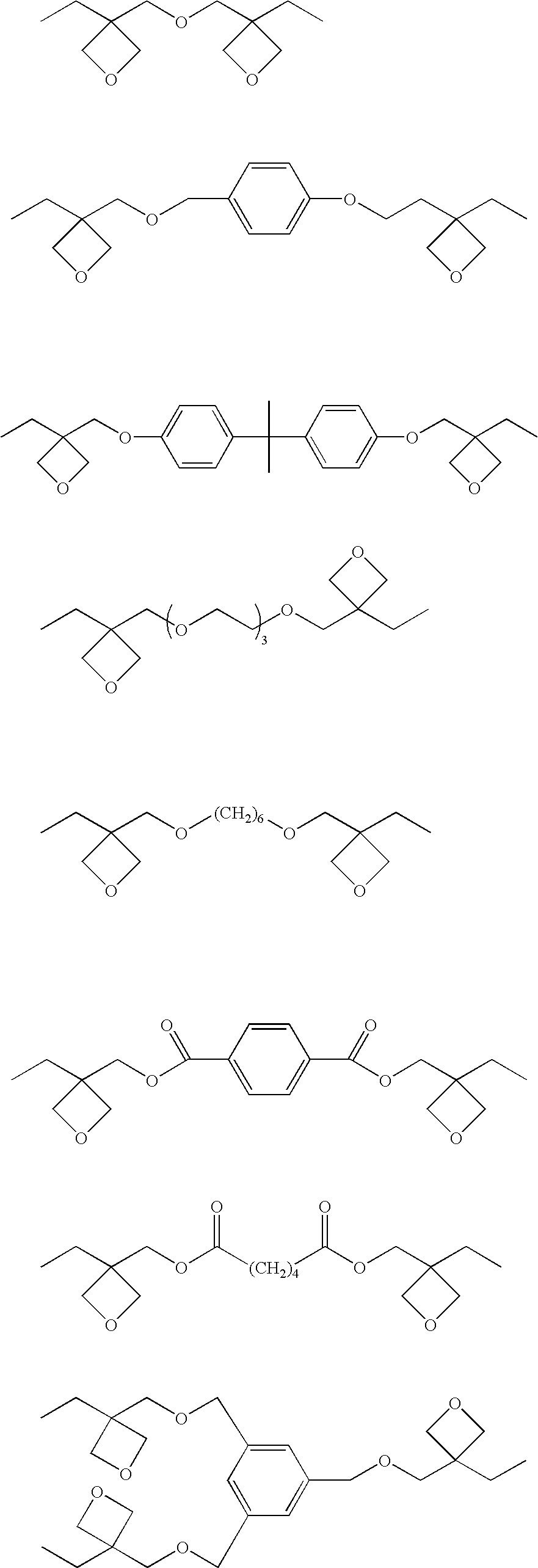
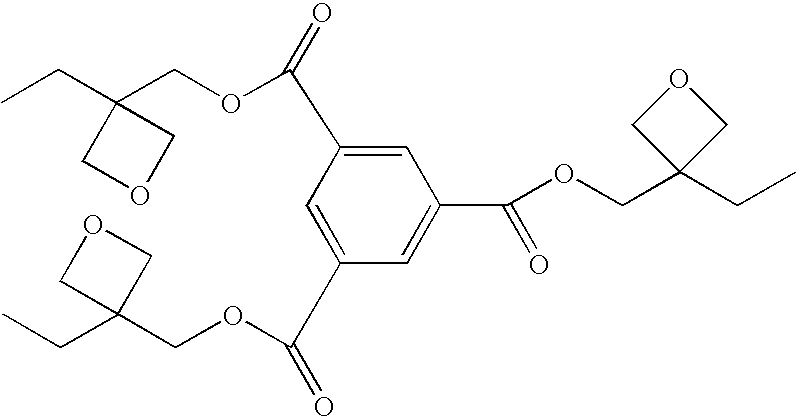
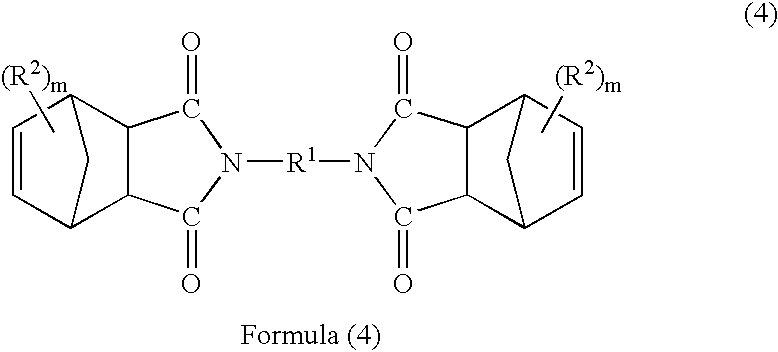
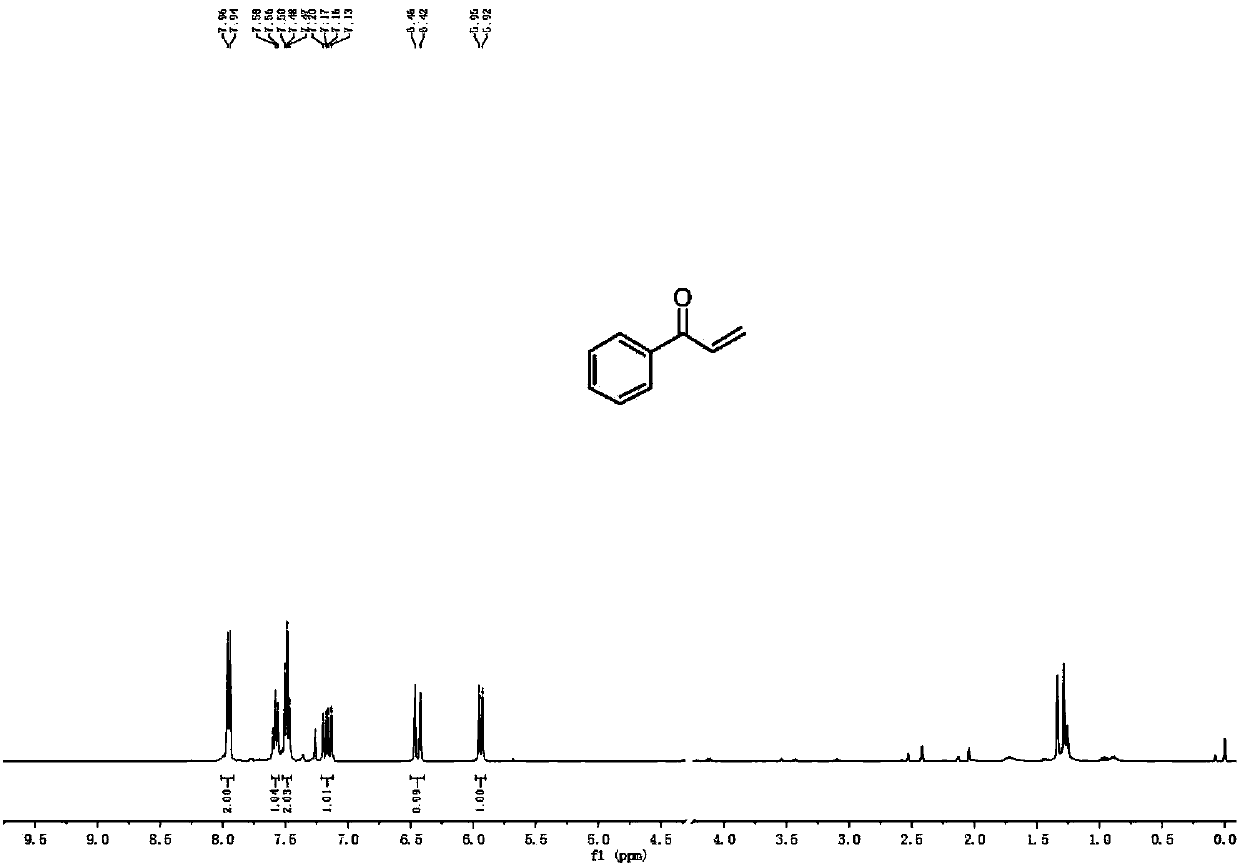
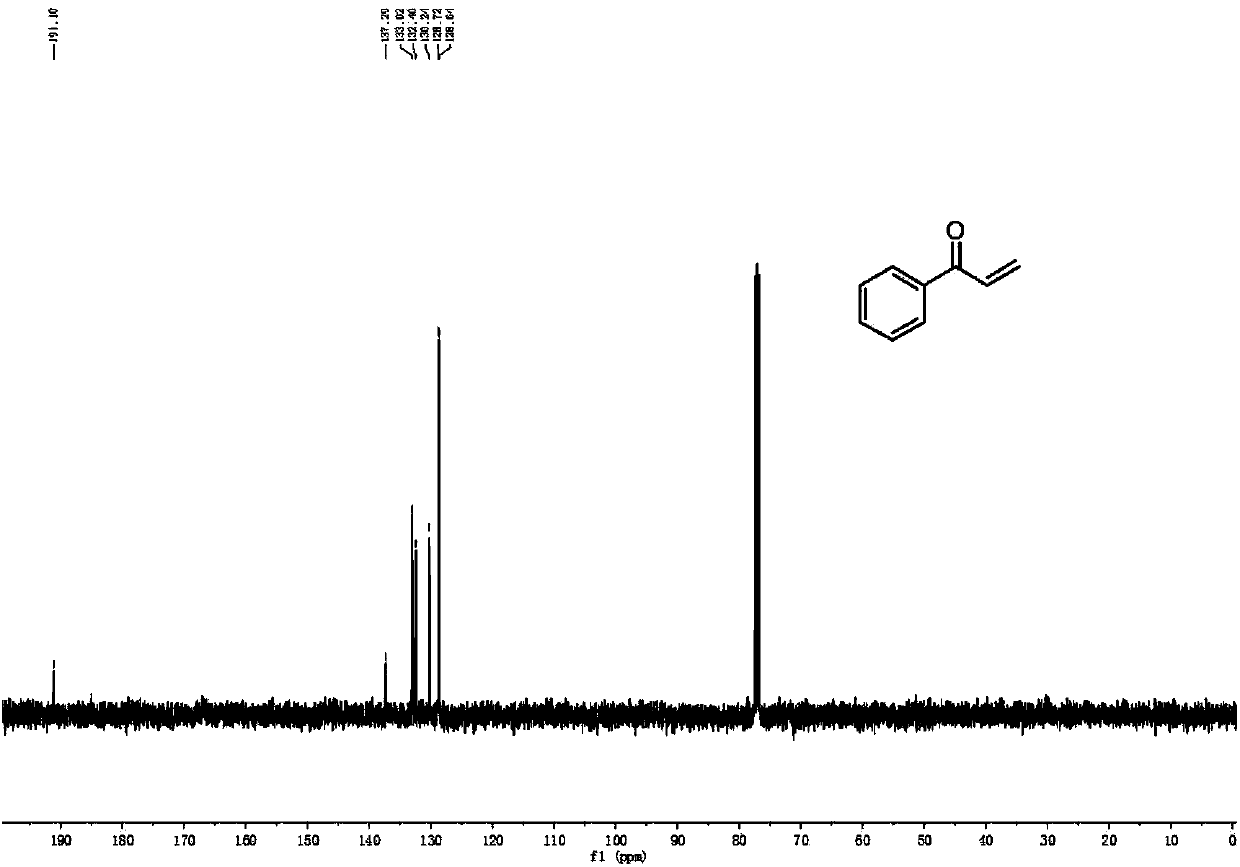
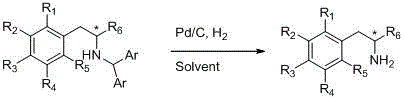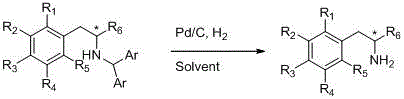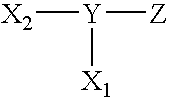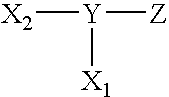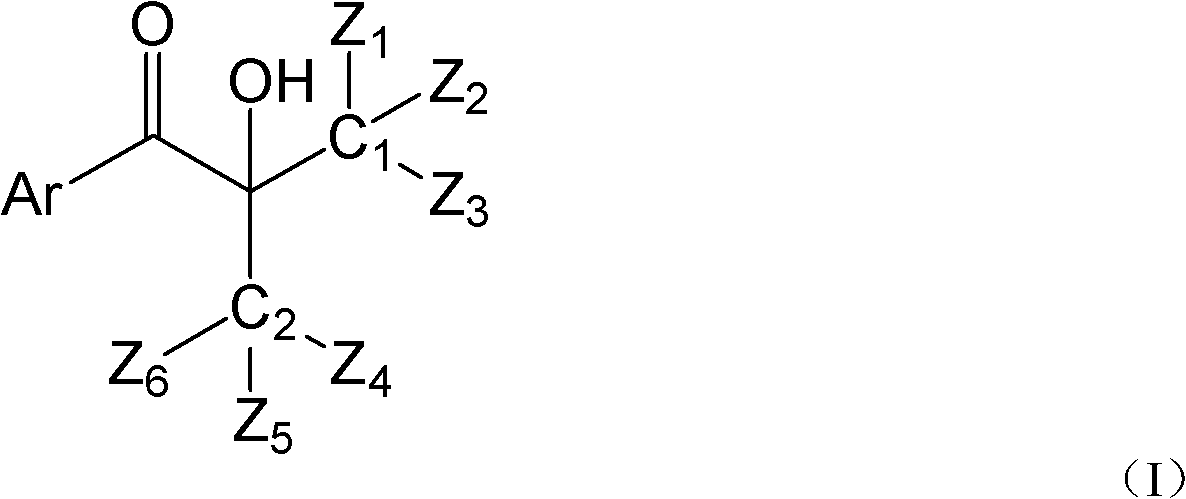Patents
Literature
179 results about "Aryl ketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
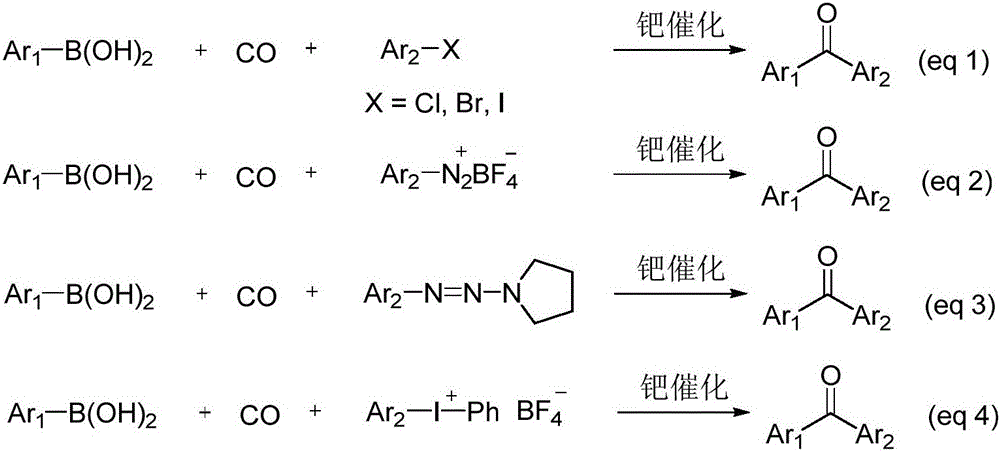
In a palladium-catalyzed one-pot procedure for the synthesis of aryl ketones, triazine esters were coupled with aryl boronic acids to provide aryl ketones in good yields in the presence of 1 mol % Pd(PPh 3) 2Cl 2 for 30 min.
