGHAI INST OF TECH
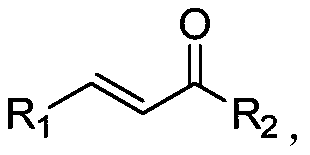
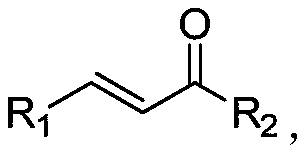
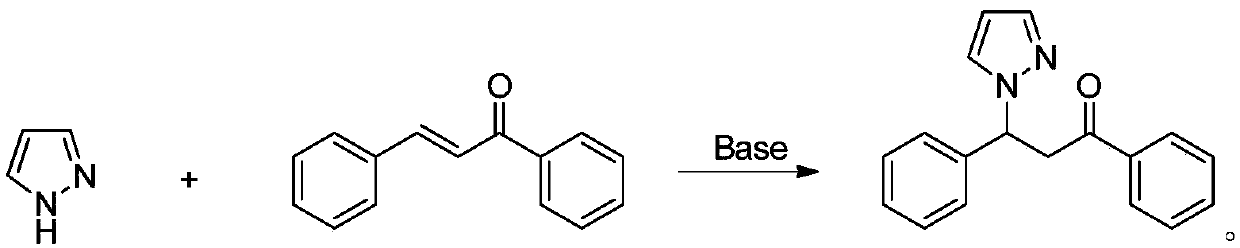
Method for synthesizing beta-(N-pyrazolyl) ketone compound through base catalysis
InactiveCN110483399AThe synthesis process is simple and greenGood choiceOrganic chemistryOrganic solventRoom temperature
The invention relates to a method for synthesizing a beta-(N-pyrazolyl) ketone compound by base catalysis, which comprises the following steps of dissolving pyrazole and alpha, beta-unsaturated ketonein an organic solvent, adding base, reacting at a room temperature for 6-12 hours, and separating and purifying to obtain the beta-(N-pyrazolyl) ketone compound. Compared with the prior art, the method has the advantages of simple and green synthesis process, excellent selectivity, higher yield and wide substrate range, and has the wide application value in the fields of biology, pharmaceutical chemistry industry and the like.
Owner:SHANGHAI INST OF TECH
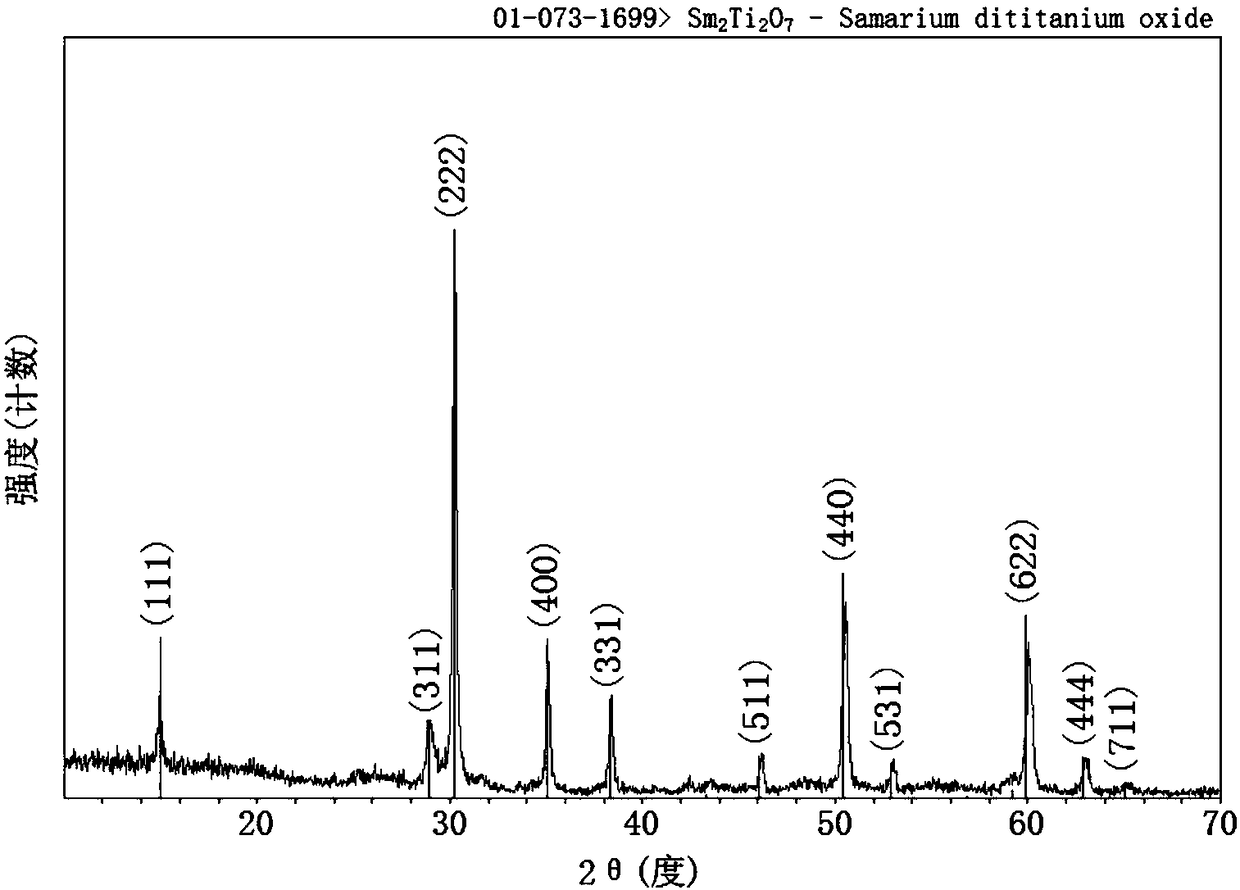
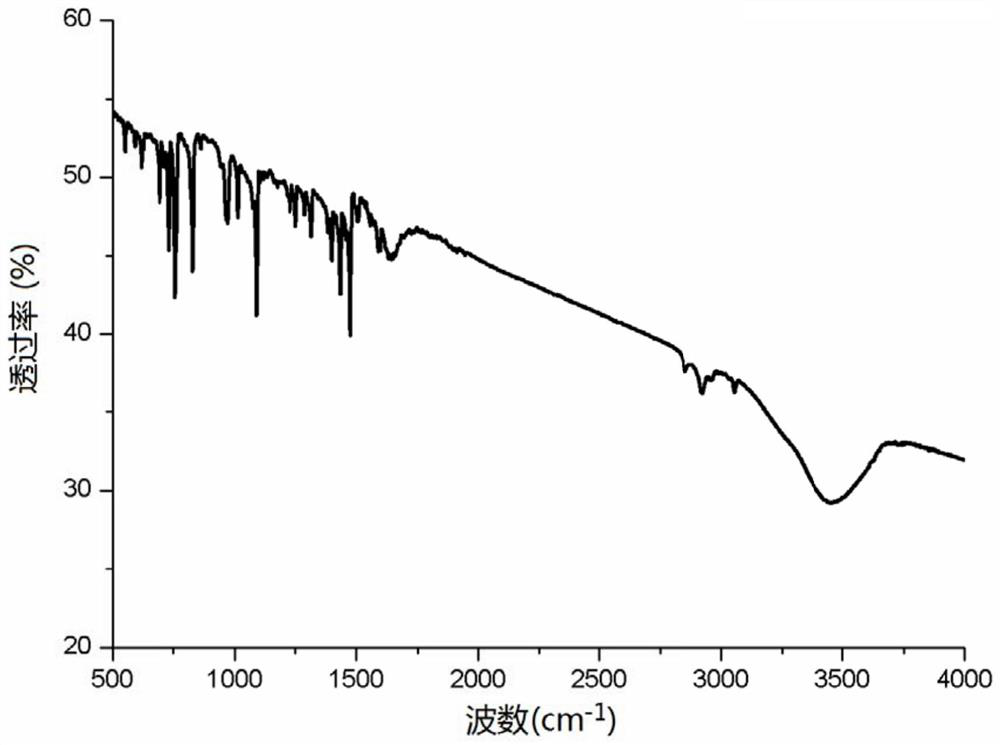
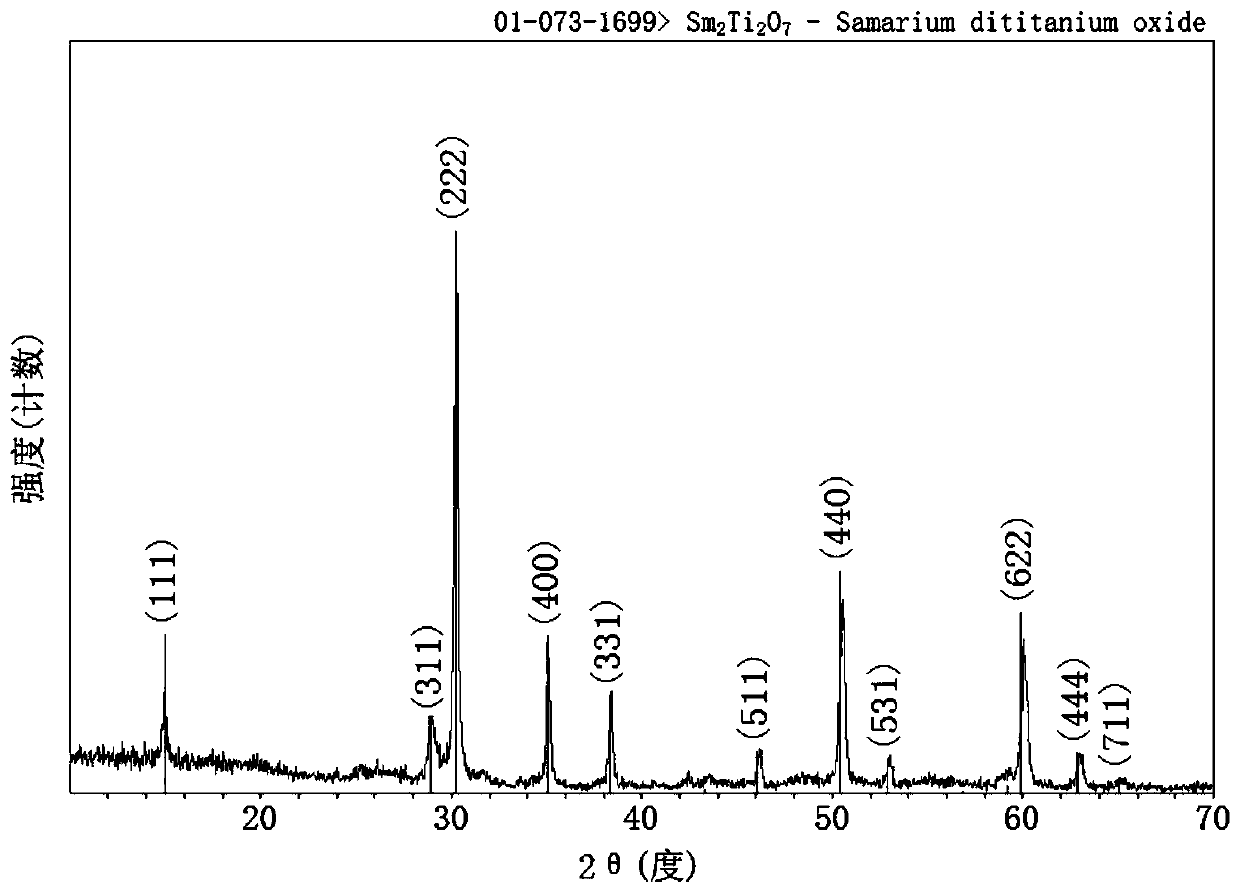
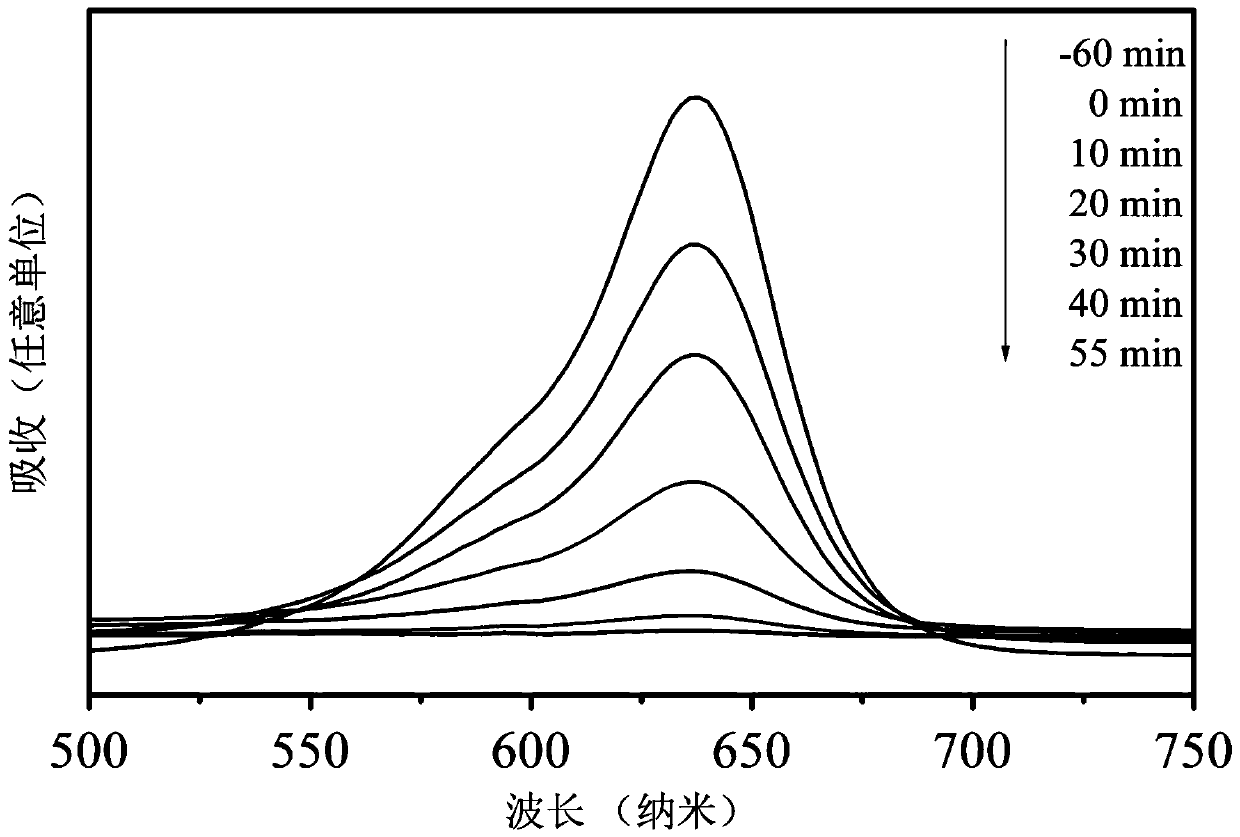
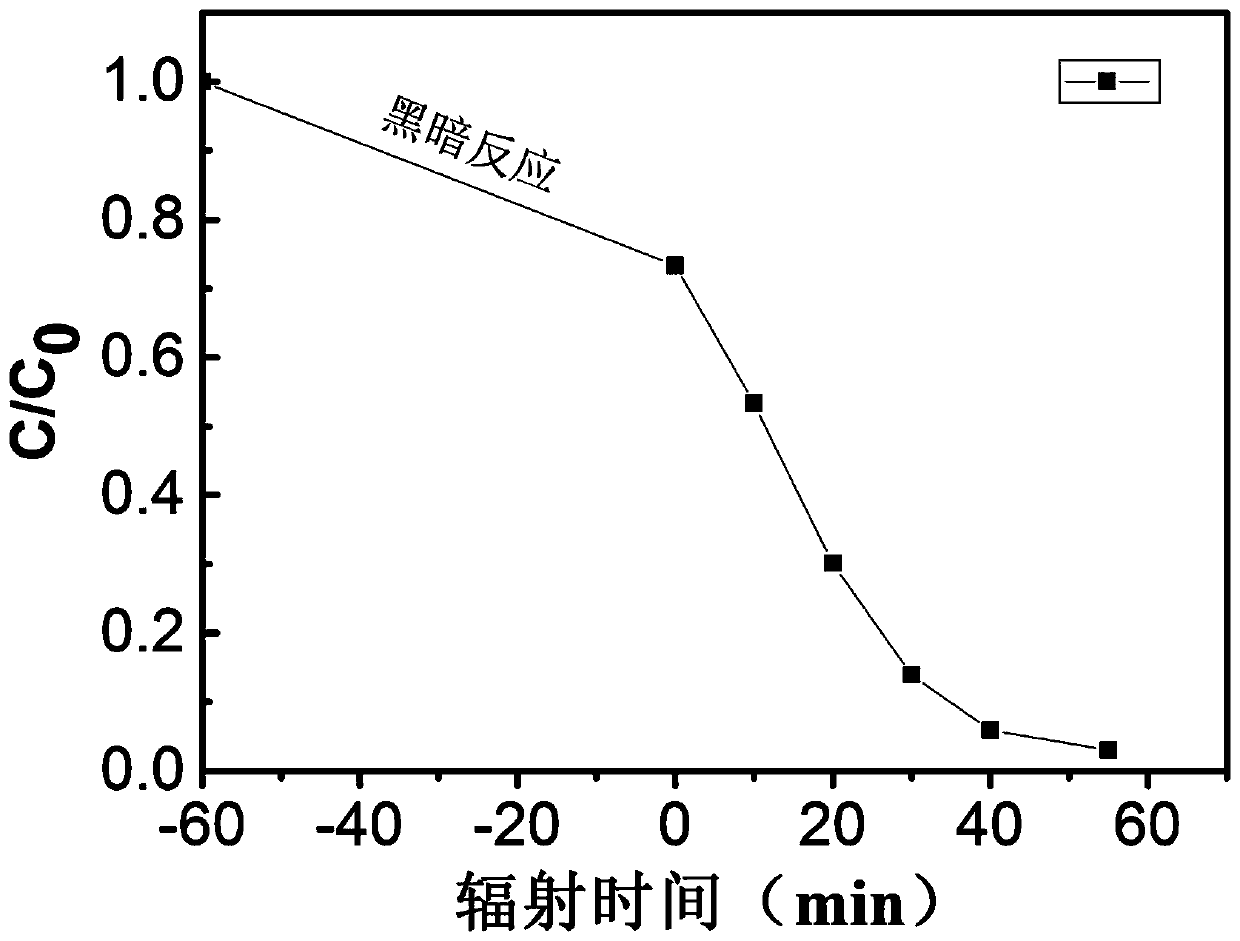
Single-phase samarium titanate nano-powder prepared by using solution method, and method for preparing single-phase samarium titanate nano-powder
ActiveCN108083328AControl stoichiometric ratioNo harmMaterial nanotechnologyTitanium compoundsPhysical chemistryWhite powder
The invention relates to single-phase samarium titanate nano-powder prepared by using a solution method, and a method for preparing the single-phase samarium titanate nano-powder. The method comprisesthe following steps: firstly dispersing a samarium source and a titanium source into a solvent according to a molar ratio of 1 to (1.1-1.3) to obtain a solution A; adjusting the pH value of the solution A to be neutral to obtain a milk-white mixed solution B; separating the milk-white mixed solution B to obtain white powder; drying the powder obtained in the third step, and then calcining to obtain the single-phase samarium titanate nano-powder. According to the method, the aim of accurately controlling the stoichiometric ratio of ternary Sm-Ti-O chemical elements is achieved by fully dissolving and dispersing the samarium source and the titanium source in a solvent medium as well as mixing and reacting the samarium source and the titanium source at an atomic scale, so that other impurities are prevented from appearing, and the aim of preparing the single-phase Sm2Ti2O7 is further realized.
