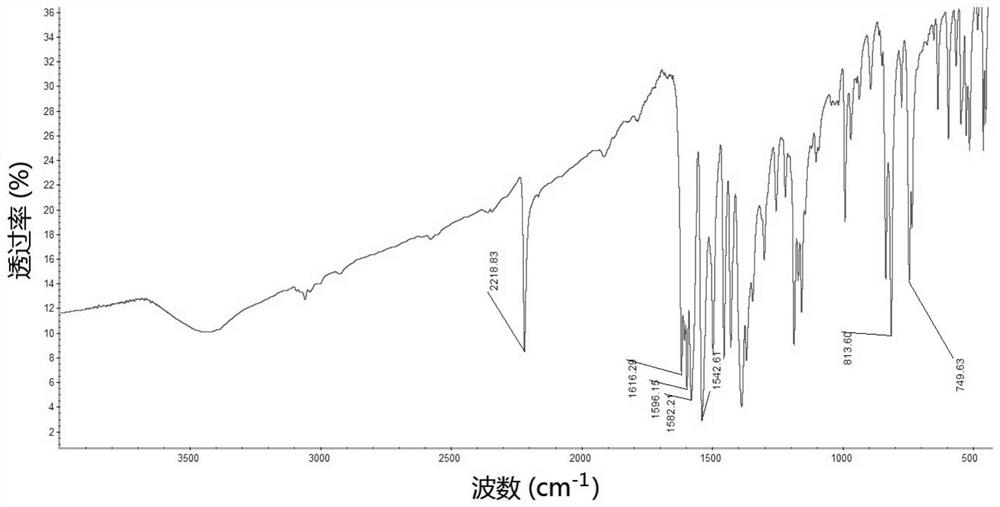
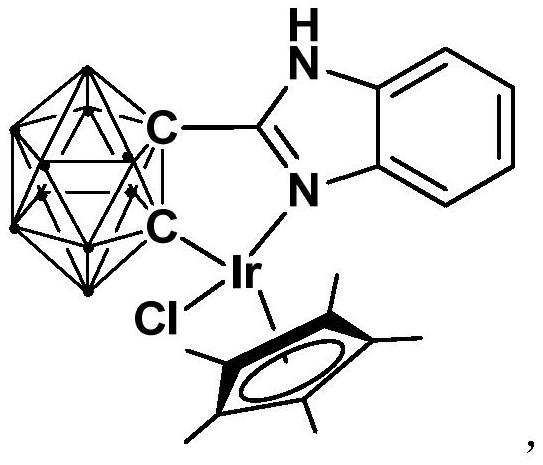
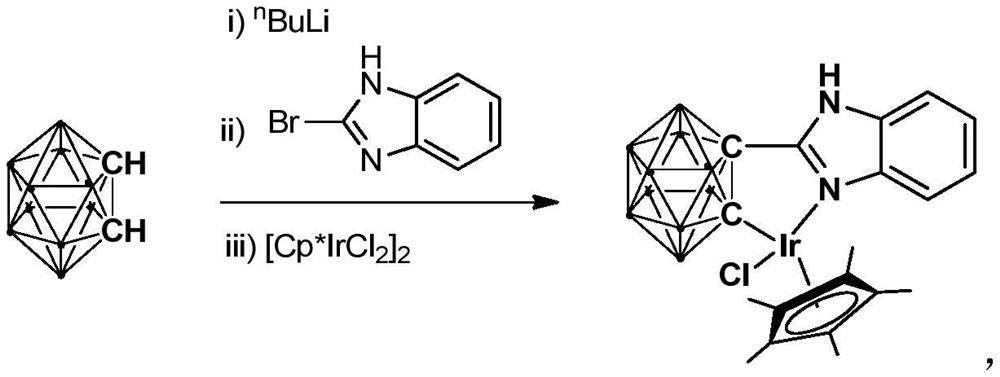
Iridium complex containing carboryl benzimidazole structure and its preparation method and application
A technology containing carboryl benzimidazole and carboryl benzimidazole, which is applied in the field of semi-sandwich iridium complexes and their preparation, can solve the problems of waste of reagents, environmental damage, cumbersome steps, etc., and achieve high stability , good universality and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of semi-sandwich iridium complex Ir containing ortho carboryl benzimidazole structure:
[0031]
[0032] At -78°C, n-BuLi (1.6M) in n-hexane (1.00 mL, 1.6 mmol) was slowly added dropwise to the o-C 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added bromobenzimidazole (126.7mg, 0.64mmol), and continued to react at room temperature for 6 Hour. Then the binuclear iridium compound [Cp*IrCl 2 ] 2 (256.0 mg, 0.32 mmol) was added to the reaction system for an additional 3 hours. After the reaction was finished, stand and filter, and dry the solvent under reduced pressure, and the obtained crude product was separated by column chromatography (petroleum ether / tetrahydrofuran=6:1) to obtain the red target product iridium (III) complex Ir (322.4 mg, yield rate 81%).
[0033] 1 H NMR (400MHz, CDCl 3 ,25℃):δ=8.03(brs,1H),7.89(d,J=7....
Embodiment 2
[0037] Iridium(III) complexes catalyze the asymmetric reduction of aryl ketones:
[0038]
[0039] The iridium complex prepared in Example 1 was used as a catalyst to catalyze the asymmetric reduction reaction of aryl ketone: in acetophenone (10mmol, 1.20g), a trivalent iridium complex (0.01mmol, 13.0 g) containing an ortho carborane structure was added mg) in ethanol solution, hydrogen was introduced under normal pressure as a reducing agent to react, the reaction temperature was 80°C, and the reaction time was 120 minutes. chiral alcohol compound C 8 h 10 O (yield 91%), ee>99%, elemental analysis: C 78.65, H 8.25 (theoretical); C 78.59, H 8.22 (actual).
Embodiment 3
[0041] Iridium(III) complexes catalyze the asymmetric reduction of aryl ketones:
[0042]
[0043] The iridium complex prepared in Example 1 is used as a catalyst to catalyze the asymmetric reduction reaction of aryl ketone: in 4-methoxyacetophenone (10mmol, 1.50g), add a trivalent iridium complex containing an ortho carborane structure (0.01mmol, 13.0mg) in ethanol solution, and feed hydrogen as a reducing agent to react, the reaction temperature is 80°C, and the reaction time is 60 minutes. After the end, the concentrated reaction solution is directly separated by silica gel column chromatography, and dried until the quality remains unchanged. , to obtain the corresponding chiral alcohol compound C 9 h 12 o 2 (96% yield), ee>99%, elemental analysis: C 71.03, H 7.95 (theoretical); C 71.00, H 7.81 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com