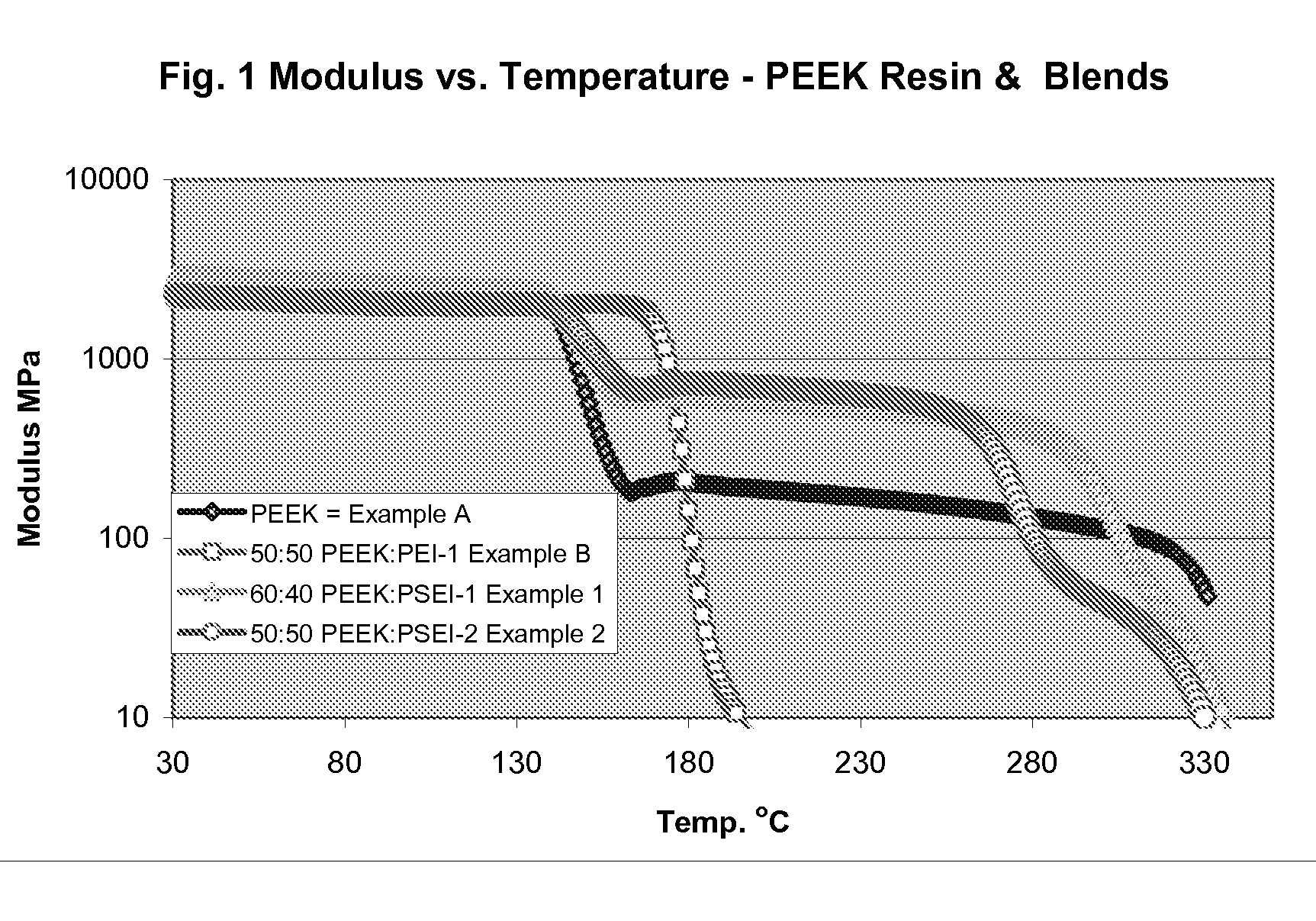
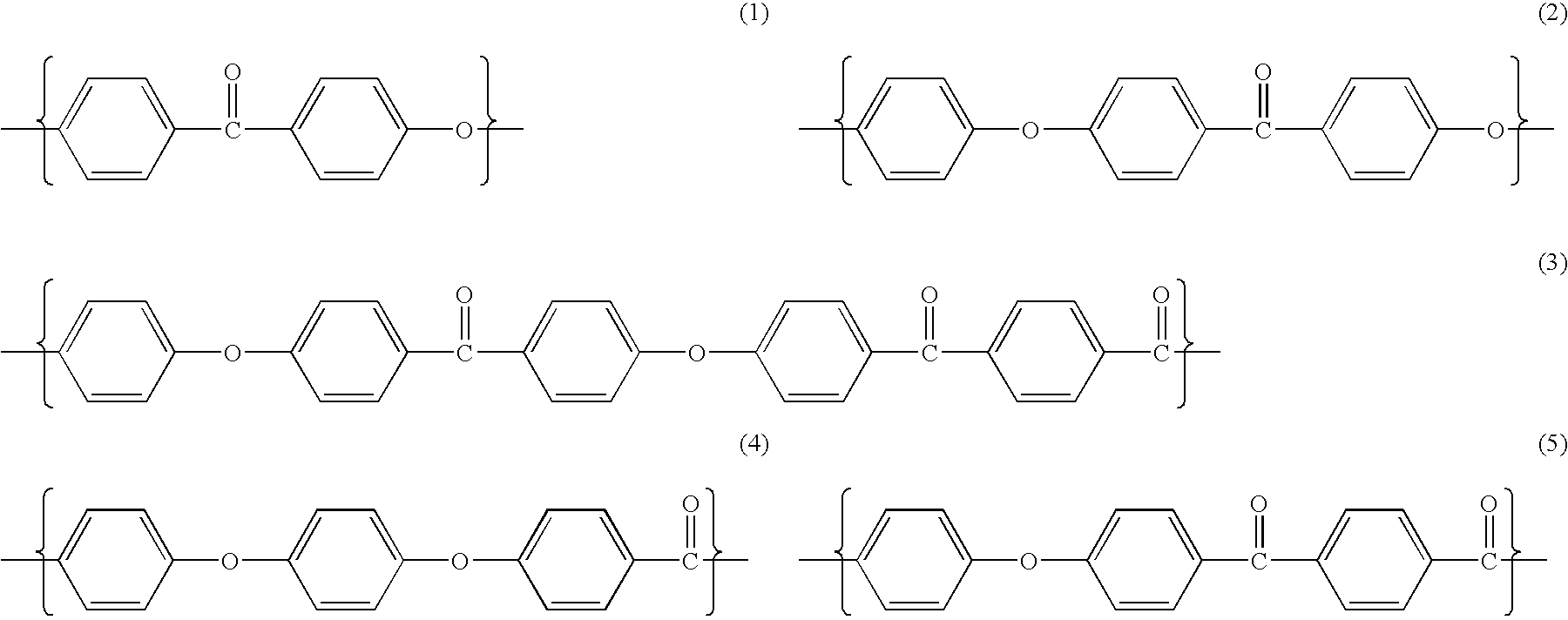
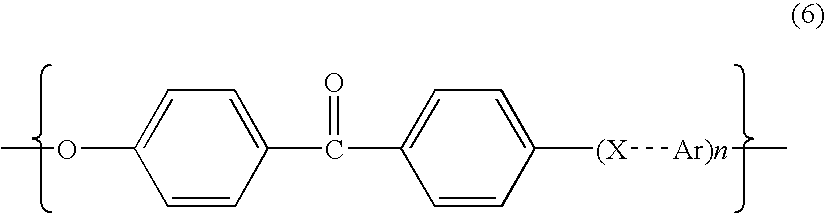
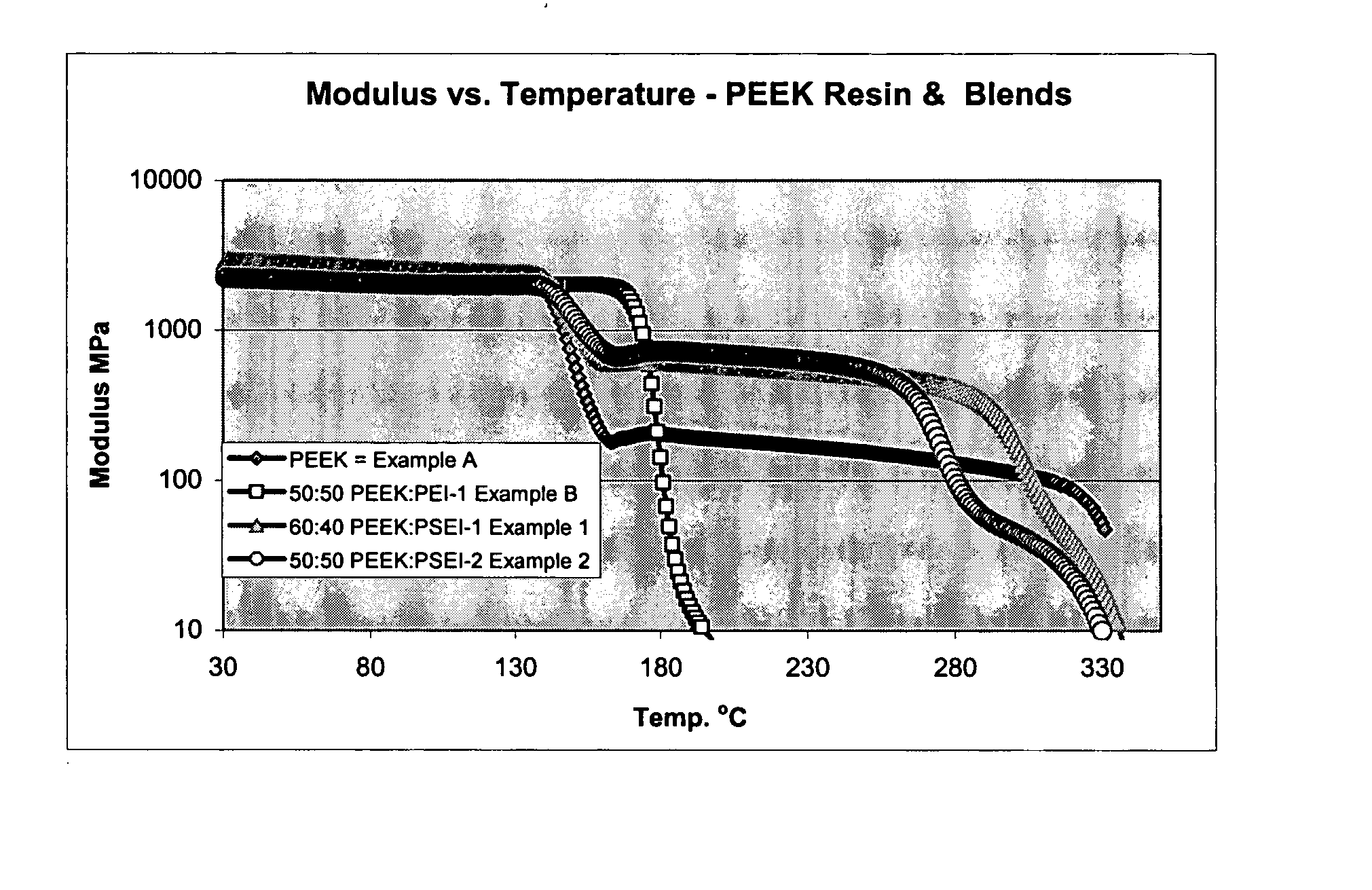
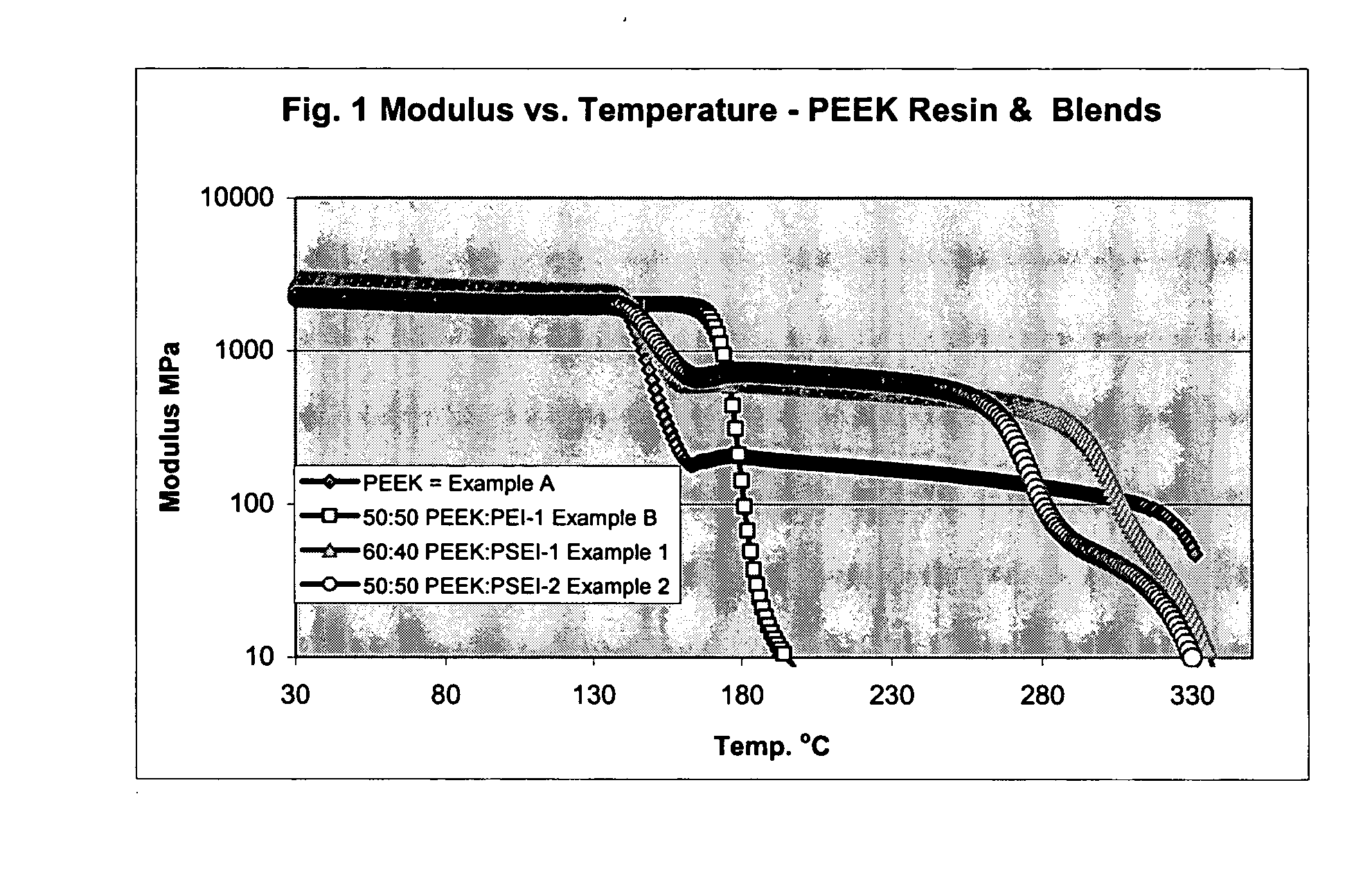
Poly aryl ether ketone polymer blends
Filled phase-separated blends of polyaryl ether ketones, polyaryl ketones, polyether ketones, polyether ether ketones and mixtures thereof with at least one polysulfone etherimide, wherein the polysulfone etherimide has greater than or equal to 50 mole % of the polymer linkages contain at least one aryl sulfone group are described. Such filled blends have improved load-bearing capability at high temperature. In another aspect the filled blends have a higher crystallization temperature, especially at fast cooling rates.
Owner:SABIC GLOBAL TECH BV
Photopolymerization initiator and photopolymerizable composition
InactiveUS20040186195A1High polymerization activityEasy to getImpression capsPhotomechanical apparatusDiketonePolycyclic compound
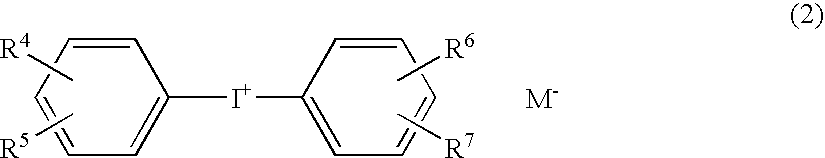
A photopolymerization initiator comprising (A) a photo acid-generating compound such as diaryliodonium salt (e.g., diphenyl iodonium, bis(p-chlorophenyl)iodonium, etc.), (B) a photo oxidation radical-generating compound such as diarylketone compound, alpha-diketone compound or ketocoumarin compound, and (C) a fused polycyclic aromatic compound such as 1,4-dimethylnaphthalene, 1-methylanthracene, 9-methylanthracene, 9,10-dimethylanthracene or 9,10-diethylanthracene. The photopolymerization initiator makes it possible to efficiently polymerize the cationically polymerizable monomer by the irradiation with visible light.
Owner:TOKUYAMA CORP +1
DOPO derivatives as well as preparation method and application thereof
ActiveCN104086593AReduce volatilityAvoid PlasticityGroup 5/15 element organic compoundsGlass/slag layered productsThermal stabilityChemical stability
The application relates to a compound capable of being used as a novel halogen-free flame retardant. The compound is prepared from aryl ketone and a DOPO compound in the presence of an acid catalyst. The compound has the advantages of good flame retardancy and good thermal stability and chemical stability.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +1