Owner:成都莒纳新材料科技有限公司
A heterojunction interface doped composite photocatalyst and its preparation method
InactiveCN107308978BEasy to separateInterface doping convenienceHydrocarbon from carbon oxidesCatalyst activation/preparationHeterojunctionMaterials science
The invention discloses a heterojunction interface doped composite photocatalyst and a preparation method. The prepared heterojunction interface is doped with Bi 12 O 17 Cl 2 / g‑C 3 N 4 Composite photocatalyst has a strong ability to convert carbon dioxide into methane under visible light. The present invention adopts g-C 3 N 4 and Bi 12 O 17 Cl 2 The composite of nanosheets makes it easier to form a large-area heterojunction, thus promoting the separation of carriers; through thermal diffusion, the Bi 12 O 17 Cl 2 The bismuth atoms on the g-C were successfully doped into 3 N 4 The lattice induces a super strong electric field at the heterojunction interface, achieving super visible light reduction performance of carbon dioxide; porous g‑C 3 N 4 The extremely high specific surface area and countless micropores facilitate interface doping; the matching band gap structure and interface doping successfully control the flow direction of carriers, achieve selective reduction of carbon dioxide into methane, and enhance the recycling of photocatalysts. Capability; material synthesis is simple, green, large-scale, and has good prospects for industrial application.
Owner:HUAIBEI NORMAL UNIVERSITY
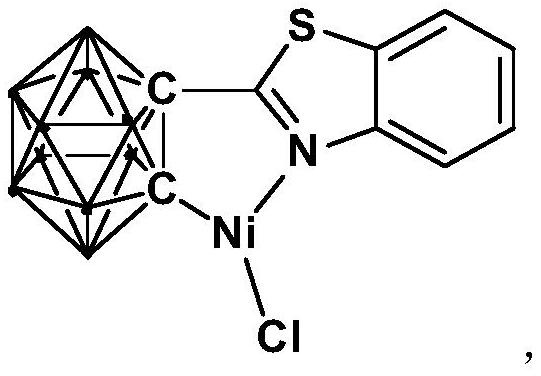
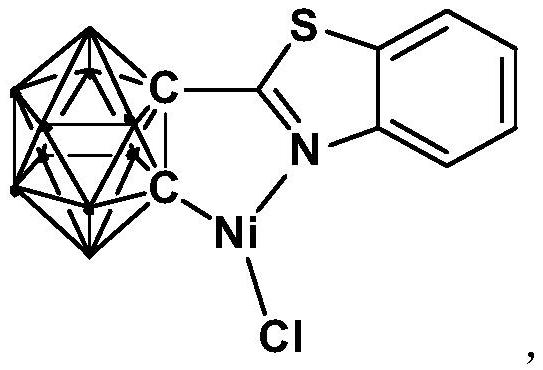
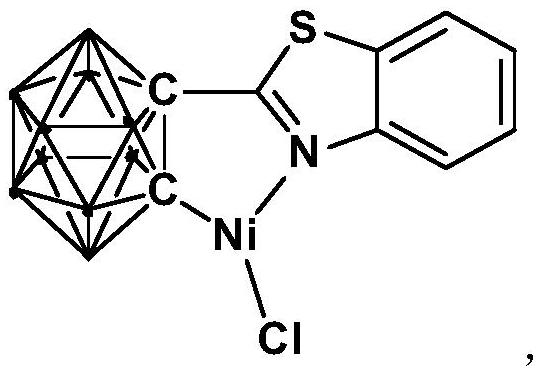
Method for catalytically synthesizing aryl oxazole compound by using nickel complex
ActiveCN114276311AImprove universalityHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsBenzoxazolePtru catalyst
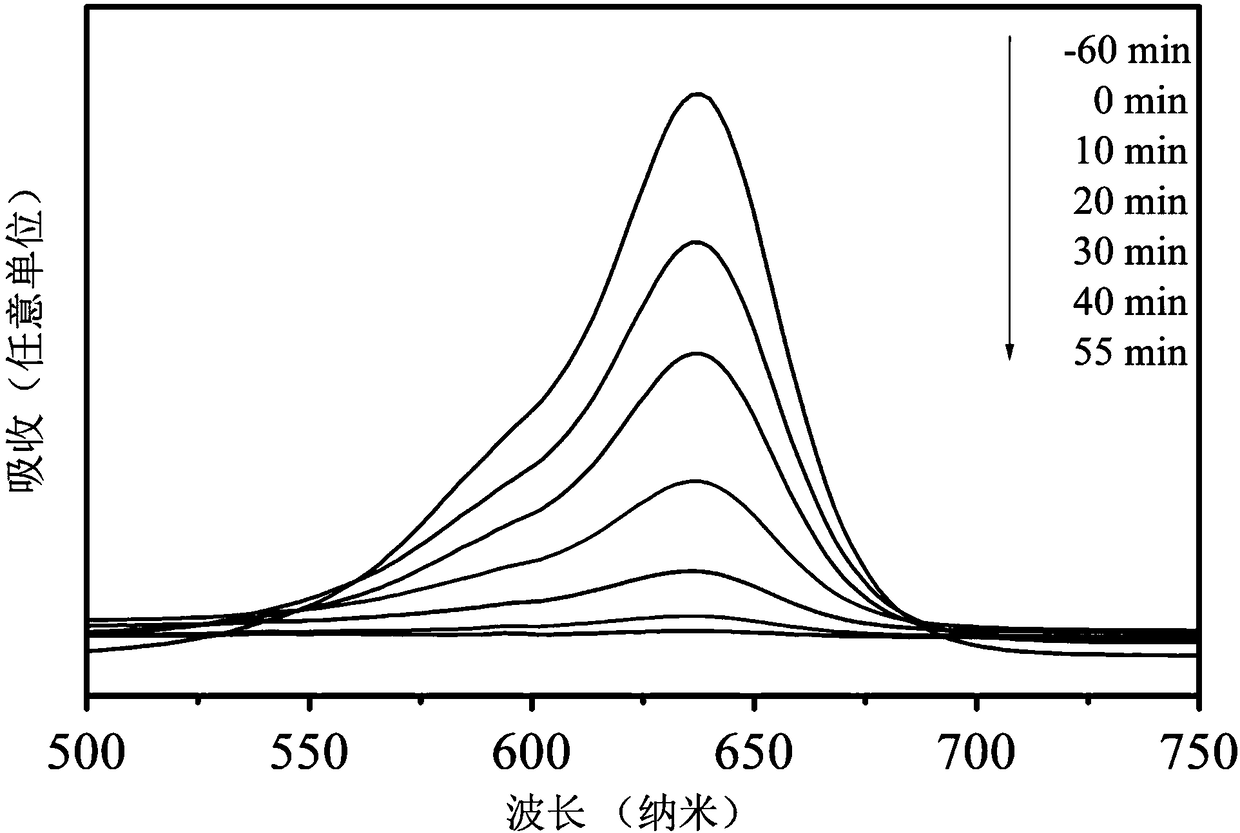
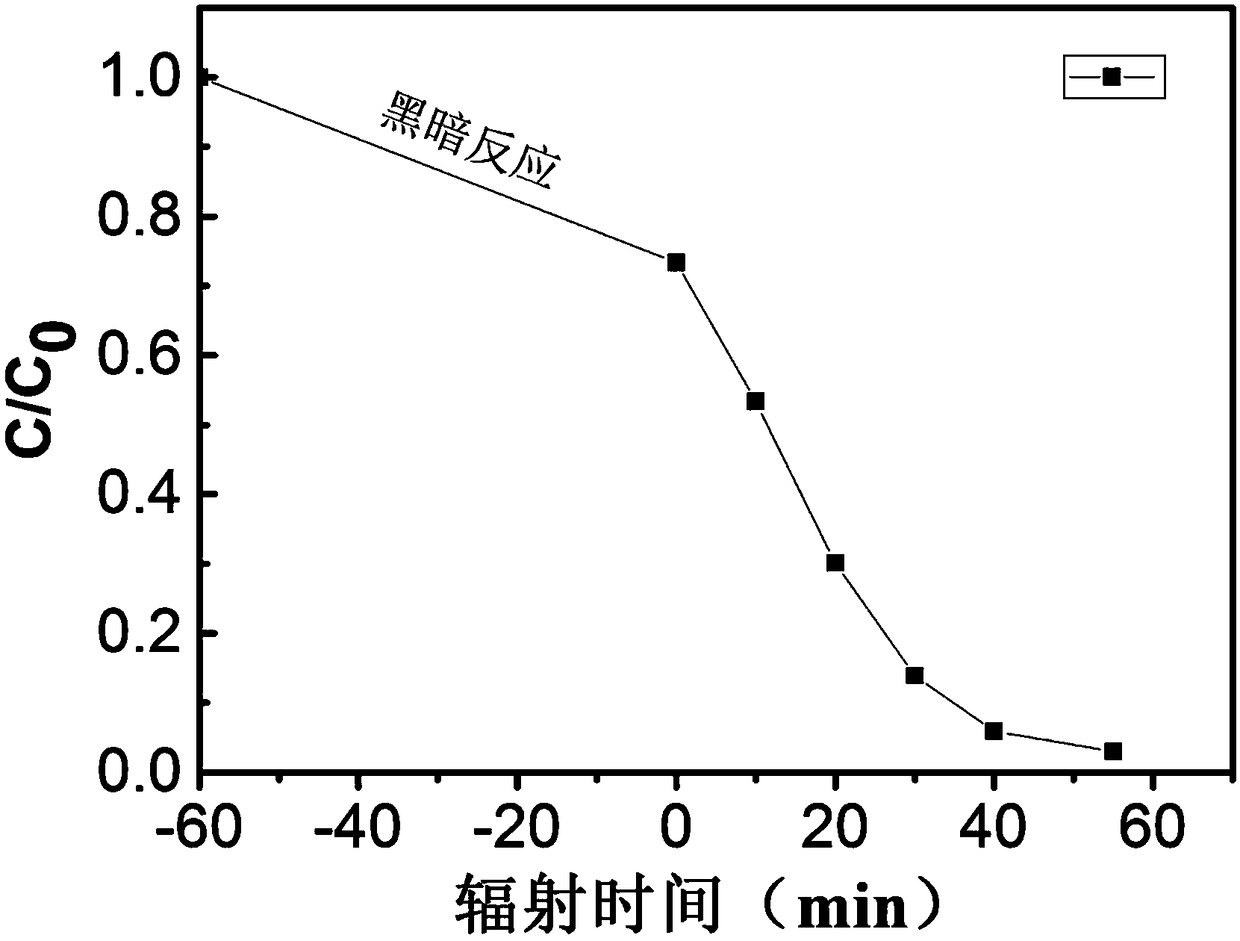
The invention relates to a method for catalytically synthesizing an aryl oxazole compound by using a nickel complex, which comprises the following steps: by taking the nickel complex containing an ortho-carborane benzothiazole structure as a catalyst and taking benzoxazole and aryl halide compounds as raw materials, carrying out oxidative coupling reaction at room temperature in the presence of alkali, thereby obtaining the aryl oxazole compound. The aryl oxazole compound is obtained. Compared with the prior art, the method has the advantages that the nickel complex containing the ortho-carborane benzothiazole structure is utilized to efficiently catalyze the multi-component reaction at room temperature to synthesize the aryl oxazole compound through the one-pot method, synthesis of the aryl oxazole compound at room temperature is achieved, the using equivalent of the catalyst is low, the reaction condition is mild, the substrate universality is high, raw materials are low in price and easy to obtain, and the method is suitable for industrial production. The product yield is high.
