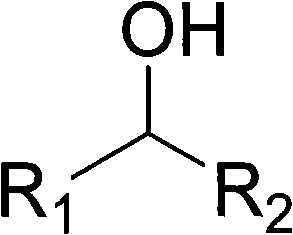
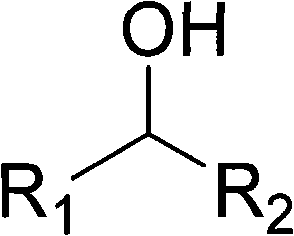
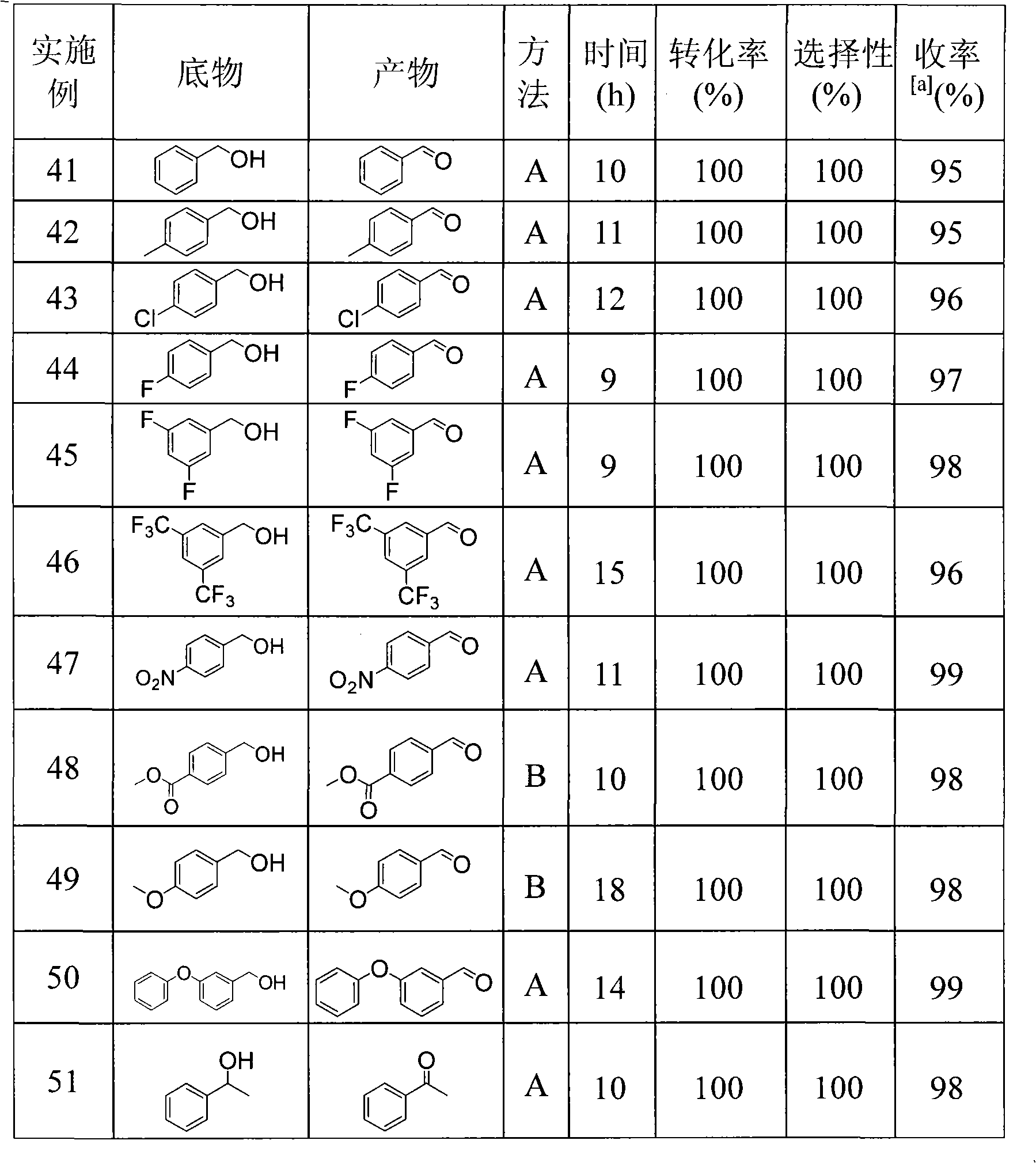
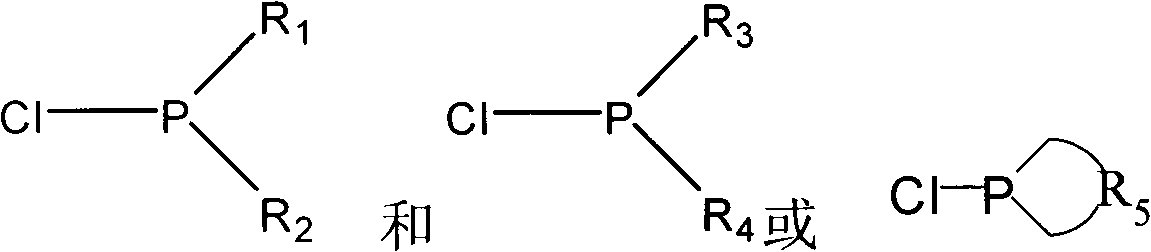
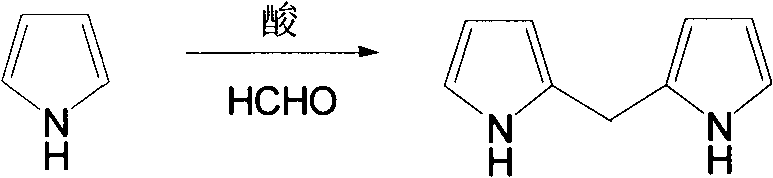
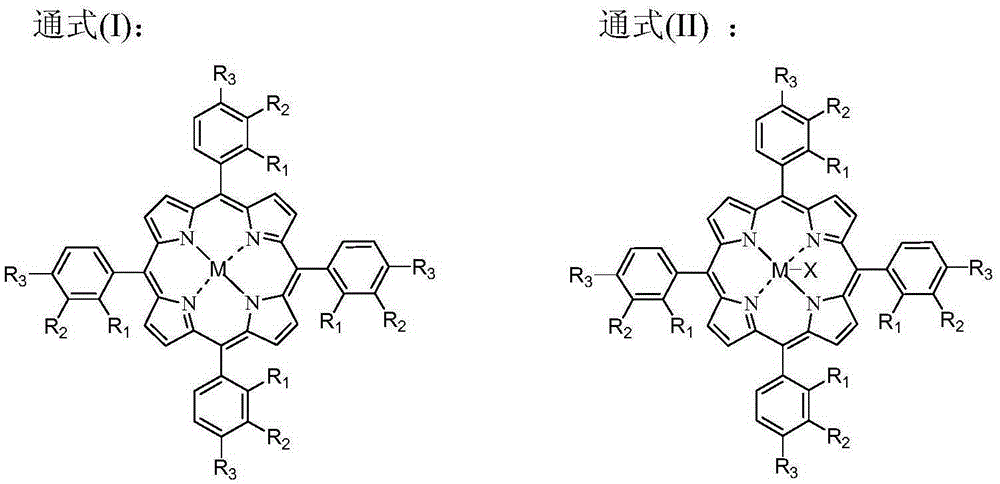
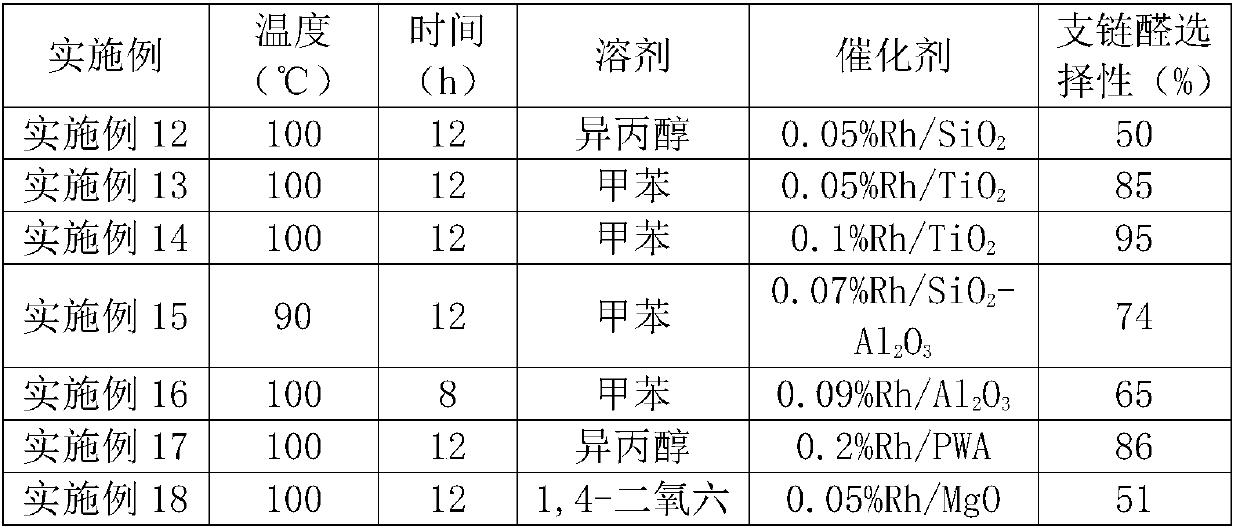