High temperature polymer blends of poly(aryl ether ketone phthalazinone)
The present invention provides high temperature compositions comprising blends of a first polymer, poly(aryl ether ketone phthalazinone)s, and a second polymer, selected from poly(aryl ether ketone)s, poly(aryl ketone)s, poly(ether ether ketone)s, poly(ether ketone ketone)s, or polybenzimidazoles, thermoplastic polyimides, polyetherimides, poly(aryl ether sulfone)s, poly(phenylene sulfide)s, and mixtures thereof. The compositions have improved high temperature characteristics, e.g., improved high temperature load capability and improved high temperature melt processibility.
Owner:POLYMER INSTR & CONSULTING SERVICES LTD
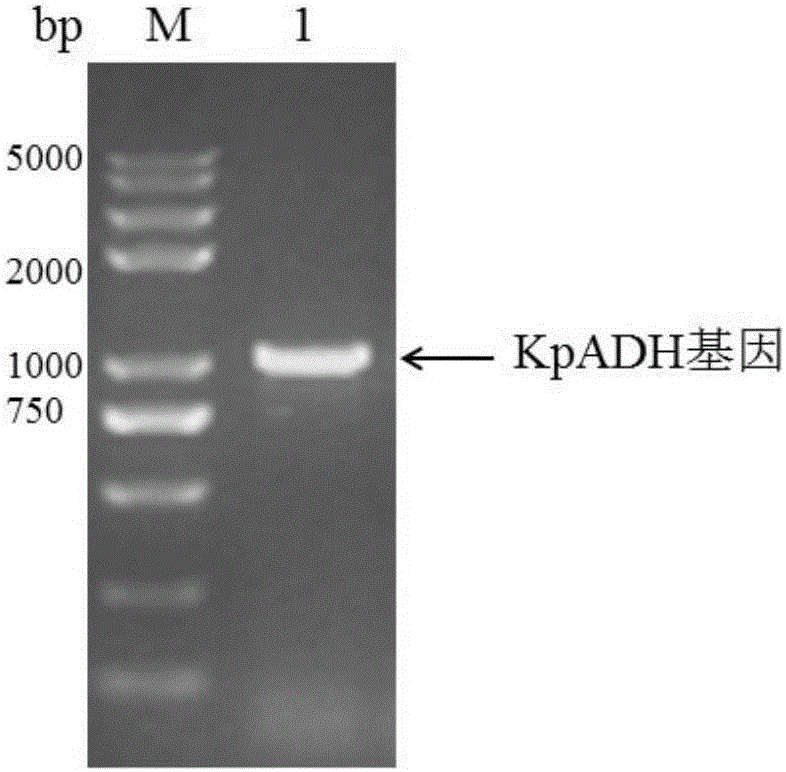
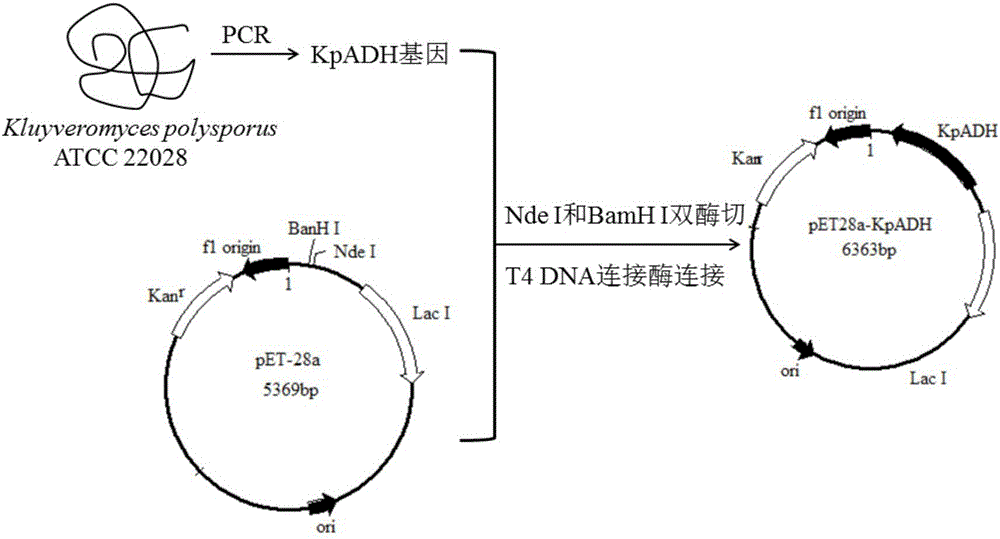
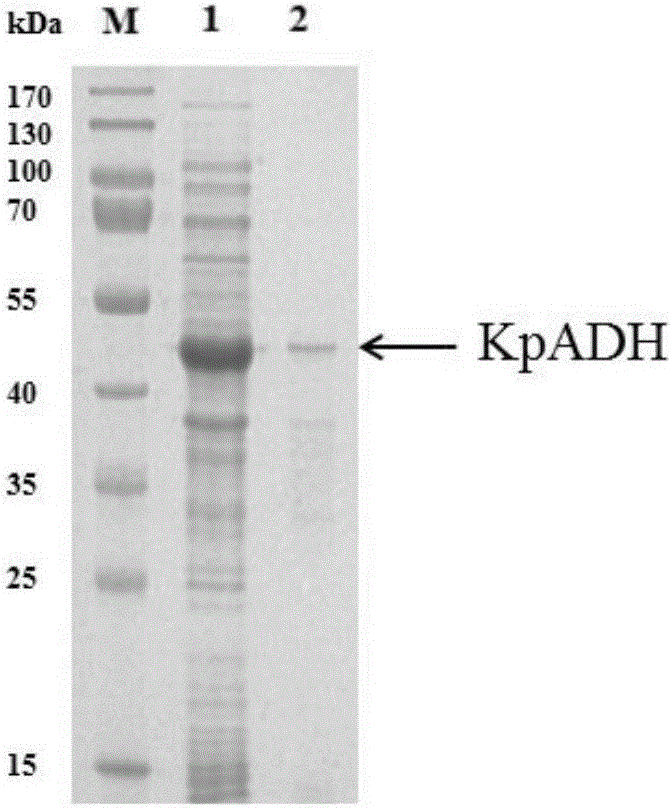
Alcohol dehydrogenase, gene and recombinase thereof, and application of alcohol dehydrogenase in synthesis of chiral diaryl secondary alcohol
InactiveCN105936909AImprove expression levelIncrease enzyme activityOxidoreductasesGenetic engineeringDiaryl ketoneGenus Kluyveromyces
The invention discloses an alcohol dehydrogenase, a gene thereof, a recombinant expression vector and a recombinant expression transformant respectively containing the gene, a recombinase of the alcohol dehydrogenase, and an application of the alcohol dehydrogenase in asymmetric reduction synthesis of chiral diaryl secondary alcohol as a catalyst, and belongs to the technical field of bioengineering. The alcohol dehydrogenase is from Kluyveromyces sp. CCTCCM2011385, has a carbonyl group reduction function, and also has a hydroxy group oxidation function. Extra addition of glucose dehydrogenase and other enzymes used for cofactor circulation is not needed when the alcohol dehydrogenase is used in the reduction of diaryl ketone into the chiral diaryl secondary alcohol as a biocatalyst, and the alcohol dehydrogenase has the advantages of high catalysis efficiency, mild reaction conditions, easy product recovery and low cost, so the alcohol dehydrogenase has very good application and exploitation prospect in the production of antihistamine medicines.
