Method for preparing diaryl ketone from aryl sulfonate
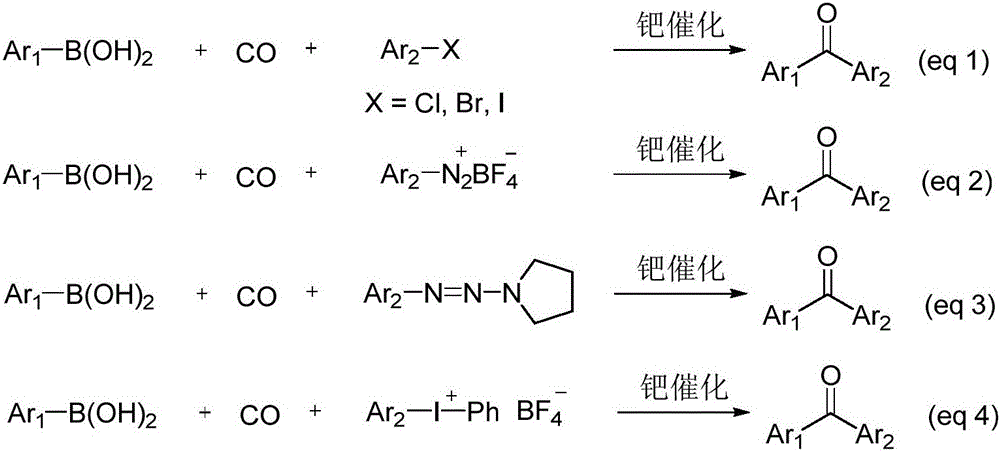
A technology of aryl sulfonate and diaryl ketone, applied in the preparation of carboxylic acid nitrile, carbon monoxide reaction preparation, chemical instruments and methods, etc., can solve the complicated steps of aryl halide, the low stability of diazo compounds, Unfavorable to large-scale production and other problems, to achieve the effects of no by-products, good functional group tolerance, and wide reaction substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12
[0037] In Examples 1-12, DMSO was used as the reaction solvent, benzenesulfonate and phenylboronic acid were used as substrates, and different catalysts were used for the reaction. The reaction conditions and results are shown in Table 1.
[0038] The reaction condition and reaction result of table 1 embodiment 1~12
[0039]
Embodiment 13~20
[0041] Examples 13-20 use triphenylphosphine palladium acetate as a catalyst, benzenesulfonate and phenylboronic acid as substrates, and react by using different solvents. See 2 for the reaction conditions and reaction results.
[0042] The reaction conditions and reaction result of table 2 embodiment 13~20
[0043]
Embodiment 21~26
[0045] In Examples 21 to 26, triphenylphosphine palladium acetate was used as a catalyst, DMSO was used as a reaction solvent, and different tosylates were used as substrates for the reaction. See 3 for the reaction conditions and results.
[0046] The reaction condition and reaction result of table 3 embodiment 21~26
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com