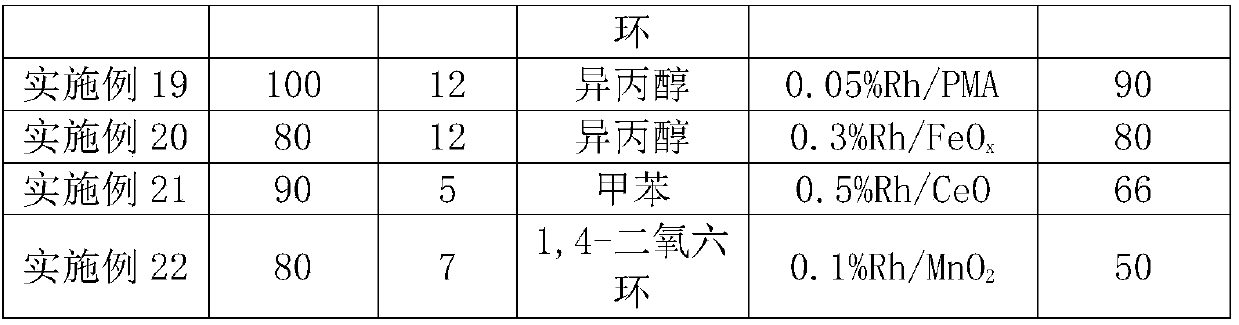
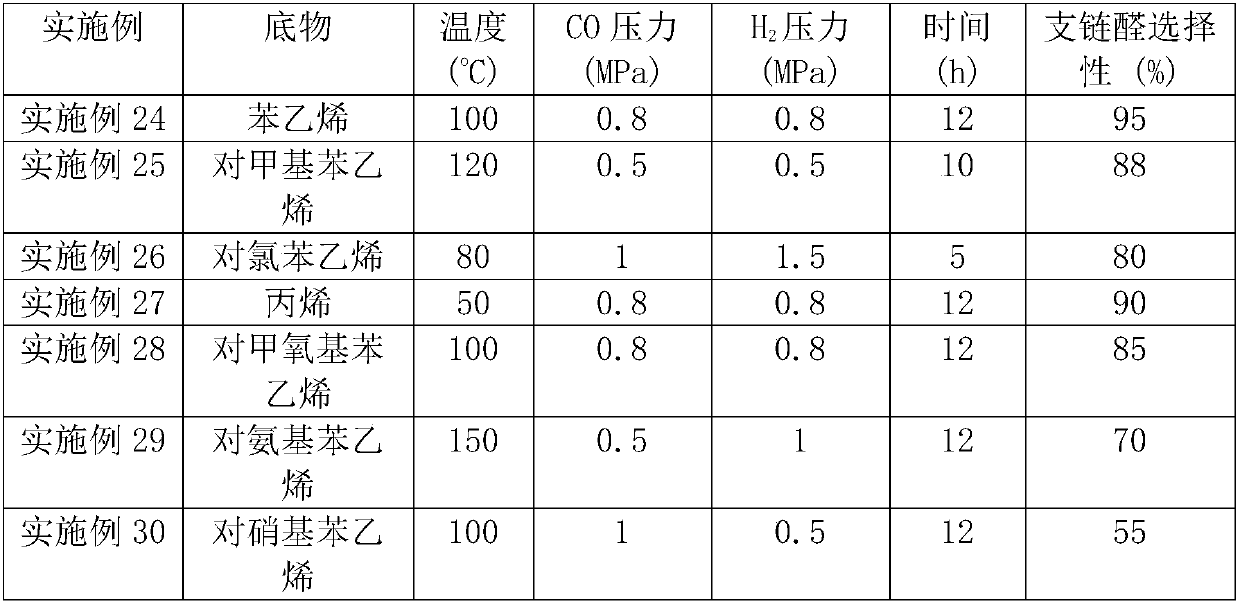
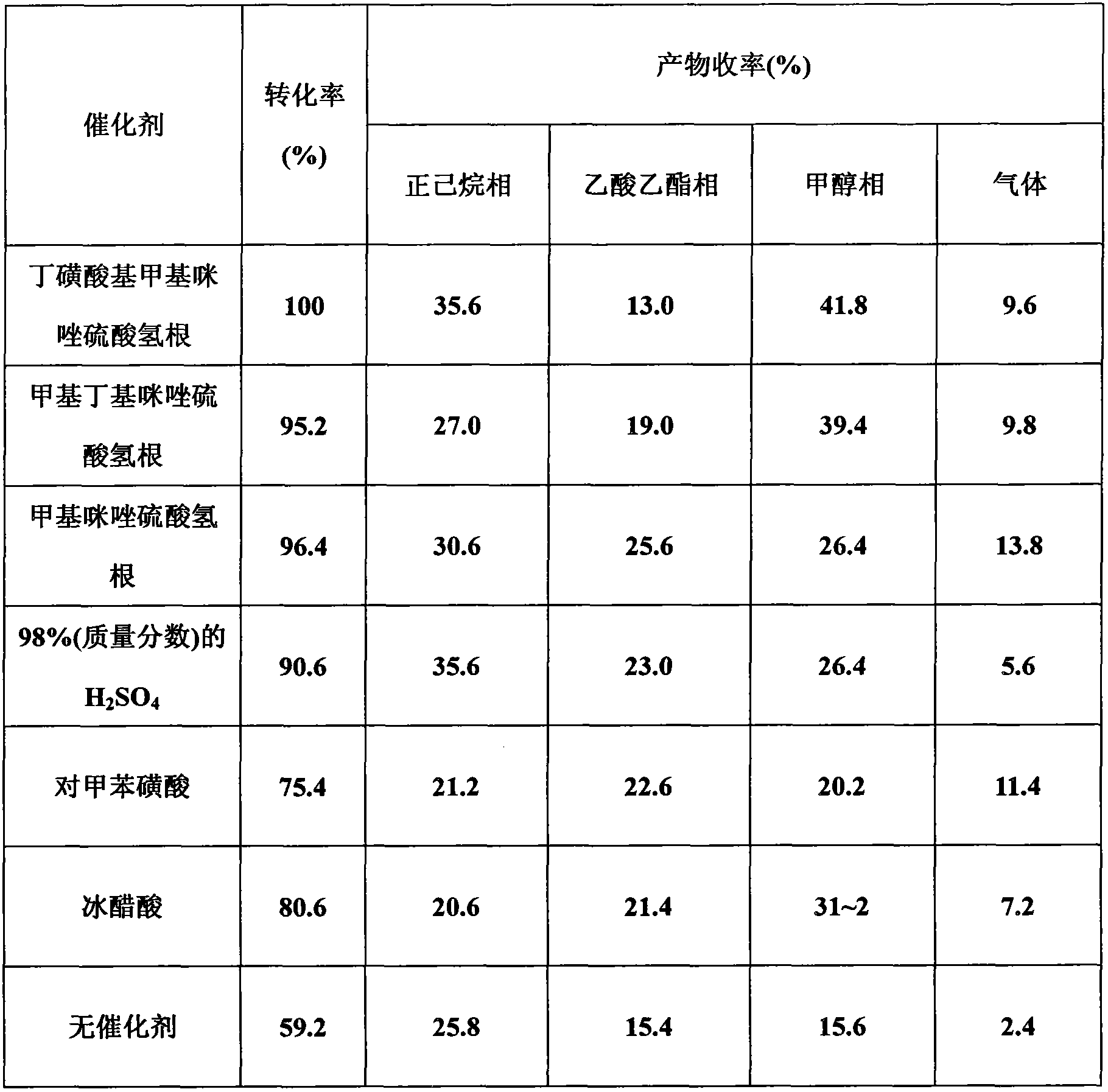
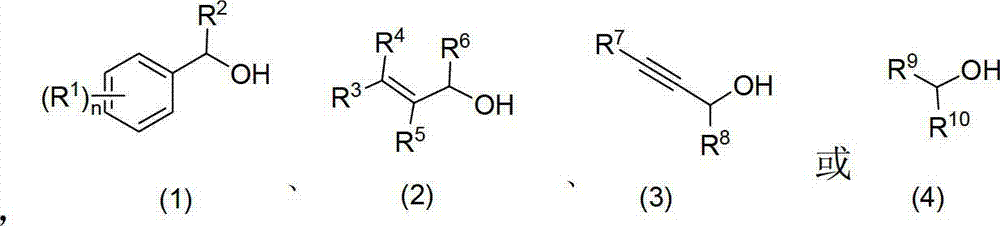
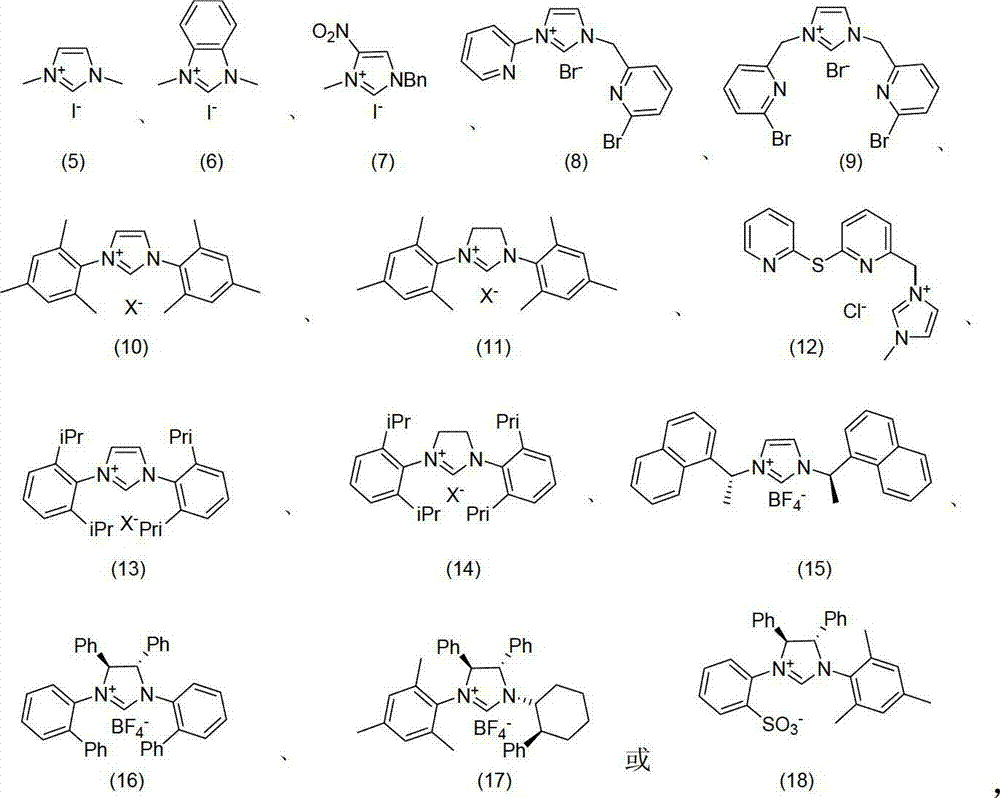
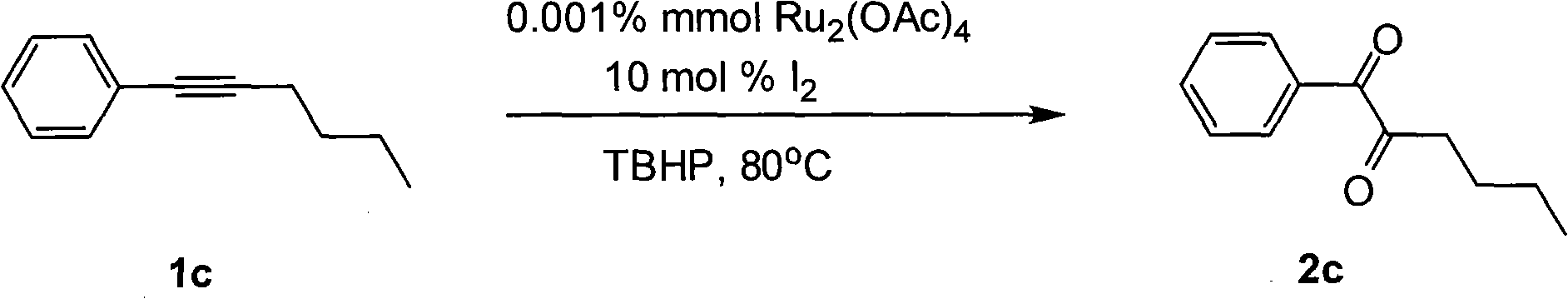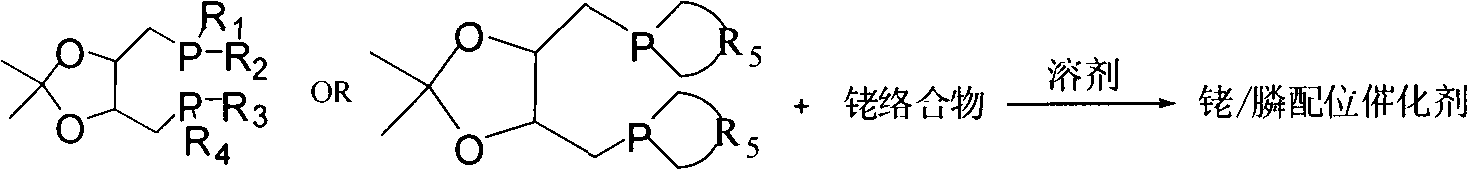