Owner:JIANGNAN UNIV
Ink compositions
An ink composition comprised of (1) an ink vehicle of (a) alkyl alkyl ketones of the formula CH3(CH2)m CO (CH2)n CH3 where m and n represent the number of segments and wherein alkyl contains from about 1 to about 25 carbons, (b) alkyl aryl ketones where each alkyl contains from about 1 to about 20 carbons, and the aryl is anthracene, naphthalene or phenyl, (c) arylaryl ketones where each aryl is benzyl, phenyl or naphthyl, (2) an ink viscosity component, (3) a conductive compound, (4) an antioxidant compound, (5) a lightfastness component, and (6) a colorant.
Owner:XEROX CORP
Poly aryl ether ketone polymer blends
Owner:SHPP GLOBAL TECH BV
Method of Chiral alkamine ligand used as catalyst of asymmetric addition process for terminal alkyne to fluoroalkylaryl ketone
InactiveCN1449865AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCarboxyl radical
The present invention provides a kind of chiral ligand (1R, 2R)-2-N,N-substituted amino-1-(4-substituted phenyl)-1-ethanol or its enantiomorph and a method using the above-mentioned chiral ligand or its enantiomorph as catalyst for asymmetric addition of acetylene copper or acetylene zinc to trifluoromethylarylketone. Said invention provides its structural general formula. Said invention adopts the asymmetric addition process so as to can high-effectively and high enantiomeric-selectively create the chiral quanternary carbon center in HIV transfrase high-activity inhibitor Efavirenz (Sustiva TM), so that it can high-effectively synthesize Efavirenz (Sustiva TM).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Chiral beta-arylamine compounds prepared by asymmetric reductive amination reaction and preparation method of chiral beta-arylamine compounds
ActiveCN105712812AHigh catalytic efficiencyFew reaction stepsOrganic compound preparationSulfonic acid amide preparationPtru catalystCombinatorial chemistry
The invention relates to chiral beta-arylamine compounds prepared by asymmetric reductive amination reaction and a preparation method of the chiral beta-arylamine compounds. The chiral beta-arylamine compounds are prepared by hydrogenised asymmetric reductive amination reaction under the action of chiral catalysts on the basis of alpha-aryl ketone and aminodiphenylmethane. By the synthesis method, catalyst consumption can be reduced to 5*10<-5>, enantioselectivity of products is up to 98%, and a diaryl methyl group can be removed from N-diaryl methyl-beta-arylamine obtained from the reaction under mild conditions to finally obtain first-grade beta-arylamine, so that the problem of synthesis of the chiral beta-arylamine compounds is solved. In addition, since relevant raw materials are very cheap, the chiral beta-arylamine compounds and the preparation method have extremely high industrial application potential.
Owner:NORTHWEST A & F UNIV
Method for preparing aryl ketone
ActiveCN102153434AMild reaction conditionsReduce usageCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystRuthenium Compounds
The invention relates to the field of catalysis, in particular to a method for preparing aryl ketone through reacting aldehyde with aryl boric acid under the catalysis of a ruthenium catalyst. In the method, an organic phosphide is used as a ligand, potassium phosphate is used as alkali, pinacolone or acetone is used as an additive, toluene or / and water is (are) used as a solvent(s), aldehyde and aryl boric acid which are used as reaction substrates react at 95-100 DEG C for 10-24h in the presence of a ruthenium compound used as a catalyst to prepare aryl ketone, wherein the catalyst is one of [Ru(cymene)Cl2]2, [Ru(CO)3Cl2]2, RuH2(CO)PPh3, Ru2(OAc)4, [Ru(benzene)Cl2]2, Ru(S-BINAP)Cl2 or Ru3(CO)12. In the invention, the used catalyst has relatively low price and low toxicity, thereby reducing the preparation cost and being more environmentally friendly.
Owner:铜陵市官作文化有限公司
Aryl ketone compounds and compositions for delivering active agents
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
A kind of dopo derivative, its preparation method and application
ActiveCN104086593BReduce volatilityAvoid PlasticityGroup 5/15 element organic compoundsGlass/slag layered productsThermal stabilityChemical stability
The application relates to a compound capable of being used as a novel halogen-free flame retardant. The compound is prepared from aryl ketone and a DOPO compound in the presence of an acid catalyst. The compound has the advantages of good flame retardancy and good thermal stability and chemical stability.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +1
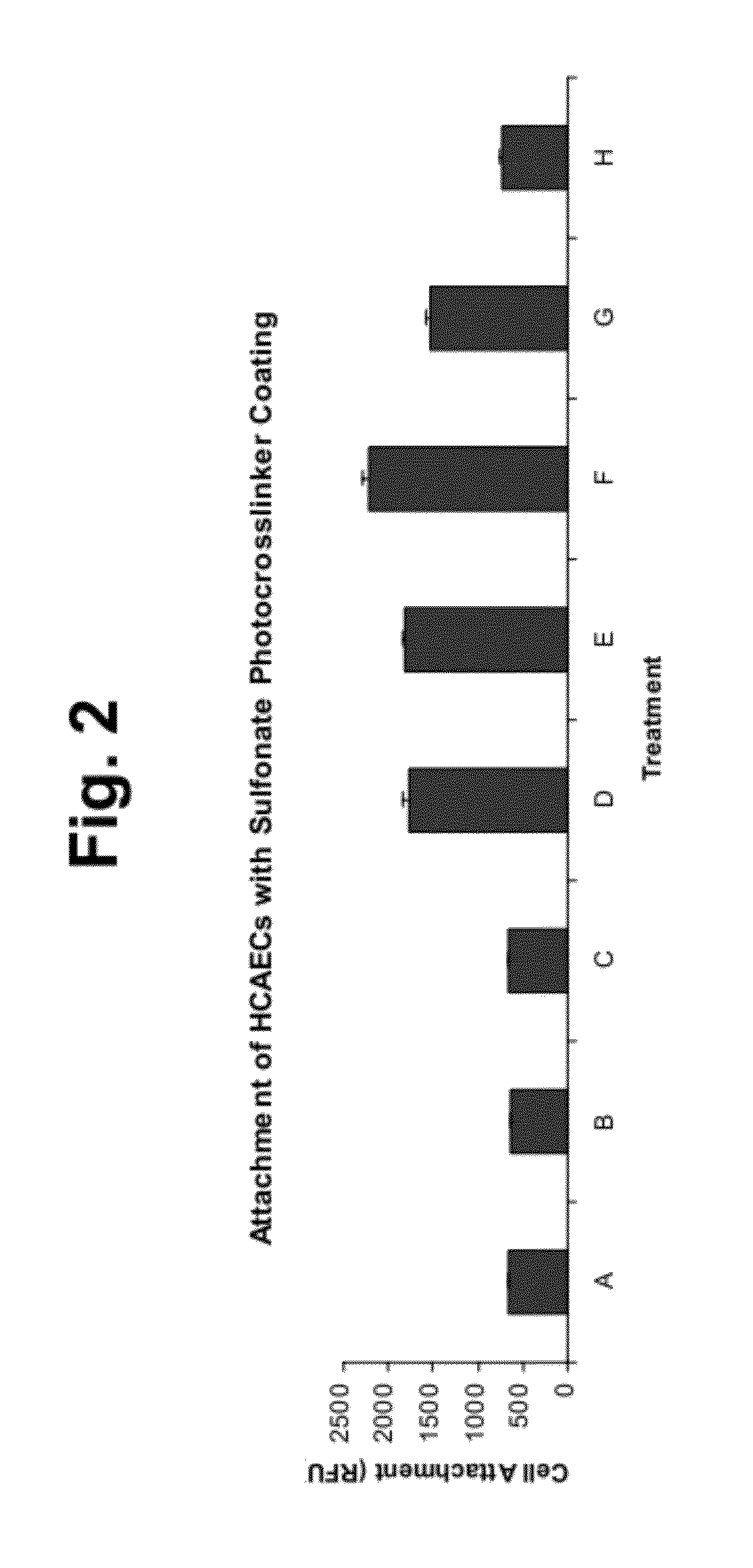
Surface coating agents
InactiveUS6924390B2Wide applicabilityGood water solubilityLiquid surface applicatorsSamplingPotassiumPhosphoric acid
Compounds useful as surface coating agents, including compounds of the formula: wherein X1 comprises a first photoreactive species; X2 comprises a second photoreactive species; Y comprises a nonpolymeric core molecule comprising an aromatic group; and Z comprises at least one charged group. The Y core can include an aromatic group such as a benzene radical, the charged groups Z can be independently selected from the organic acids that include sulfonic acid, carboxylic acid, and phosphoric acid, and the photoreactive species of X1 and X2 can independently be aryl ketones, such as those selected from the group acetophenone, benzophenone, anthraquinone, anthrone, and anthrone-like heterocycles, and their substituted derivatives. Examples of such coating agents include 4,5-bis(4-benzoylphenylmethyleneoxy)benzene-1,3-disulfonic acid di(potassium and / or sodium) salt, 2,5-bis(4-benzoylphenylmethyleneoxy)benzene-1,4-disulfonic acid di(potassium and / or sodium) salt (Compound II), and 2,5-bis(4-benzoylphenylmethyleneoxy)benzene-1-sulfonic acid monopotassium and / or monosodium salt.