Owner:SHANGHAI INST OF TECH
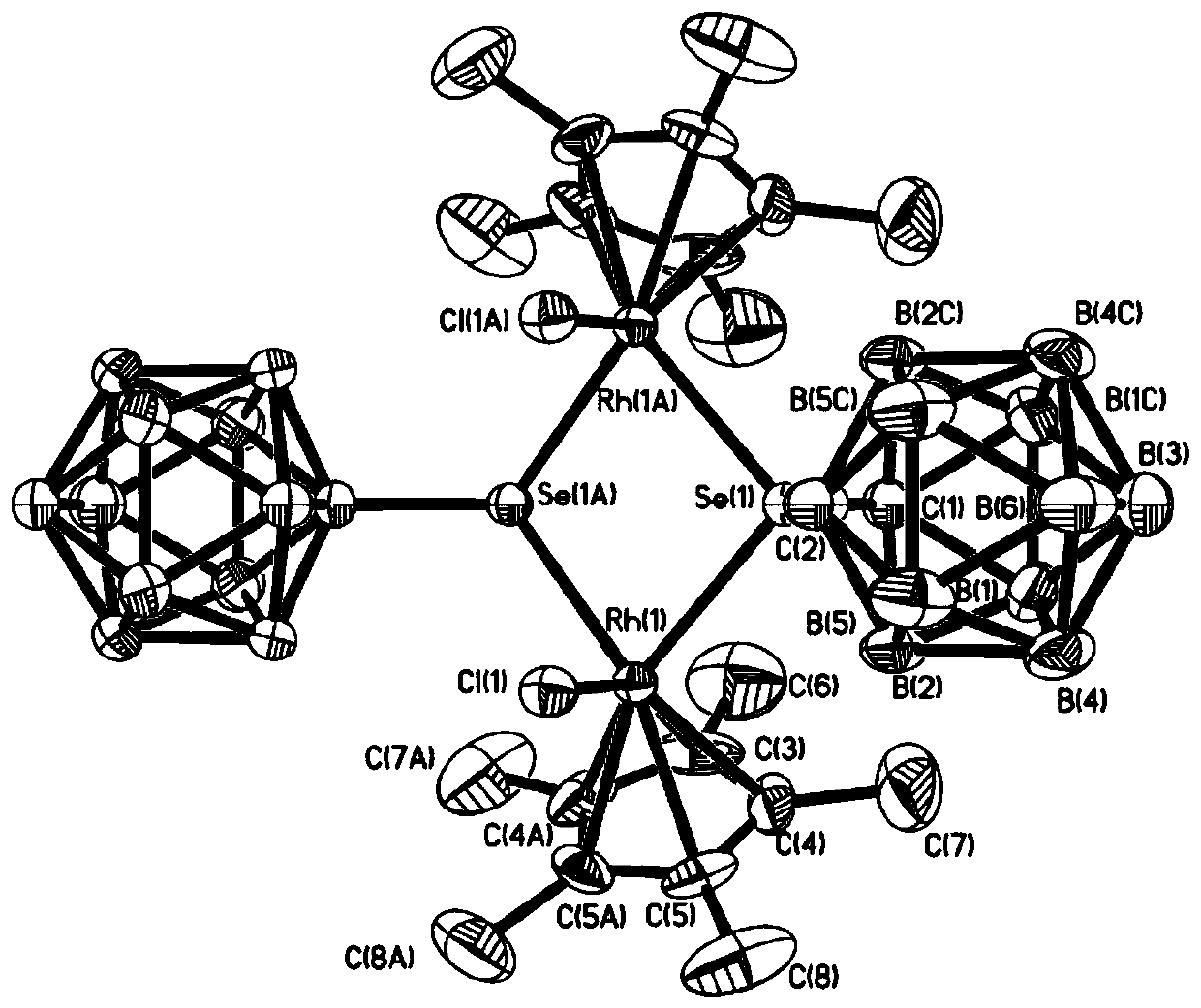
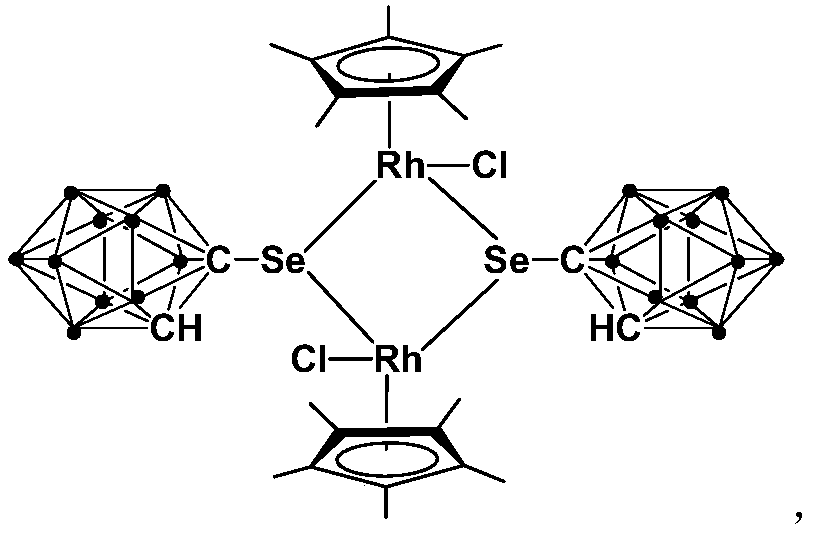
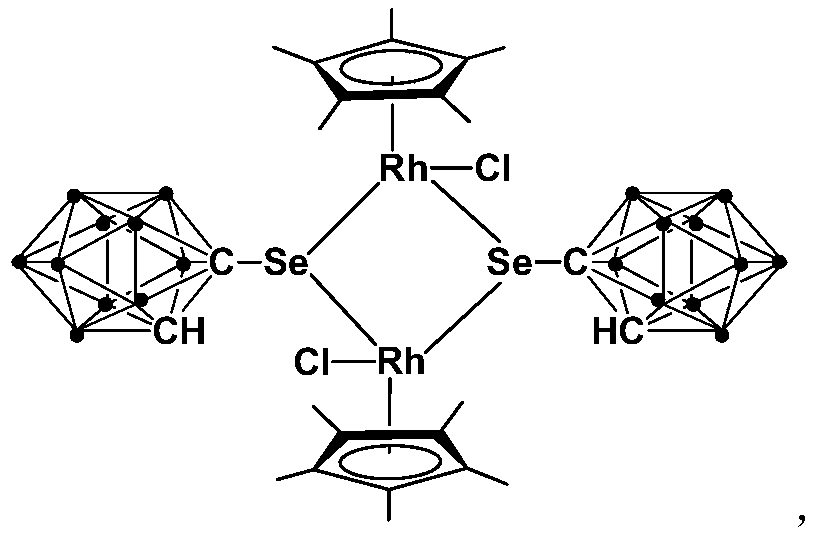
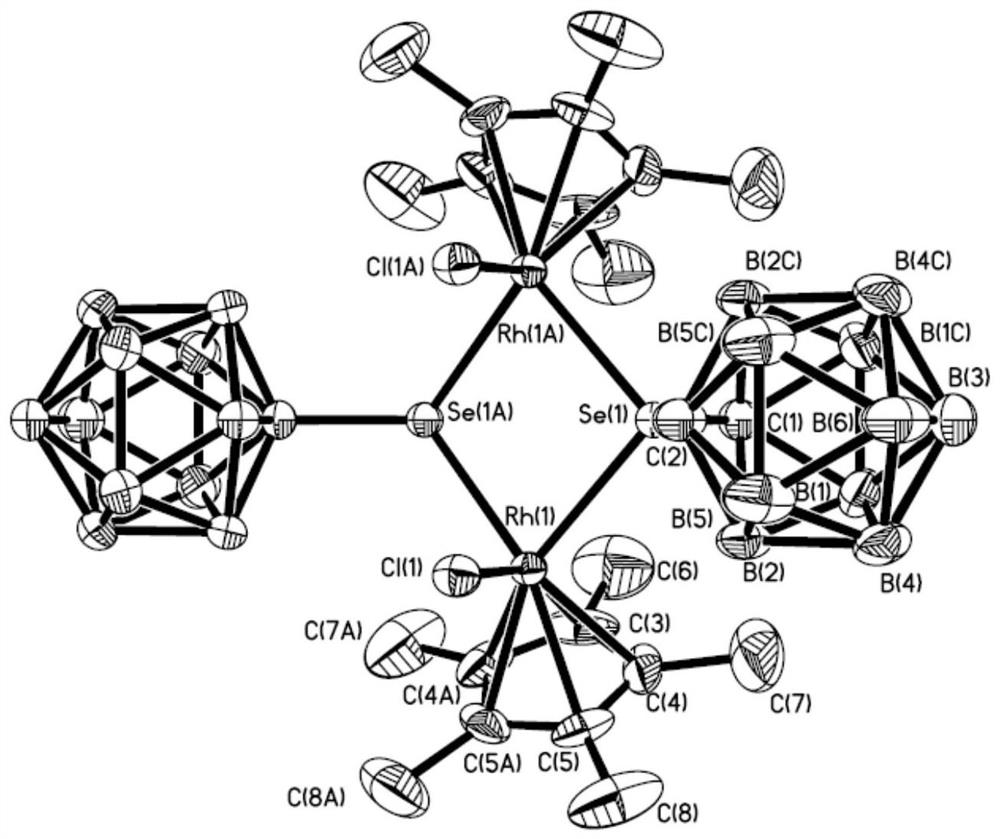
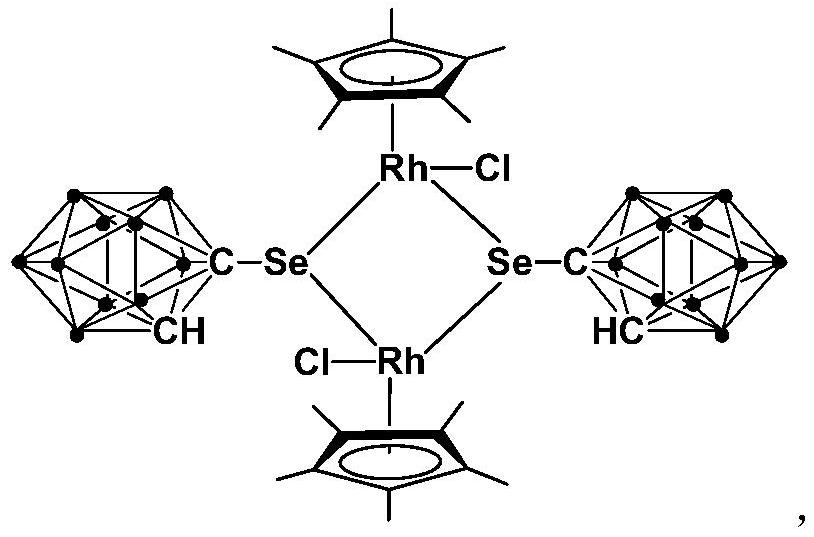
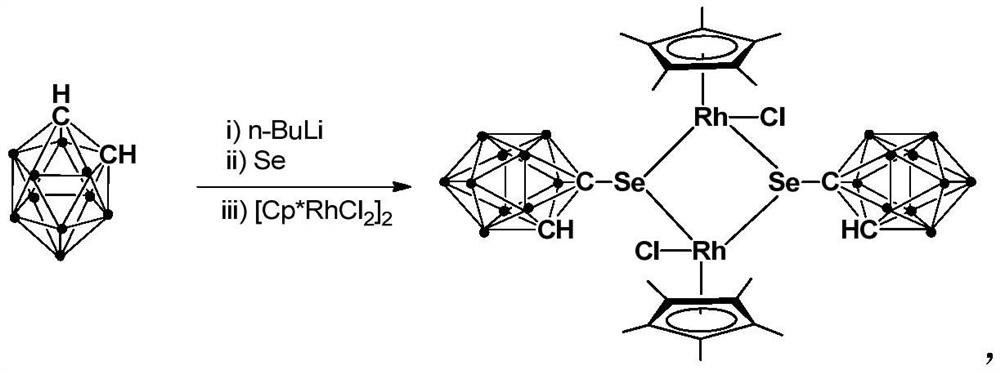
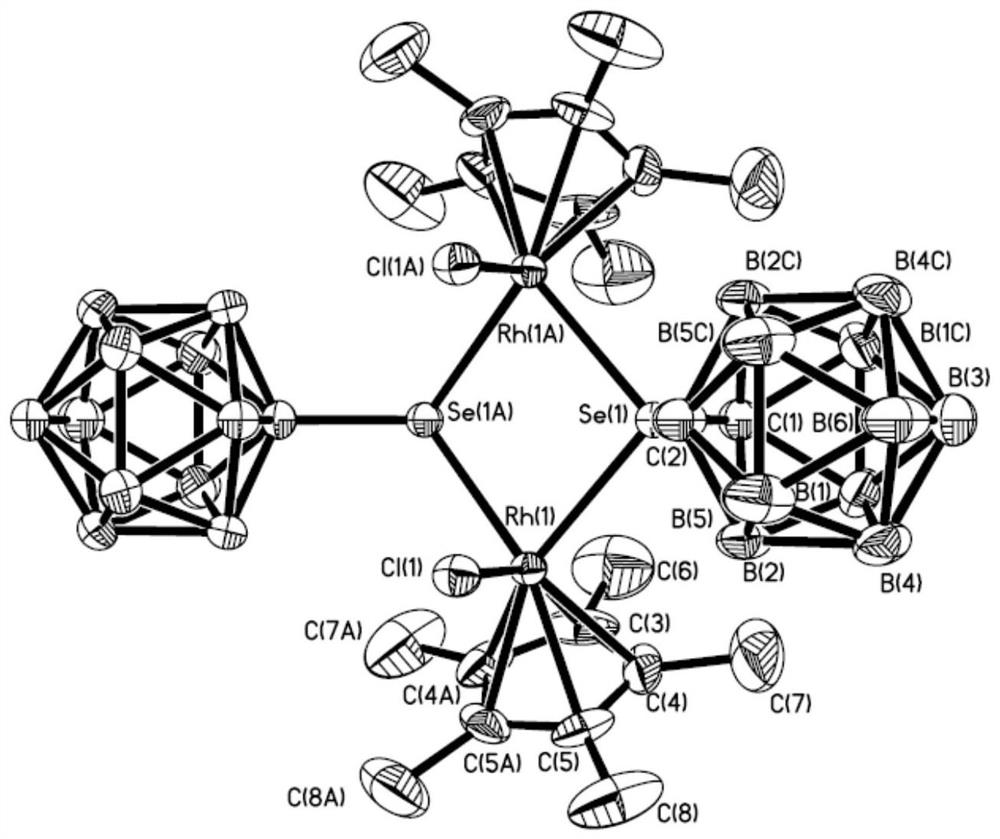
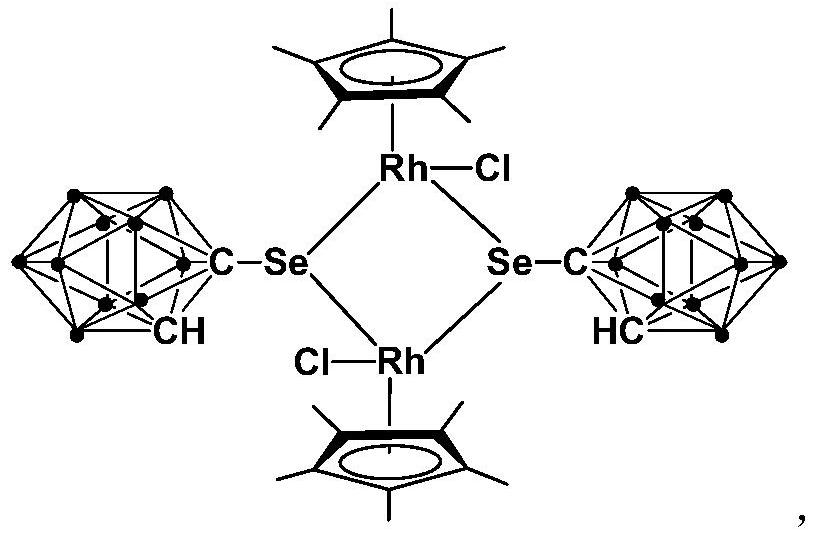
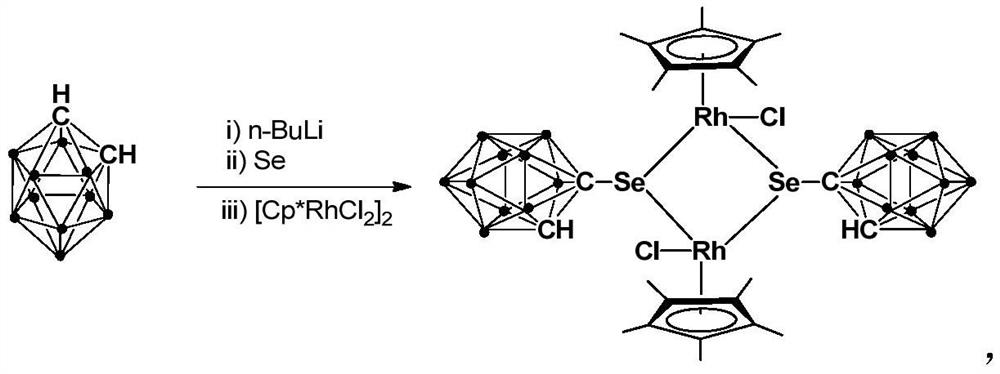
A kind of binuclear rhodium complex containing ortho carborane structure and its preparation and application
ActiveCN110240620BThe synthesis process is simple and greenGood choiceRhodium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrtho positionCombinatorial chemistry
The invention relates to a binuclear rhodium complex containing an ortho-carborane structure and its preparation and application. The preparation method of the rhodium complex comprises the following steps: 1) adding n-BuLi solution to the ortho-carborane solution, Then react at room temperature for 30-60min; 2) add selenium, and react at room temperature for 1-2h; 3) add [Cp*RhCl 2 ] 2 , and react at room temperature for 3‑6h, and obtain the rhodium complex after post-treatment; the rhodium complex is used to catalyze the N-methylation reaction of arylamine to prepare arylamine N-methylated derivatives. Compared with the prior art, the preparation method of the binuclear semi-sandwich rhodium complex containing the ortho-carborane structure in the present invention is simple and green, has excellent selectivity and higher yield, and the prepared rhodium complex is stable at room temperature It has high catalytic activity, can be used to catalyze the N-methylation reaction of arylamines to prepare N-methylated derivatives of arylamines, has high catalytic reaction yield, and has wide industrial application prospects.