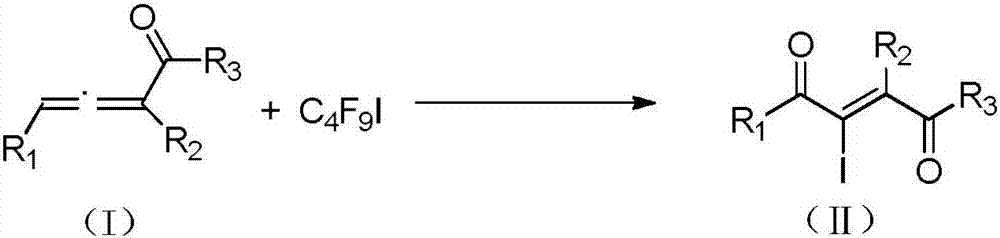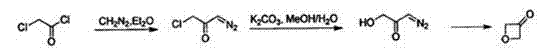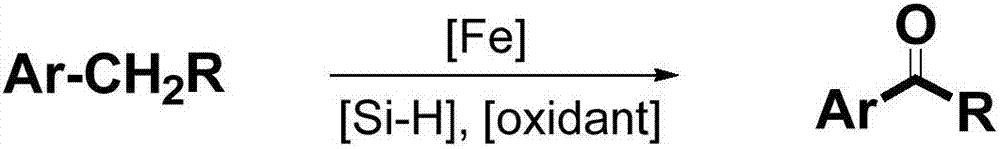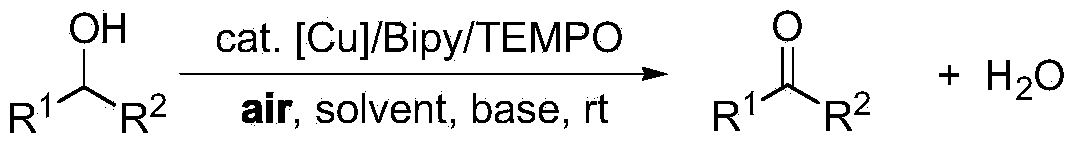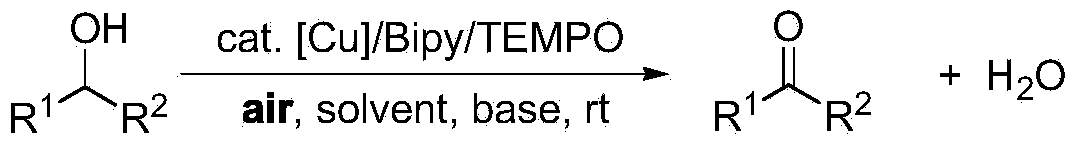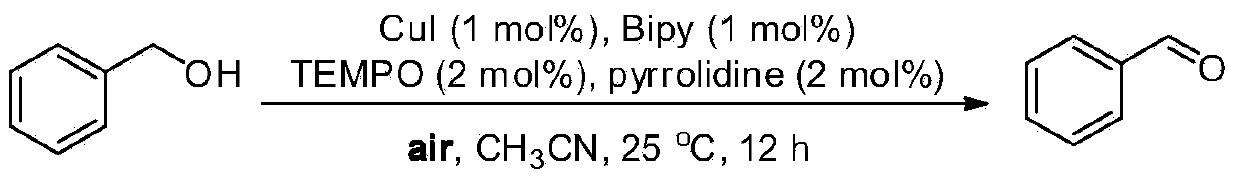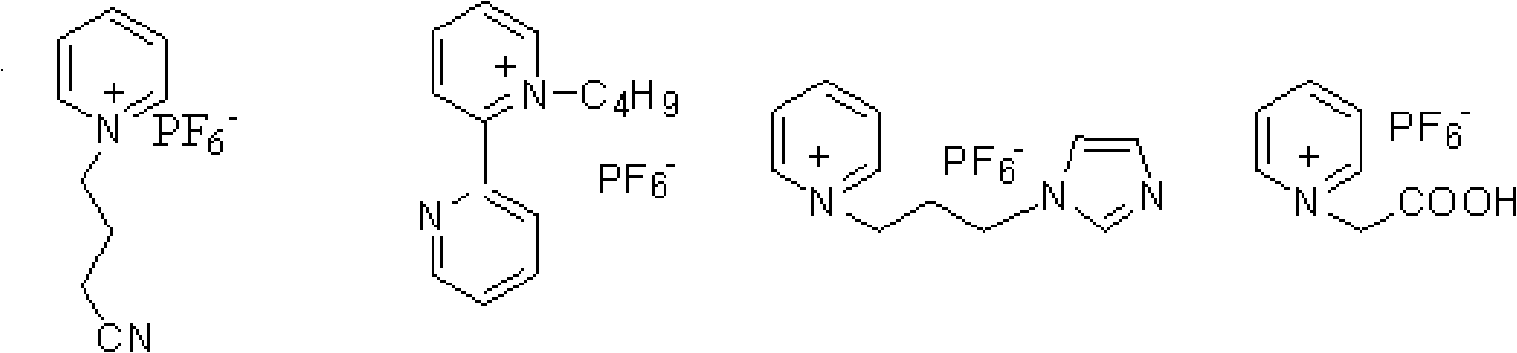