Owner:SURMODICS INC
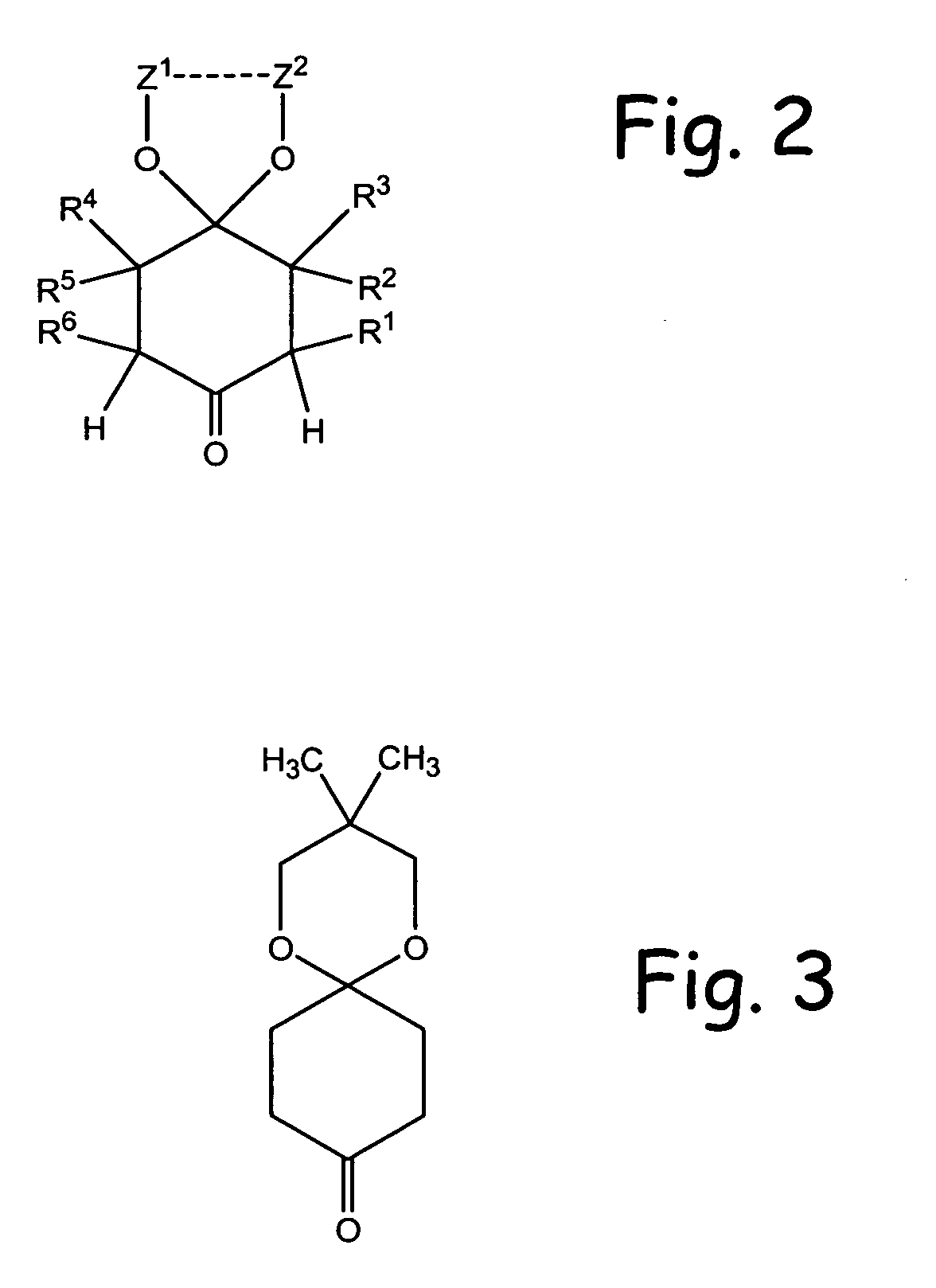
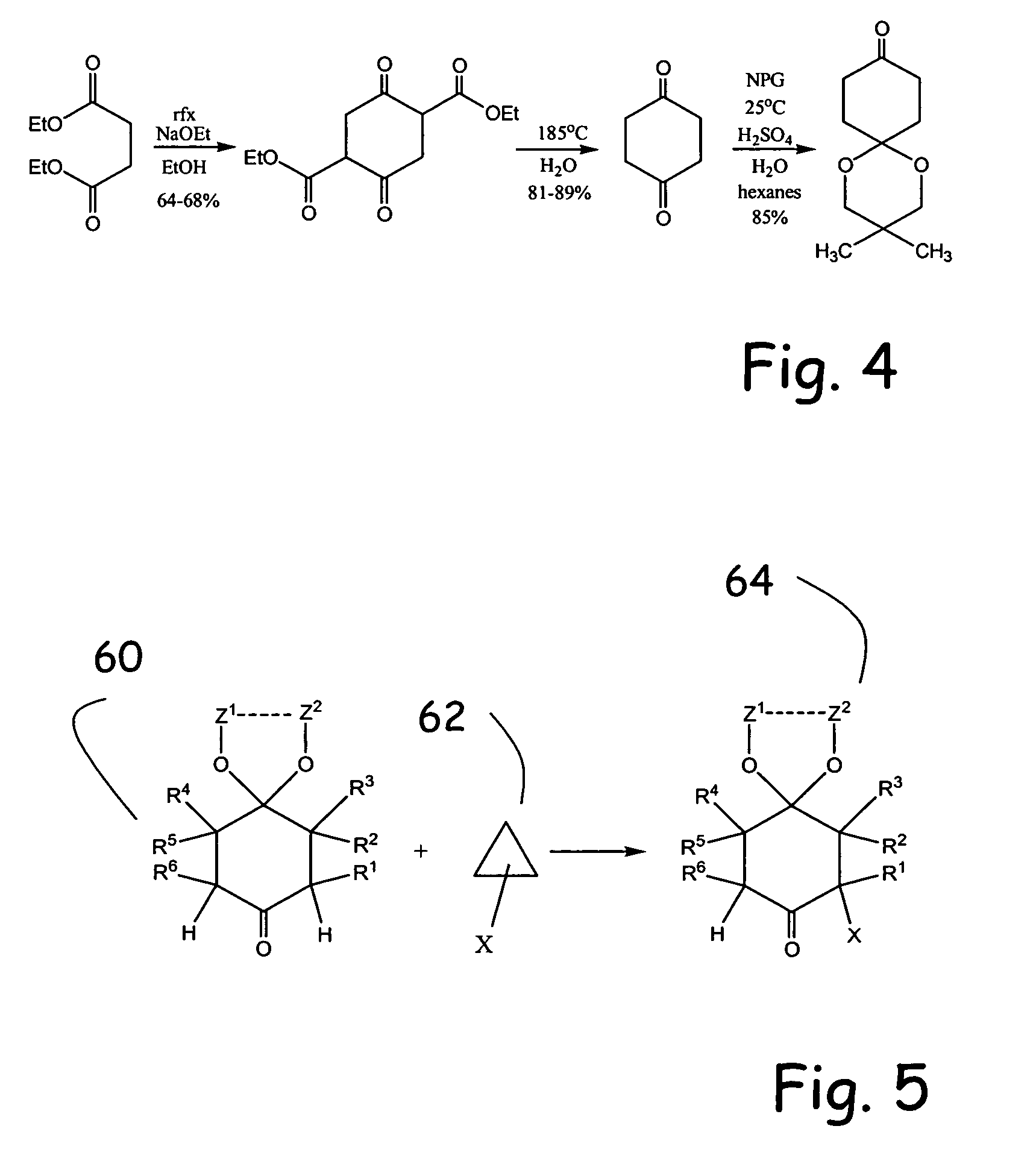
Alpha functionalization of cyclic, ketalized ketones and products therefrom
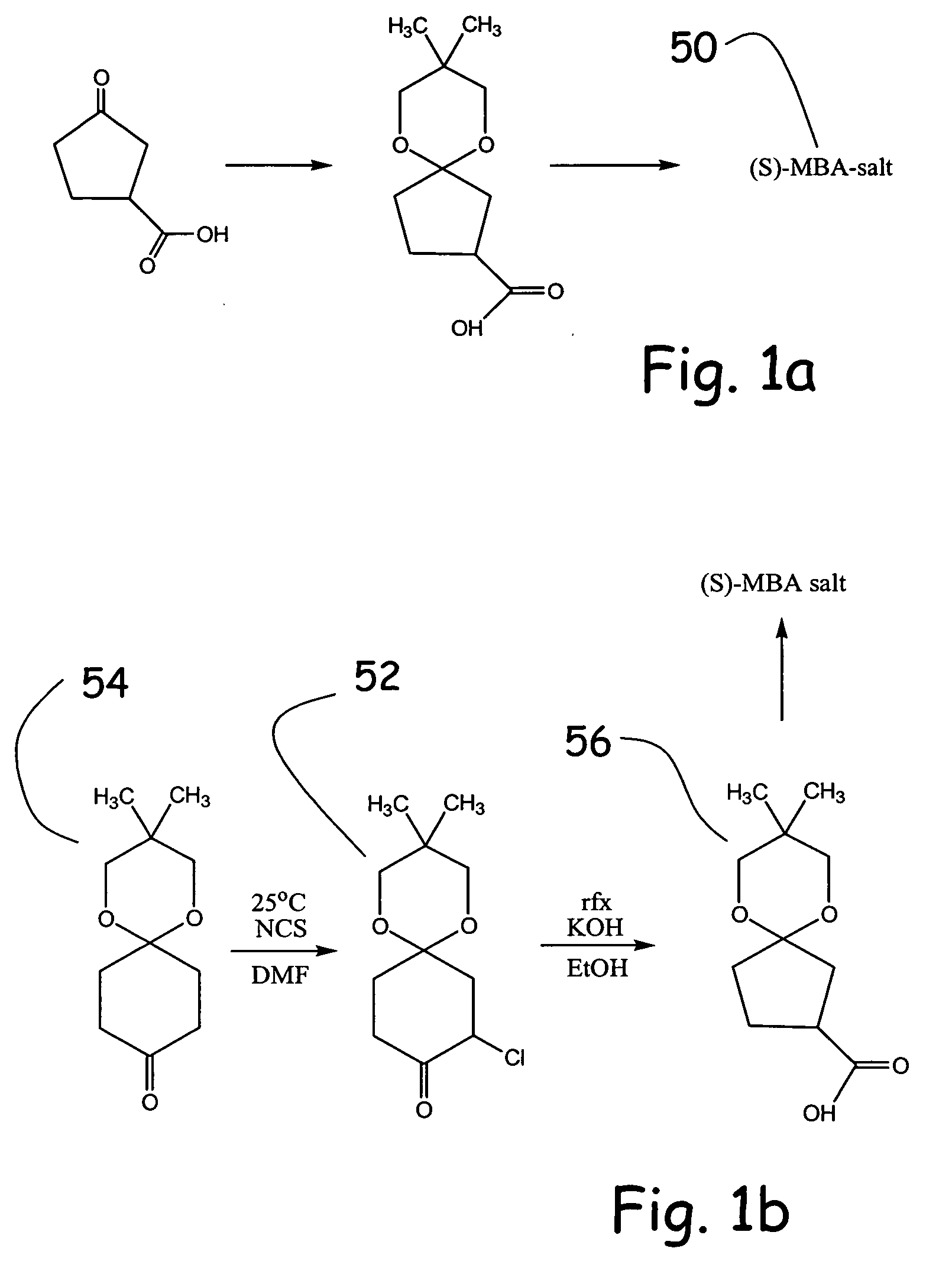
Methodologies for the alpha-monohalogenation of acid sensitive ketones, especially cyclic, acid-sensitive, ketalized ketones. As one approach, the ketone is reacted with a halogen donor compound, e.g., N-chlorosuccinimide, in anhydrous, highly polar organic reagents such as dimethylformamide (DMF). As another monohalogenation approach, it has been observed that organic salts generated from amines and carboxylic acids catalyze the monohalogenation of ketalized ketone in reagents comprising alcohol solvent (methanol, ethanol, isopropanol, etc.). The monohalogenation is fast even at −5° C. The salt can be rapidly formed in situ from ingredients including amines and / or carboxylic acids without undue degradation of the acid sensitive ketal. Aryl ketones are monooxygenated using iodosylbenzene. This methodology is applied to monohalogenation of an acid sensitive monoketal ketone. The ability to prepare monohalogenated, acid sensitive ketones facilitates syntheses using halogenated, acid sensitive ketones. As just one example, facile synthesis of halogenated, acid sensitive ketones provides a new approach to synthesize the S-ketal-acid S-MBA (S-methylbenzylamine) salt useful as an intermediate in the manufacture of a glucokinase activator. As an overview of this scheme, a monohalogenated, cyclic, ketalized ketone is prepared using monohalogenation methodologies of the present invention. The halogenated compound is then subjected to a Favorskii rearrangement under conditions to provide the racemic acid counterpart of the desired chiral salt. The desired chiral salt is readily recovered in enantiomerically pure form from the racemic mixture.
Owner:HARRINGTON PETER J +2
Alkyl and aryl ketone compound preparation method
InactiveCN103274916AImprove responseSimple and fast operationOrganic compound preparationCarbonyl compound preparationOrganic solventTrifluoroacetic acid
The invention relates to an alkyl and aryl ketone compound synthetic method. The method comprises a step of enabling an aliphatic nitrile compound to react with aryl sodium trifluoroborate in an organic solvent in the presence of a palladium catalyst, dipyridyl and trifluoroacetic acid so as to prepare the target product through one step. The method has the advantages of high product yield, convenience in operation, simple method and the like; and the method has a good scientific research value and an industrial application prospect.
Owner:WENZHOU UNIVERSITY
Delivery of hydrophobic active agent particles
Embodiments of the invention include drug delivery coatings and devices including the same. In an embodiment, the invention includes a drug delivery coating including a polymeric layer. The polymeric layer can include a hydrophilic outer surface. The coating can also include a matrix contacting the hydrophilic outer surface. The matrix can include a particulate hydrophobic therapeutic agent and a cationic agent. The polymeric layer can further include a hydrophilic polymer having pendent photoreactive groups and a photo-crosslinker including two aryl ketone functionalities. Other embodiments are also included herein.