Owner:SHANGHAI INST OF TECH
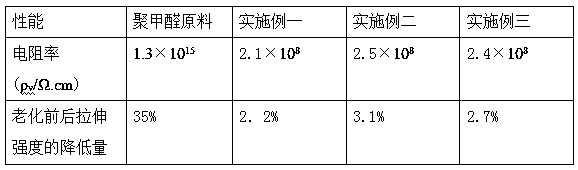
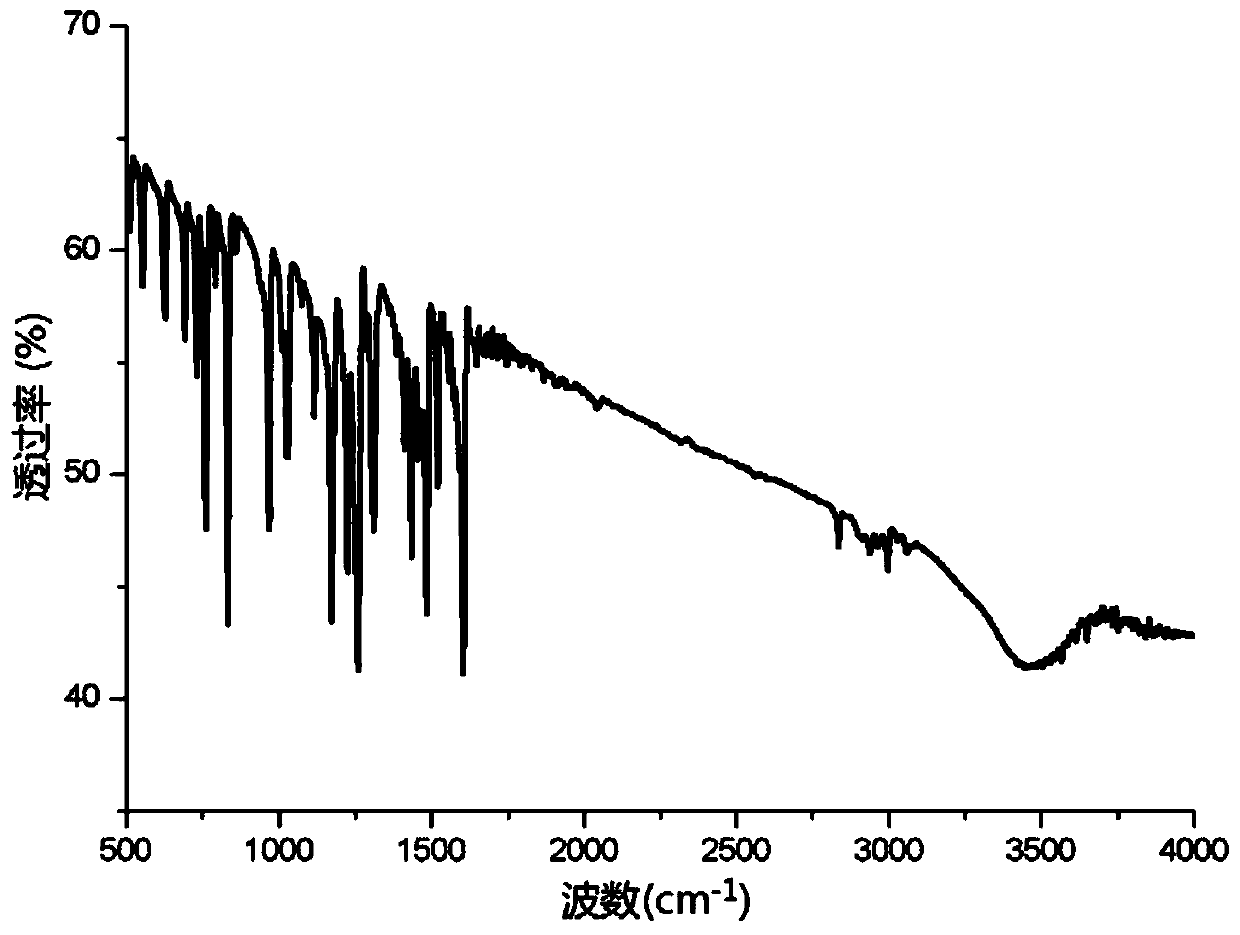
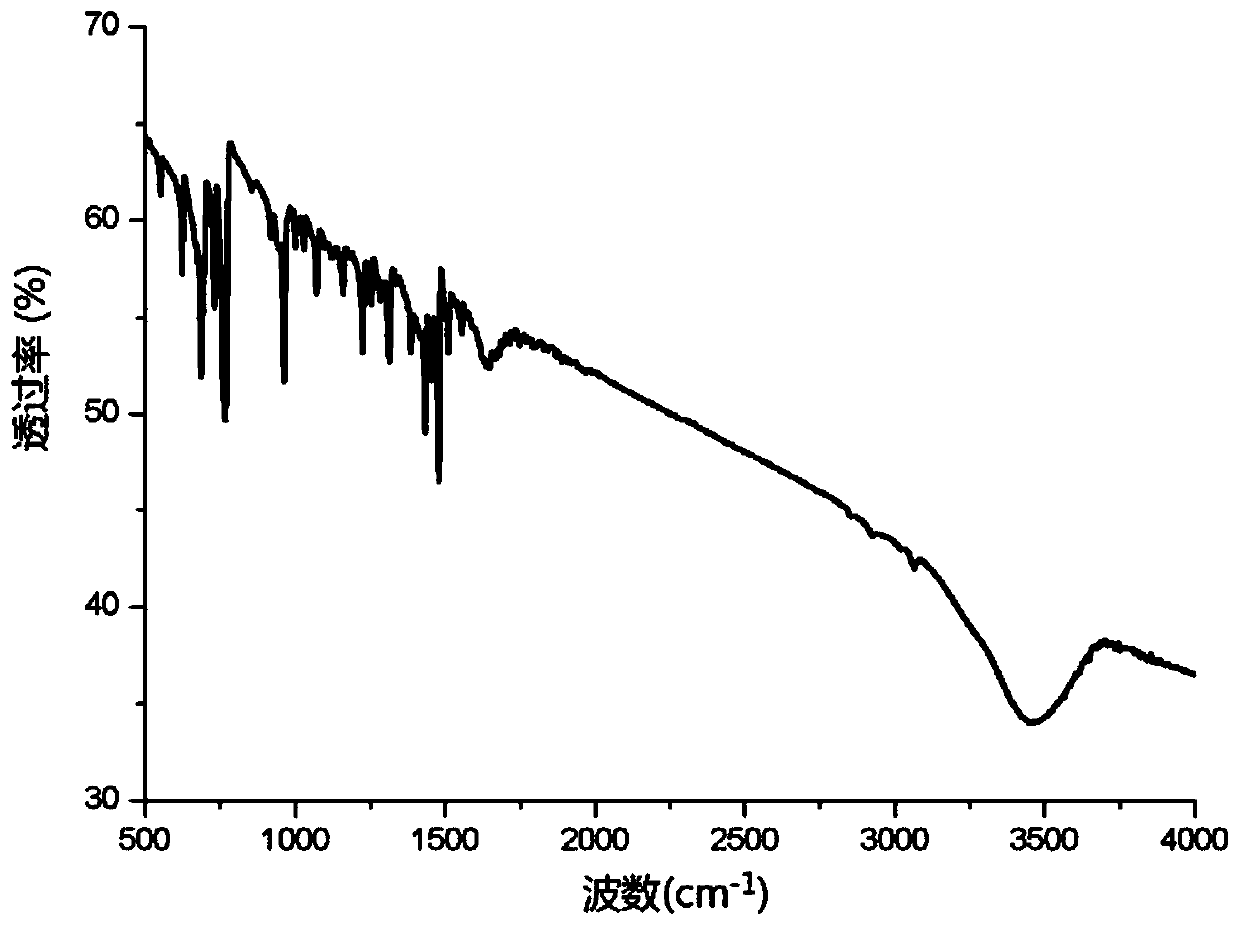
A kind of preparation method of light-stable antistatic polyoxymethylene material
ActiveCN107099115BImprove antistatic performanceImprove photostabilityAntistatic agentPolymer science
The invention discloses a preparation method of a photostable antistatic polyformaldehyde material. In the method, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxygen free radical (TEMPO), which has photostability and is used as a hydrogen bond donor and a hydrogen bond receptor, is used for preparing a photostable antistatic low-cofusion reagent, which is then mixed with an antistatic agent and polyformaldehyde uniformly, and the mixture is subjected to extruding granulation in a twin-screw extruder to obtain antistatic master batches; the antistatic master batches are uniformly mixed with a thermal stabilizer, an antioxidant and pure polyformaldehyde, and the mixture is subjected to extruding granulation in the twin-screw extruder; the granules finally are dried and injection-moulded to form the photostable antistatic polyformaldehyde material. The photostable antistatic polyformaldehyde material can significantly improve antistatic performance and photostability of a material in the field of electronics industry.