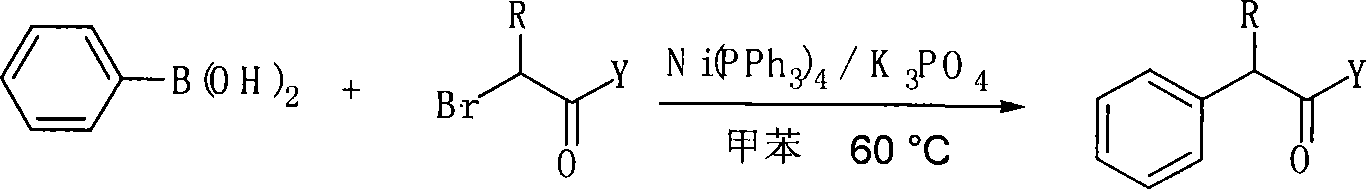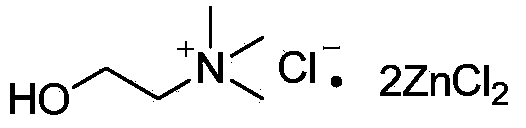Patents
Literature
426results about "Carbonyl group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
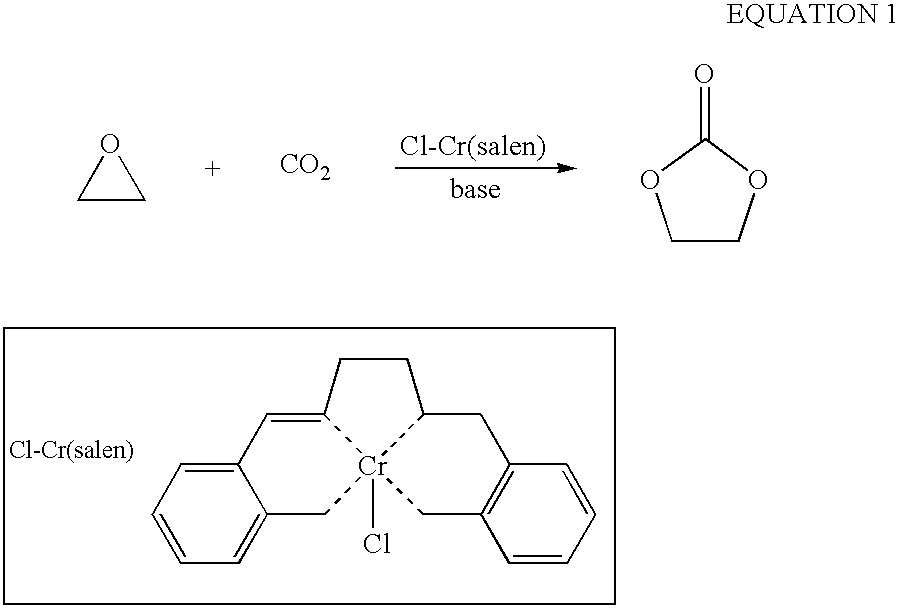
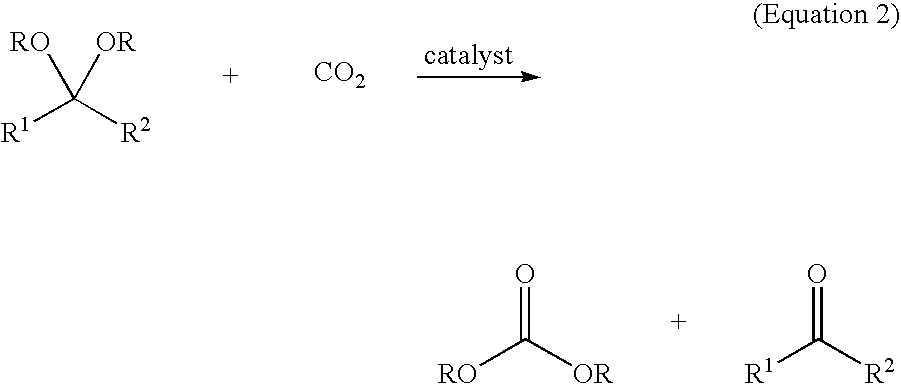
Catalytic Composition for the Insertion of Carbon Dioxide Into Organic Compounds
The invention relates to a catalytic composition comprising: a first component which is at least a component with one or more metals from groups 3A, 4A, 5A, 6A, 7A, 8, 1B, 2B, 3B, 4B; and a second component selected from (1) at least one ionic liquid which consists of a compound formed by cations and anions and which is a liquid at ambient temperature, (ii) a matrix to which the first component is bound or on which it is supported, and (iii) a combination of the two. The invention relates to the use of said catalytic composition in a method for the insertion of carbon dioxide into an organic compound and, preferably, a compound selected from epoxides, acetals and orthoesters. The invention also relates to catalytic compositions comprising said metallic compounds.