Owner:SURMODICS INC
Optical activity di(heteto)aryl methanol and asymmetric synthesis method thereof
InactiveCN106831550AEasy to synthesizeLow priceOrganic compound preparationHydroxy compound preparationIridiumSynthesis methods
The invention relates to optical activity di(heteto)aryl methanol and an asymmetric synthesis method thereof. The method comprises the following steps: taking mono-sulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as catalysts, taking sodium formate or formic acid / triethylamine or isopropanol as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction of di(heteto)aryl ketone first, thereby obtaining the optical activity di(heteto)aryl methanol. The method is mild in reaction conditions, easy and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspects of synthesis of antihistamine chiral drugs such as diphenhydramine, methyldiphenhydramine, carbinoxamine and bepotastine.
Owner:CHINA THREE GORGES UNIV
Method for preparing aromatic ketone or aromatic aldehyde by selectively oxidizing alkyl arene
InactiveCN101100418AEasy to handleRaw materials are cheap and easy to getOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationBromineAryl radical
A non-metallic catalytic system with pyramine compound, molecule bromine and N-hydroxy-o-dimethyl-acylimine is prepared by catalyzing aryl radical oxide with molecular oxygen as oxygen source to generate aryl ketone or aryl aldehyde under gentle condition and oxidation reacting selectively. It costs low, is non-toxic and has less environmental pollution.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing chirality halohydrin in copper-catalyzed asymmetry hydrosilation mode
InactiveCN103524307AHigh yieldHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholNitrogen
The invention discloses a method for preparing chirality halohydrin in a copper-catalyzed asymmetry hydrosilation mode. In the prior art, mostly a noble metal catalyst is needed, the research is generally limited in catalysis asymmetry reduction of alpha-haloketone, and documents about catalysis asymmetry reduction of beta-, gamma-, epsilon- or other haloketone substrates are less related. The method disclosed by the invention comprises preparing chirality gamma-, delta- or zeta-haloalkyl aryl alcohol from beta-, gamma-, epsilon-haloalkyl aryl ketone in a copper catalysis asymmetry hydrosilation reduction mode, or preparing chirality beta-, gamma- or delta-haloalkyl aryl alcohol from alpha-, beta- or gamma-haloalkyl aryl ketone in the copper catalysis asymmetry hydrosilation reduction mode. The method adopts a non-noble metal catalyst, the raw material is easy to obtain, nitrogen protection is not needed, the reaction condition is mild, the operation is simple, and the yield and the enantioselectivity of the reaction are high.
Owner:HANGZHOU NORMAL UNIVERSITY
High temperature polymer blends of poly(aryl ether ketone phthalazinone)
The present invention provides high temperature compositions comprising blends of a first polymer, poly(aryl ether ketone phthalazinone)s, and a second polymer, selected from poly(aryl ether ketone)s, poly(aryl ketone)s, poly(ether ether ketone)s, poly(ether ketone ketone)s, or polybenzimidazoles, thermoplastic polyimides, polyetherimides, poly(aryl ether sulfone)s, poly(phenylene sulfide)s, and mixtures thereof. The compositions have improved high temperature characteristics, e.g., improved high temperature load capability and improved high temperature melt processibility.
Owner:POLYMER INSTR & CONSULTING SERVICES LTD
Synthesis method of optically active aryl vicinal diol under catalysis of whole yeast cells
The invention relates to a preparation method of an optically active aryl vicinal diol by reducing an alpha-hydroxy aromatic ketone under catalysis of whole yeast cells, belonging to the technical field of optically active alcohol preparation utilizing a biological catalysis method. According to the invention, the whole yeast cells are used as a biocatalyst to catalyze the asymmetric reduction of the alpha-hydroxy aromatic ketone, so as to obtain high substrate conversion rate and high enantiomeric excess value of a product. Based on the invention, when the substrate concentration is 5.0-15 g / L, different conditions of whole yeast cell catalyst systems are selected according to the different substrates, thus the conversion rate of the substrate (alpha-hydroxy aryl ketone) reaches 72.4-98.4% and the enantiomeric excess value of the product (optically active aryl vicinal diol) reaches 92.2-99.7%, and the preparation has high application value.
Owner:JIANGNAN UNIV
Formulations for environmentally friendly photoresist film layers
ActiveUS8394575B2Precise alignmentImprove imaging effectPhotosensitive materialsPhotosensitive materials for photomechanical apparatusEpoxyPhotoacid generator
Owner:LEXMARK INT INC
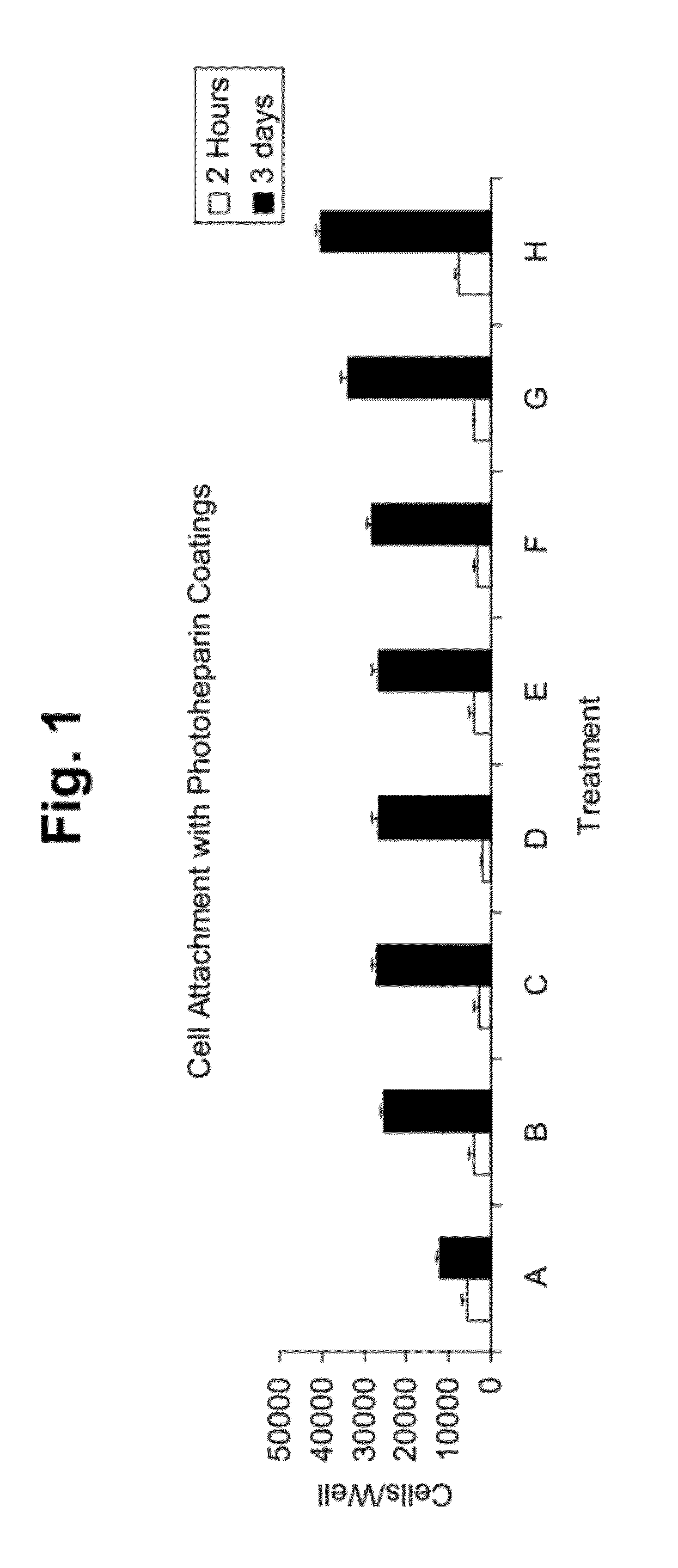
Cell attachment coatings and methods
InactiveUS20120171769A1Enhanced cell attachmentIncrease the number ofSynthetic resin layered productsSurgeryCell bindingECM Protein
Cell attachment coatings for articles such as implantable medical devices and cell culture vessels are disclosed. The coatings include an intermediate coater layer which includes a sulfonated component that is bonded in the coating by reacted aryl ketone functional groups. The coating also include a second coated layer including an immobilized ECM protein or peptide that includes an active portion of an ECM protein that is able to serve as an outer layer to contact cells during use. The coatings promoted enhanced cell binding and growth.
Owner:SURMODICS INC
Method for preparing diaryl ketone from aryl sulfonate
InactiveCN106146271AHigh yieldImprove toleranceCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventDiaryl ketone
The invention discloses a method for preparing diaryl ketone from aryl sulfonate. The method includes following steps: in the presence of a catalyst and carbon monoxide, allowing aryl sulfonate and aryl boronic acid to react in an organic solvent; after reaction is finished, performing aftertreatment to obtain diaryl ketone. Aryl sufonate is adopted as an electrophilic reagent for Suzuki cross carbonylation coupling reaction, diaryl ketone is directly synthesized through carbon monoxide, aryl sulfonate and aryl boronic acid, reaction conditions are milk, functional groups are high in tolerance, a substrate is cheap and easy to get, and diaryl ketone can be prepared with high yield.
Owner:SHAOXING UNIVERSITY
Alcohol dehydrogenase mutant and application thereof
ActiveCN110982799AImprove thermal stabilityImprove conversion efficiencyBacteriaMicroorganism based processesChlorobenzeneAlcohol dehydrogenase (NADP+)
The invention, which belongs to the technical field of enzyme engineering and microbial engineering, discloses an alcohol dehydrogenase mutant and application thereof. The alcohol dehydrogenase mutantdisclosed by the invention is high in thermal stability, and high in catalytic efficiency and conversion efficiency (namely space-time yield) of asymmetric reduction of prochiral diarylketone to produce chiral diarylalcohol. Therefore, the alcohol dehydrogenase mutant disclosed by the invention has the extremely broad application prospects in production of chiral diarylalcohol such as (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol, (R)-(4-chlorphenyl)-(pyridine-2-yl)-methanol and the like.
Owner:JIANGNAN UNIV
Resin composition of poly(aryl ketone), poly(arylene sulfide) and thermosetting imide resine
A resin composition comprises a resin component containing 40 to 99% by mass of a poly(aryl ketone) and 1 to 60% by mass of a poly(arylene sulfide), and 0.1 to 5 parts by mass, per 100 parts by mass of the resin component, of at least one thermosetting imide resin selected from the group consisting of a polyfunctional unsaturated imide compound and its thermoset product. The resin composition is improved in terms of compatibility, moldability or formability, melt flowability, mechanical properties, etc. while maintaining high levels of various properties such as heat resistance, flame retardancy, chemical resistance and dimensional stability.