Owner:广东盟信塑胶实业有限公司
Method for synthesizing enamine under catalysis of palladium imine complex containing pyridine ligands
ActiveCN110183331AImprove stabilityImprove thermal stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsBenzenePhenyl-acetylene
The invention relates to a method for synthesizing enamine under catalysis of a palladium imine complex containing pyridine ligands. The method comprises steps as follows: phenylacetylene and secondary amine are taken as raw materials, the palladium imine complex containing the pyridine ligands is taken as a catalyst, a hydroamination reaction is performed in a solvent, aftertreatment is performed, and enamine is obtained. Compared with the prior art, the method for preparing enamine compounds through the hydroamination reaction of phenylacetylene under the catalysis of a divalent palladium imine complex containing the pyridine ligands is provided, raw materials are simple and easy to obtain, a synthesis process is simple and green, the method has excellent selectivity and high yield, theatom economy is high, three wastes are reduced, and the method is environmentally friendly, simple and convenient to operate and suitable for industrial synthesis of the high-value enamine compounds.
Owner:SHANGHAI INST OF TECH
A method for improving the efficiency of cellulose enzymatic hydrolysis of rice straw by using lactic acid and guanidine hydrochloride
InactiveCN106480128BImprove enzymatic hydrolysis efficiencyHigh yieldFermentationBiotechnologyEnzymatic hydrolysis
The invention belongs to the fields of lignocellulose utilization and energy and chemical application, and discloses a green method for increasing a cellulose enzymolysis efficiency in rice straws by utilizing lactic acid / guanidine hydrochloride to extract hemicellulose. The green method includes: taking the lactic acid / the guanidine hydrochloride as a solvent to preprocessing the rice straws, filtering and separating to obtain residues, and drying to obtain preprocessed rice straws; taking the rice straw residues as a base material, and subjecting the base material to enzymolysis by cellulose so as to obtain a liquid sugar which mainly comprises glucose. The green method has the advantages that the enzymolysis efficiency of the rice straws can be increased effectively, a yield rate of fermentable reducing sugar is increased, and the defects of high cost and environmental unfriendliness of an ionic liquid preprocessing process are overcome.
Owner:GUANGDONG UNIV OF TECH
Method for catalytically synthesizing N-arylated derivative of pyrimidine-2-amine
ActiveCN114315737AThe synthesis process is simple and greenGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsPtru catalystCombinatorial chemistry
The invention relates to a method for catalytically synthesizing an N-arylated derivative of pyrimidine-2-amine, which comprises the following steps: by taking an N, N-coordinated meta-carborane ligand-containing cuprous complex as a catalyst and taking pyrimidine-2-amine compounds and aryl halide compounds as raw materials, carrying out coupling reaction at room temperature to obtain the N-arylated derivative of pyrimidine-2-amine. The N-arylation derivative of the pyrimidine-2-amine is obtained. Compared with the prior art, the preparation method disclosed by the invention has the advantages that the N, N-coordinated cuprous complex containing the meta-carborane ligand is used as the catalyst, has higher catalytic activity under a mild condition, can be used for catalyzing Buchwald-Harwig coupling reaction of the pyrimidine-2-amine compound and the aryl halide compound to prepare the N-arylated derivative of pyrimidine-2-amine, is good in universality, and can be used for preparing the N-arylated derivative of pyrimidine-2-amine. The method has the advantages of mild reaction conditions, high catalytic efficiency, few byproducts, lower cost and easiness in separation of products.
Owner:SHANGHAI INST OF TECH
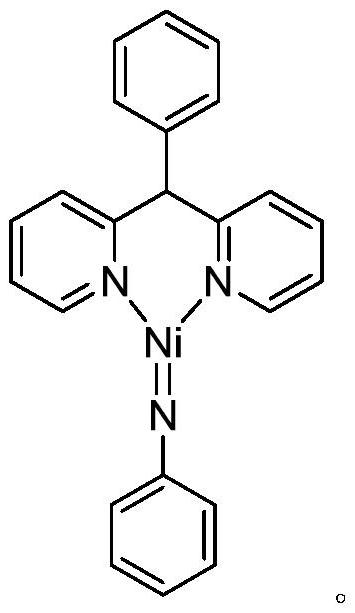
A kind of method that catalyzes the hydroamination reaction of cyclohexylacetylene to prepare enamine compounds
ActiveCN109896964BImprove stabilityImprove thermal stabilityAmino preparation from aminesPtru catalystEnamine
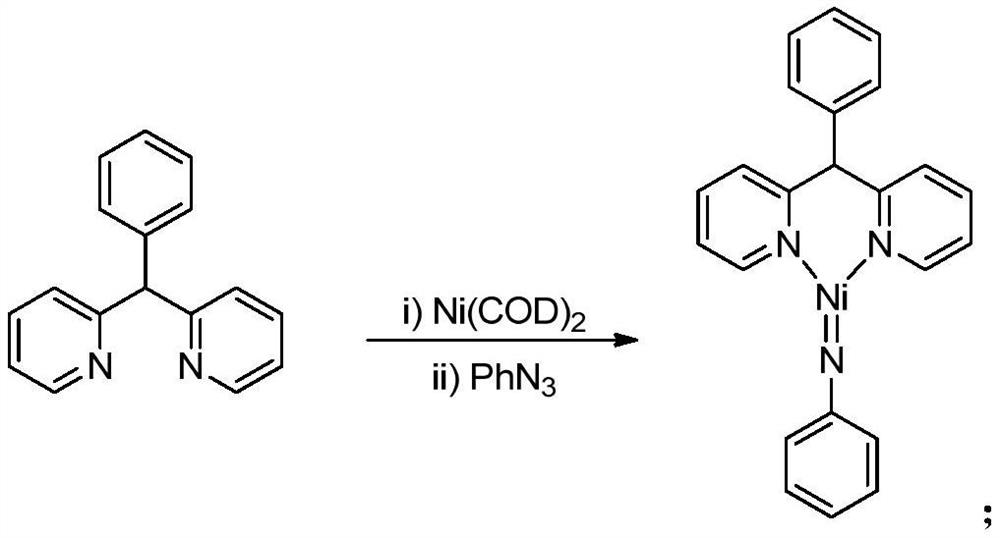
The invention provides a method for preparing enamine compounds by catalyzing the hydroamination reaction of cyclohexylacetylene, which is characterized in that, under the catalysis of the catalyst, cyclohexylacetylene and secondary amine undergo hydroamination reaction in a solvent , to obtain enamine compounds; the molecular formula of the catalyst is [R 1 R 2 C(C 5 h 4 N) 2 ] Pd=NPh, wherein, R 1 and R 2 independently selected from H, CH 3 and Ph, the solvent is an aromatic hydrocarbon, the reaction temperature is 60-100°C, and the reaction time is 6-12h. The invention has mild reaction conditions, high catalytic efficiency, high atom economy, low cost, easy separation of products, no large amount of waste residue, and is suitable for industrial production.
Owner:SHANGHAI INST OF TECH
Single-phase samarium titanate nanopowder prepared by a solution method and method thereof
ActiveCN108083328BControl stoichiometric ratioNo harmMaterial nanotechnologyTitanium compoundsBarium titanateChemical element
The invention relates to a single-phase samarium titanate nanopowder prepared by a solution method and a method thereof. First, the samarium source and the titanium source are dispersed in a solvent according to a molar ratio of 1: (1.1 to 1.3) to obtain solution A; solution A is adjusted to neutral pH value to obtain a milky white mixed solution B; separate the milky white mixed solution B to obtain a white powder; dry the powder obtained in step three and then calcine to obtain single-phase samarium titanate nanopowder . In the present invention, the samarium source and titanium source are fully dissolved and dispersed in the solvent medium, and the samarium source and titanium source are mixed and reacted at the atomic scale to achieve precise control of the chemistry between the ternary Sm-Ti-O chemical elements. The purpose of the stoichiometric ratio is to prevent the appearance of other impurities and achieve the preparation of single-phase Sm 2 Ti 2 O 7 The goal.