Owner:KUREHA KAGAKU KOGYO KK
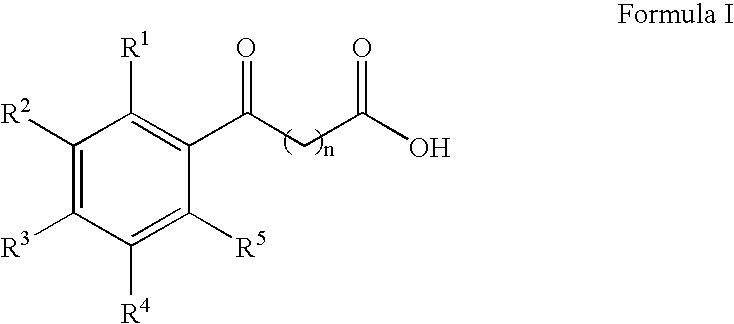
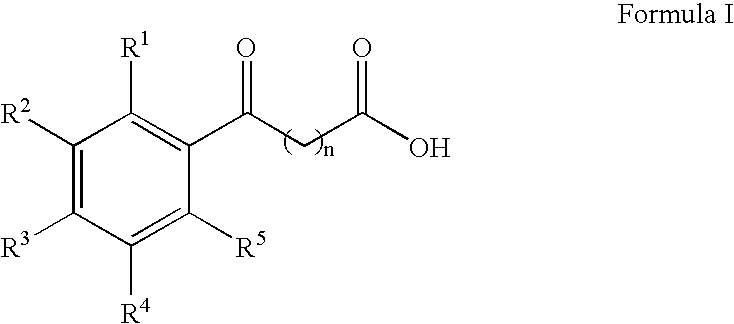
Aryl ketone compounds and compositions for delivering active agents
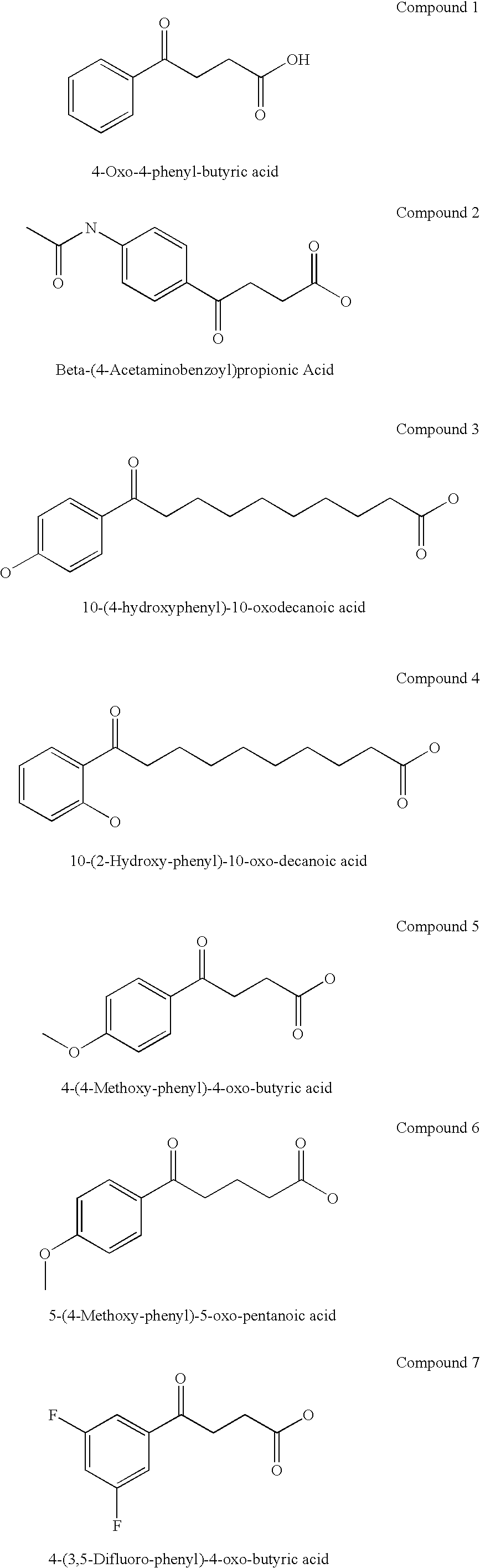
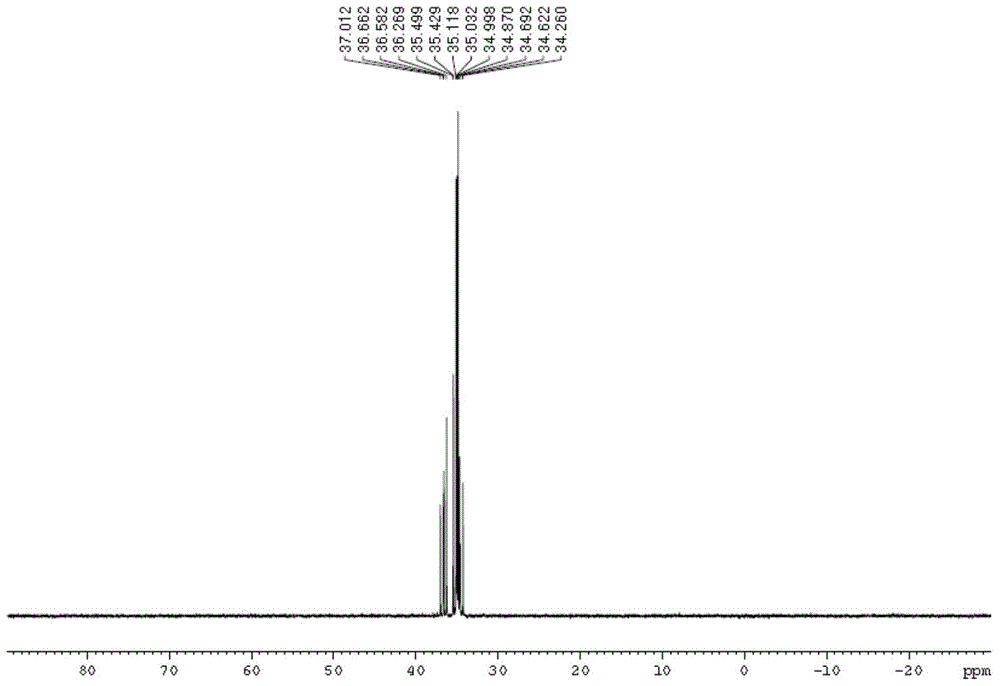
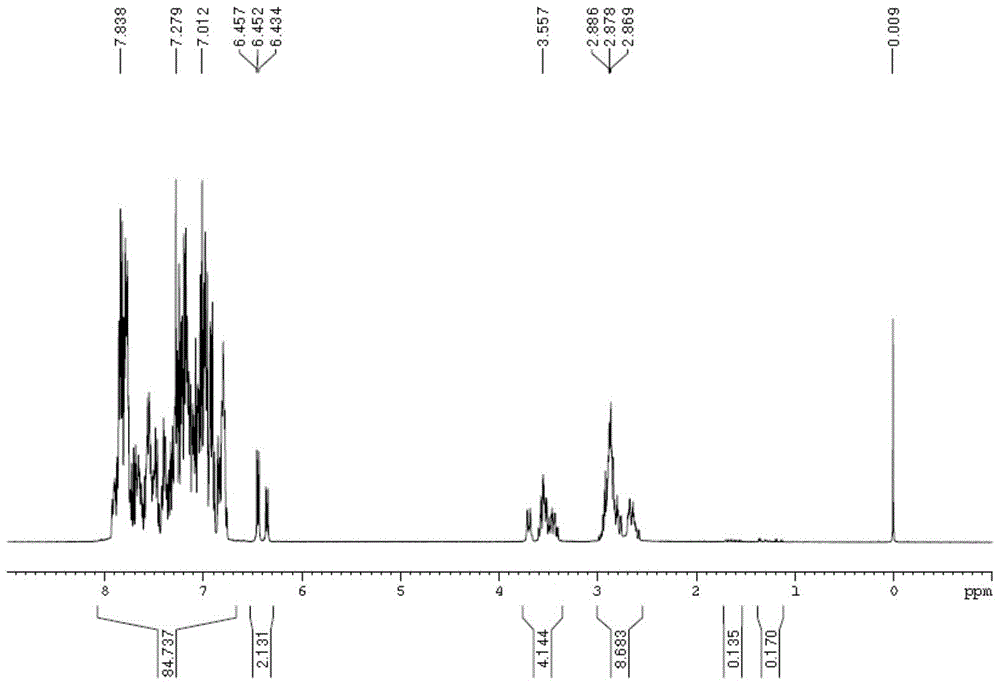
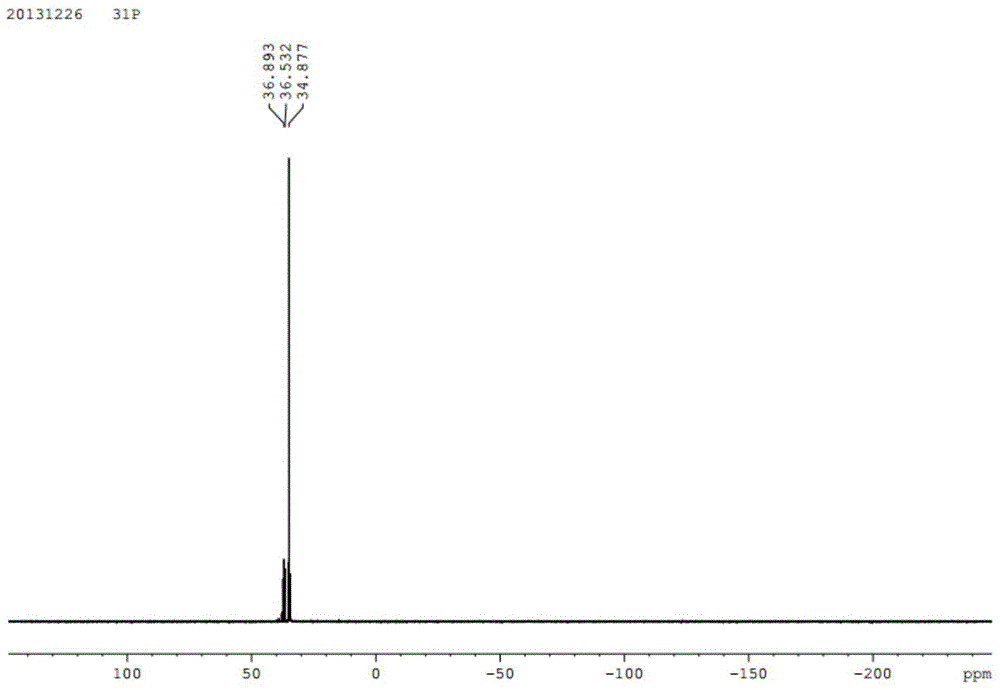
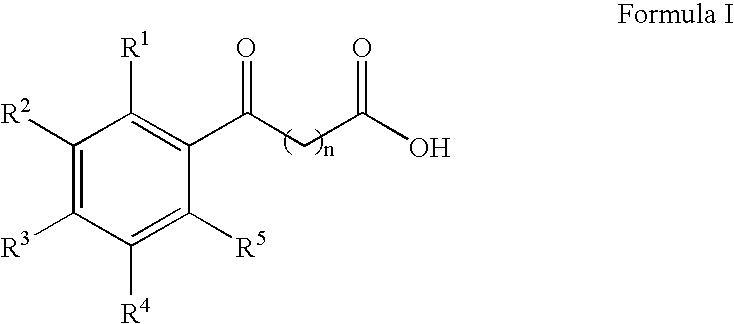
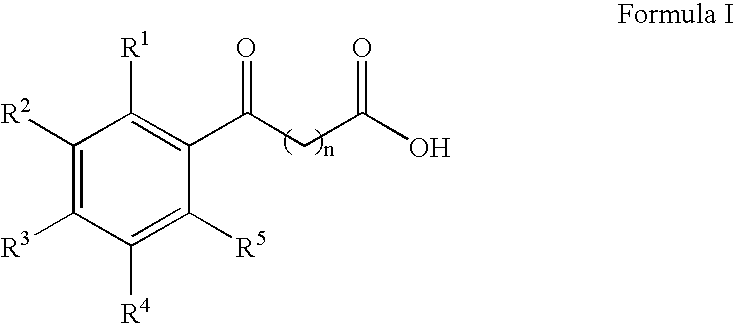
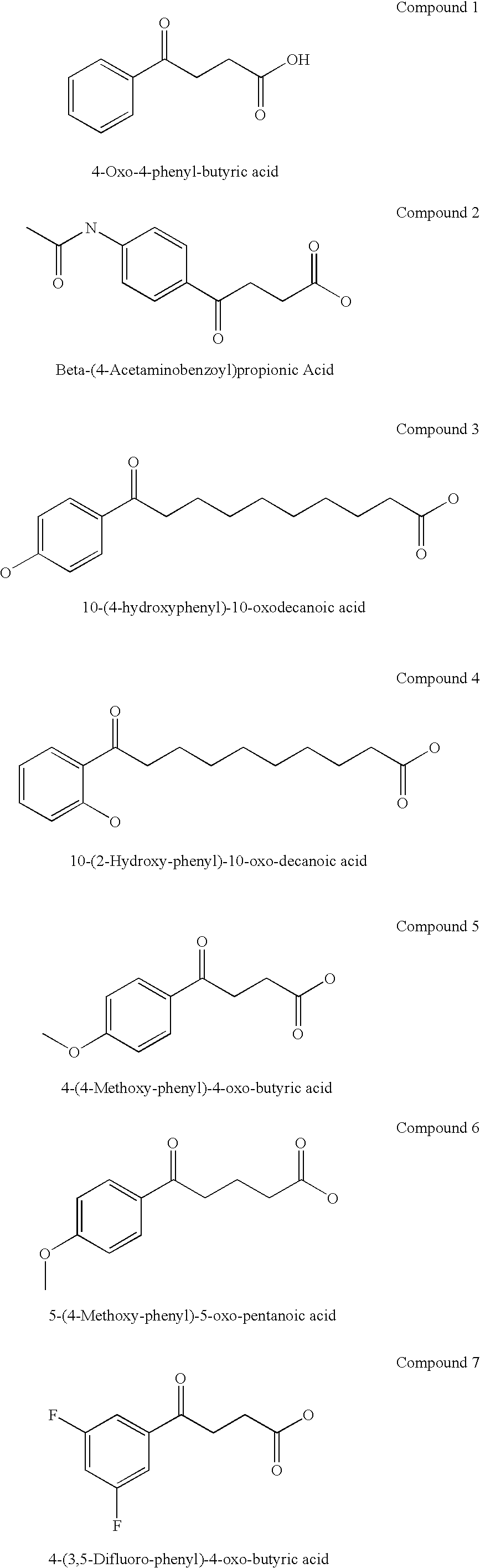
The present invention provides aryl ketone compounds and compositions containing them which facilitate the delivery of active agents. The aryl ketone compounds have the formulaor a salt thereof, where n=1 to 9, and R1 to R5 are independently hydrogen, C1 to C4 alkyl, C1 to C4 alkoxy, C2 to C4 alkenyl, halogen, hydroxyl, —NH—C(O)—CH3, or —O—C6H5.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
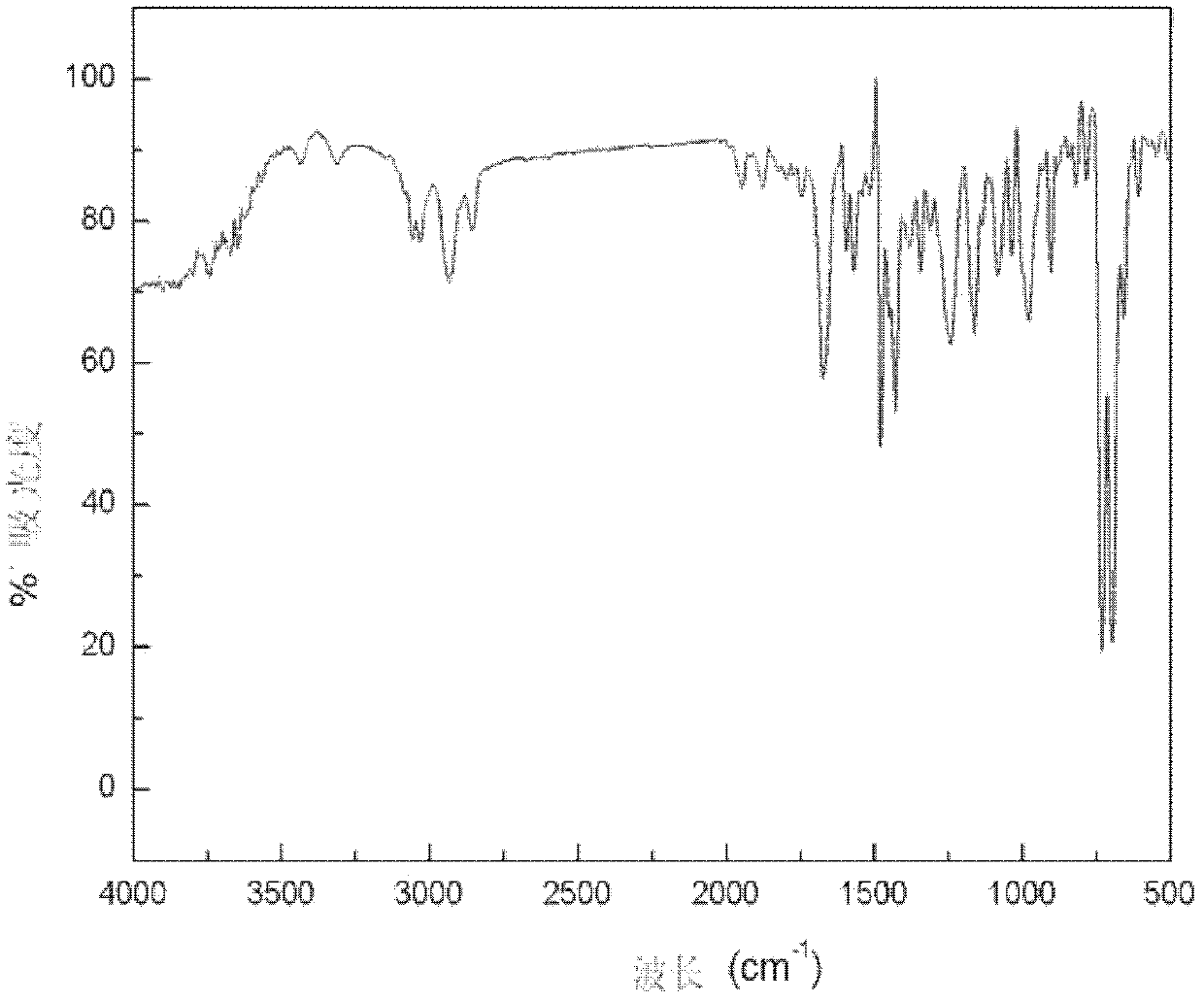
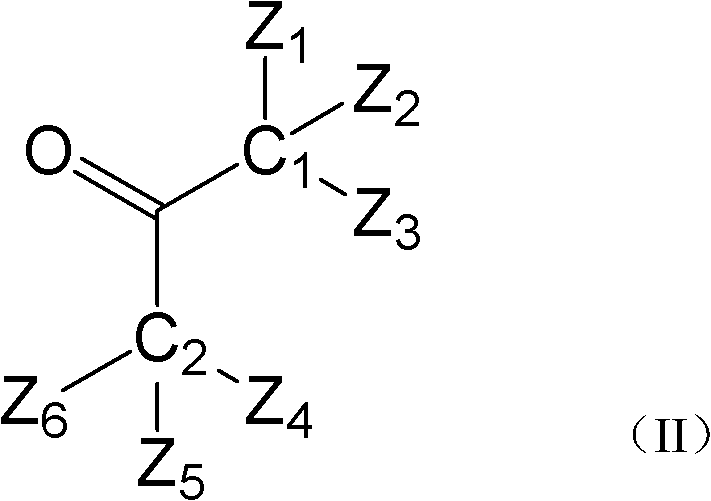
Hydroxyalkyl aryl ketone photoinitiator capable of reducing volatile organic compound (VOC) discharge until elimination of VOC discharge
ActiveCN102417553AHigh atmospheric pressure boiling pointLow mobilitySilicon organic compoundsOrganic compound preparationPrinting inkLight cure
The invention discloses a hydroxyalkyl aryl ketone photoinitiator capable of reducing volatile organic compound (VOC) discharge until elimination of VOC discharge and a preparation method thereof. The hydroxyalkyl aryl ketone photoinitiator capable of reducing VOC discharge until elimination of VOC is shown in the formula I. In the formula I, Ar of the hydroxyalkyl aryl ketone photoinitiator capable of reducing VOC discharge until elimination of VOC represents a substituted or a non-substituted aryl group; Z1, Z2, Z3, Z4, Z5 and Z6 do not simultaneously represent hydrogen atoms; six groups represented by the Z1, the Z2, the Z3, the Z4, the Z5 and the Z6 are organic groups, wherein the sum of the number of carbon atoms of each one of the six groups and the sum of the number of heteroatoms of each one of the six groups are great than or equal to 5; and C1 and C2 represent carbon atoms. The hydroxyalkyl aryl ketone photoinitiator capable of reducing VOC discharge until elimination of VOCis characterized in that through simultaneous change of an aryl structure and a hydroxyalkyl structure, a structure and characteristics of a by-product produced by compound photolysis are changed finally, wherein the improved by-product has low VOC discharge performances and even VOC discharge complete elimination performances. The hydroxyalkyl aryl ketone photoinitiator capable of reducing VOC discharge until elimination of VOC has high initiation efficiency, overcomes the defects of strong smell, toxicity and a migration capability belonging to a traditional photoinitiator of which the typeis the same as the type of the hydroxyalkyl aryl ketone photoinitiator provided by the invention, and has good application prospects in the fields of light-cured paint and printing ink.