Owner:成都莒纳新材料科技有限公司
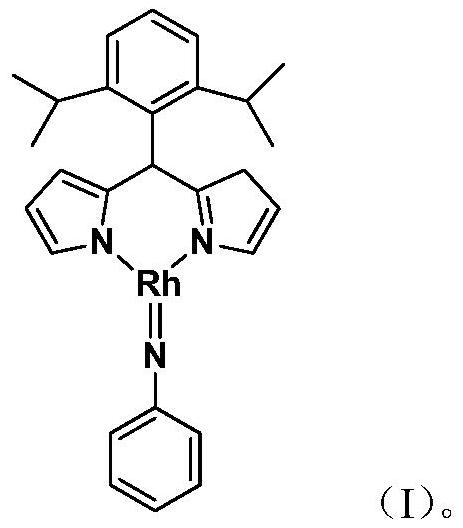
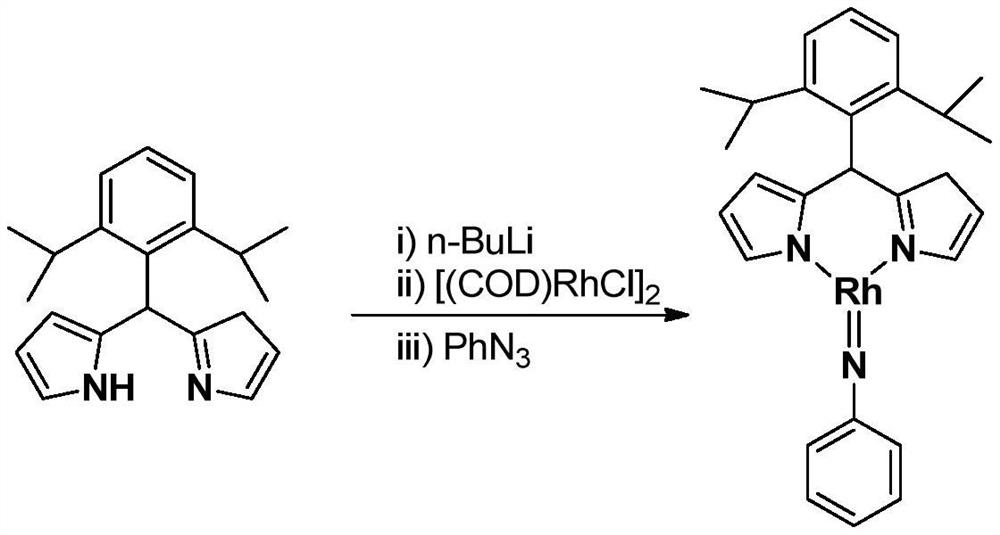
Preparation and application of a large sterically hindered trivalent rhodium imine complex
ActiveCN109651445BEasy to prepareHigh yieldRhodium organic compoundsOrganic compound preparationImideRegioselectivity
Owner:SHANGHAI INST OF TECH
n,n-Coordinated Palladium Complex Containing Meta-Carborane Ligand and Its Preparation and Application
ActiveCN110372755BThe synthesis process is simple and greenGood choiceOrganic compound preparationGroup 8/9/10/18 element organic compoundsFormylation reactionCombinatorial chemistry
The present invention relates to N,N-coordinated palladium complexes containing meta-carborane ligands and preparation and application thereof. The preparation method of palladium complexes comprises the following steps: 1) adding n-BuLi solution to meta-carbon In the borane solution, react at room temperature for 30-60min; 2) add 3-chloromethylpyridine, and react at room temperature for 3-5h; 3) add PdCl 2 , and reacted at room temperature for 2-5 h, and the palladium complex is obtained after post-treatment; the palladium complex is used to catalyze the arylamine formylation reaction to prepare the arylamine carboxamide compounds. Compared with the prior art, the synthesis process of the palladium complex in the present invention is simple and green, and has excellent selectivity and high yield; the palladium complex has the characteristics of stable physical and chemical properties, thermal stability, etc. It exhibits excellent catalytic activity in the acylation reaction.
Owner:SHANGHAI INST OF TECH
Divalent nickel-imine complexes containing nickel-nitrogen double bond structure and its preparation and application
ActiveCN110204580BImprove thermal stabilityThe synthesis process is simple and greenOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAzidobenzeneRegioselectivity
The invention relates to a nickelous imine complex with a nickel nitrogen double-bond structure and preparation and application of the nickelous imine complex. The preparation method of the nickelousimine complex comprises the following steps that 1, a phenyldipyridine ligand solution is added into a zero-valent nickel precursor solution for a reaction for 3-5 hours at room temperature; 2, azidobenzene is added for a reaction for 2-5 hours at room temperature, and through aftertreatment, the nickelous imine complex is obtained. The nickelous imine complex is used for catalyzing an anti-Markovnikov hydroamination reaction of styrene to prepare a linear-chain amine compound. Compared with the prior art, the synthesizing technology is simple and green, the selectivity and the yield are high,the nickelous imine complex prepared by using the method has the advantages of stable physicochemical properties, thermal stability and the like, and the nickelous imine complex can show excellent activity and regioselectivity in the reaction of catalyzing the anti-Markovnikov hydroamination reaction of the styrene.
Owner:SHANGHAI INST OF TECH
Application of a dinuclear rhodium complex in n-methylation reaction of aliphatic amines
ActiveCN110201720BThe synthesis process is simple and greenGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsAmino preparation by functional substitutionPtru catalystOrganic solvent
The present invention relates to the application of a kind of binuclear rhodium complex in fatty amine N-methylation reaction, with binuclear rhodium complex as catalyst, with CH 3 I, as a methylating agent, catalyzes the N-methylation reaction of fatty amines in an organic solvent to prepare N-methylated derivatives of fatty amines. Compared with the prior art, the binuclear rhodium complex in the present invention has higher catalytic activity at room temperature, and can be used to catalyze the N-methylation reaction of fatty amines to prepare N-methylated derivatives of fatty amines, and the catalytic reaction The yield is high (90‑97%), the reaction conditions are mild, no strong base is required, all reagents are stable to air and water, the requirements for reaction equipment are not high, and there is a wide range of industrial application prospects.
Owner:SHANGHAI INST OF TECH
Application of a kind of palladium complex in fatty carbamylation reaction
ActiveCN110368989BThe synthesis process is simple and greenGood choiceOrganic compound preparationGroup 8/9/10/18 element organic compoundsPtru catalystFormylation reaction
The invention relates to the application of a palladium complex in fatty carbamylation reaction, using N,N-coordinated palladium complex containing meta-carborane ligand as catalyst, and N,N-dimethyl Formamide is used as a formylating reagent and solvent to catalyze the formylation reaction of fatty amines to prepare fatty amine formamide compounds. Compared with the prior art, in the present invention, the divalent palladium complex containing meta-carborane ligands coordinated by N,N has higher catalytic activity under mild conditions, and can catalyze the formylation reaction of aliphatic amines , DMF is used as a formylating reagent and a solvent at the same time, it is cheap, low-toxic and easy to separate, and the yield is high (88‑96%).
Owner:SHANGHAI INST OF TECH
A kind of method that utilizes the palladium imine complex containing pyridine ligand to catalyze the synthesis of enamine
ActiveCN110183331BImprove stabilityImprove thermal stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsEnamineCombinatorial chemistry
The invention relates to a method for catalyzing and synthesizing enamines by utilizing palladium imine complexes containing pyridine ligands. , the hydroamination reaction occurs in the solvent, and the enamine is obtained after post-treatment. Compared with the prior art, the present invention provides a method for preparing enamine compounds by utilizing a divalent palladium imine complex containing a pyridine ligand to catalyze the hydroamination reaction of phenylacetylene. The raw materials are simple and easy to obtain, and the synthesis process is simple and green. , has excellent selectivity and high yield, high atom economy, reduces the generation of three wastes, is environmentally friendly, easy to operate, and is suitable for industrial synthesis of high-value enamine compounds.
Owner:SHANGHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

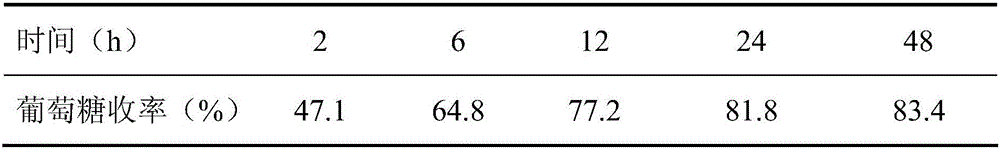
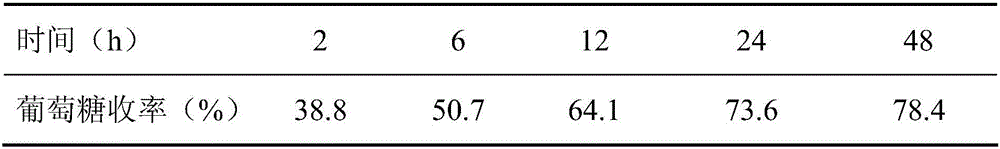

![Synthesis method of pyrrole[1,2-a]quinoxaline derivative Synthesis method of pyrrole[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/6f4f8bbf-a925-4a14-a63b-c794410a128c/HDA0002853513550000011.png)
![Synthesis method of pyrrole[1,2-a]quinoxaline derivative Synthesis method of pyrrole[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/6f4f8bbf-a925-4a14-a63b-c794410a128c/FDA0002853513530000011.png)
![Synthesis method of pyrrole[1,2-a]quinoxaline derivative Synthesis method of pyrrole[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/6f4f8bbf-a925-4a14-a63b-c794410a128c/BDA0002853513540000021.png)