Owner:CHANGSHANG NEWSUN CHEM IND
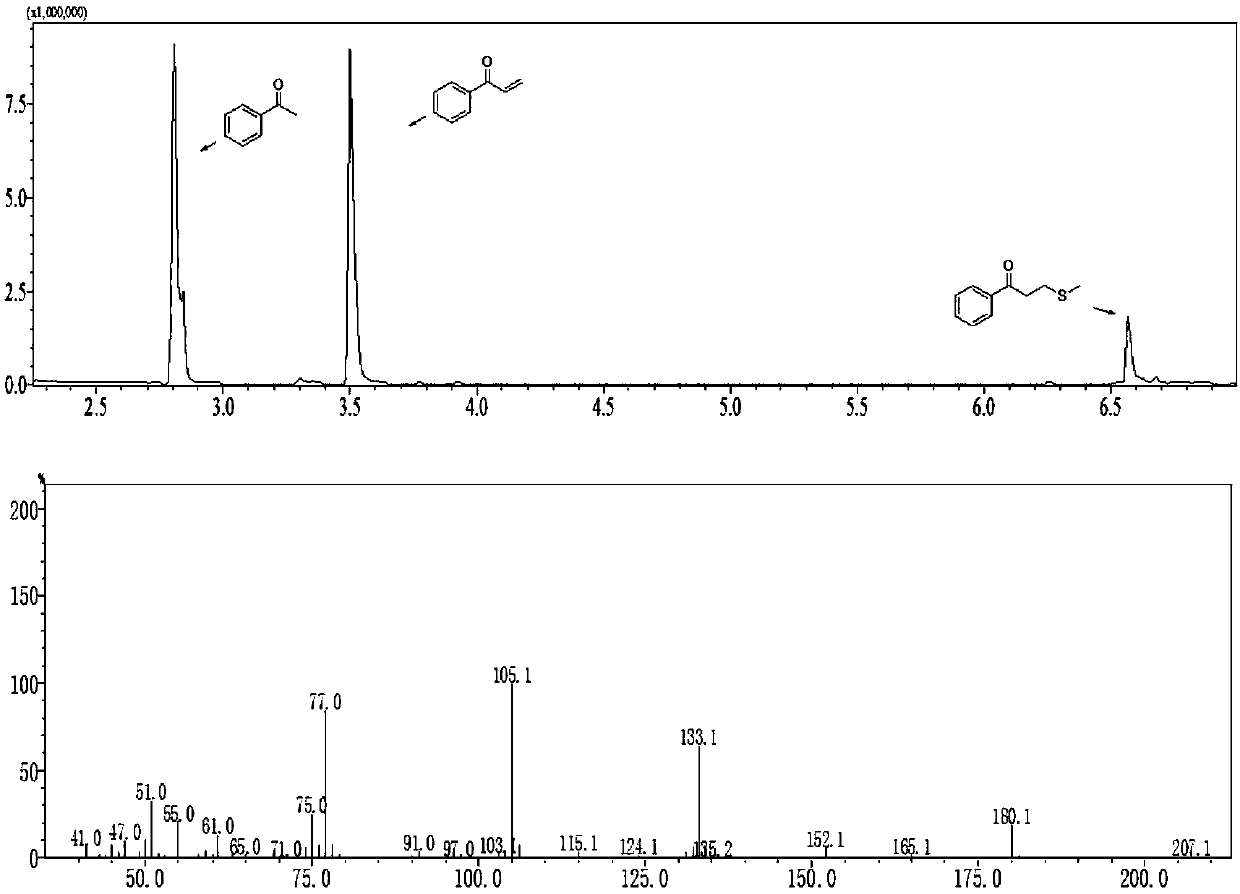
Method for synthesizing alpha,beta-unsaturated aryl ketone compound from dimethyl sulfoxide and arylethanone
ActiveCN107759458AHigh selectivityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationPersulfateCarboxylic salt
The invention discloses a method for synthesizing an alpha,beta-unsaturated aryl ketone compound from dimethyl sulfoxide and arylethanone. According to the method, dimethyl sulfoxide and arylethanoneare subjected to a one-pot reaction in the presence of carboxylate and persulfate so as to produce the alpha,beta-unsaturated aryl ketone compound. The method uses dimethyl sulfoxide as a methylenation reagent and employs a one-pot process for high-selectivity high-yield synthesis of the alpha,beta-unsaturated aryl ketone compound from arylethanone; and the method is simple to operate, low in cost, friendly to environment and beneficial for industrial production.
Owner:YUANJIANG HUALONG CATALYST TECH
Elastic body for actuator, and piezoelectric actuator
InactiveCN104956584AImprove injection efficiencyImprove the delivery effectPiezoelectric/electrostriction/magnetostriction machinesPiezoelectric/electrostrictive devicesElastomerPower flow
An elastic member 43 comprises a crystalline resin. The elastic member is fixed to a piezoelectric element 42 that generates vibration in response to application of an AC voltage, is in contact with a movable body 45 in use, and flexurally vibrates in response to the vibration of the piezoelectric element 42 to drive the movable body 45. The elastic member 43 may have a tooth 43b on an opposite side with respect to a side fixed to the piezoelectric element 42. The crystalline resin may be a poly(aryl ketone) resin or a poly(phenylene sulfide) resin. The piezoelectric actuator has an excellent transmission of flexural vibration. In a case where a displacement expansion element of a displacement-expanding piezoelectric actuator is formed from a crystalline resin, the actuator greatly amplifies an expansion and contraction displacement of a piezoelectric element. In a case where a resonance member of a Langevin transducer is formed from a crystalline resin, the transducer allows the vibration of the surface at a high speed even in application of a low electric current (or a low voltage).
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com