Owner:RODRIGUEZ MERCEDES ALVARO +3
Preparation method of benzaldehydes compound and novel double-metal catalyst loaded by mesoporous carbon for preparation method
ActiveCN102513104AHigh catalytic activityGood synergyCatalyst carriersOrganic compound preparationBenzeneBenzaldehyde
The invention relates to a preparation method of a benzaldehydes compound and a catalyst for the preparation method. The catalyst is composed of 0.01-90 wt% of metal grains and 10-99.99 wt% of mesoporous carbon carrier; the metal grains are selected from any two of Pd, Au, Ag, Pt, Ru, Rh, Ni, Cu, Fe, Co, Cr, W, Mo, Ti and Ta; the weight ratio of two types of the metal is 1: (0.01-100) and the average grain diameter of the metal grains is 1-100 nm; and the mesoporous carbon carrier is prepared from a heteroatom-doped mesoporous carbon material. The content of heteroatoms in the mesoporous carbon material is 0.01-80 wt%. The catalyst provided by the invention is stable for water, air and heat and has an excellent catalytic activity; particularly, the catalyst has a high selectivity when being used for catalyzing an alcohol oxidation reaction to prepare aldehyde or ketone. The preparation method of the benzaldehydes compound provided by the invention has the advantages of high conversion rate of raw materials and good selectivity of a target product.
Owner:ZHEJIANG UNIV
Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole
InactiveUS6268502B1Effect of the acidic medium on omeprazole decomposition isPoorly solubleOrganic active ingredientsDigestive systemAcetic acidBenzoic acid
An improved process of synthesis of 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl-1H-benzimidazole (omeprazole) by oxidation of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]thio]benzimidazole with 3-chloroperoxy-benzoic acid in ethyl acetate wherein omeprazole is poorly soluble, at a temperature between -10° C. and 5° C. is disclosed. The second step is a purification of the crude product by dissolution and reprecipitation of the final product.
Owner:LEK TOVARNA FARM & KEMICNIH IZDELKOV D D
Method for preparing benzaldehyde or substituted benzaldehyde by catalytically oxidizing methylbenzene or substituted methylbenzene
ActiveCN102070382AReduce dosageSimple processOrganic compound preparationCarbonyl group formation/introductionBenzaldehydeCatalytic oxidation
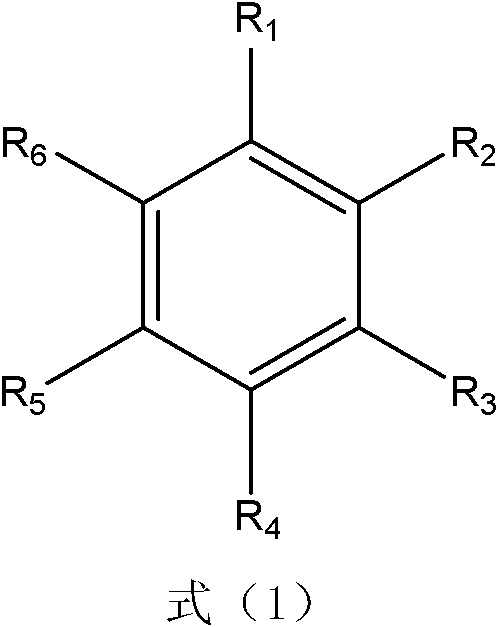
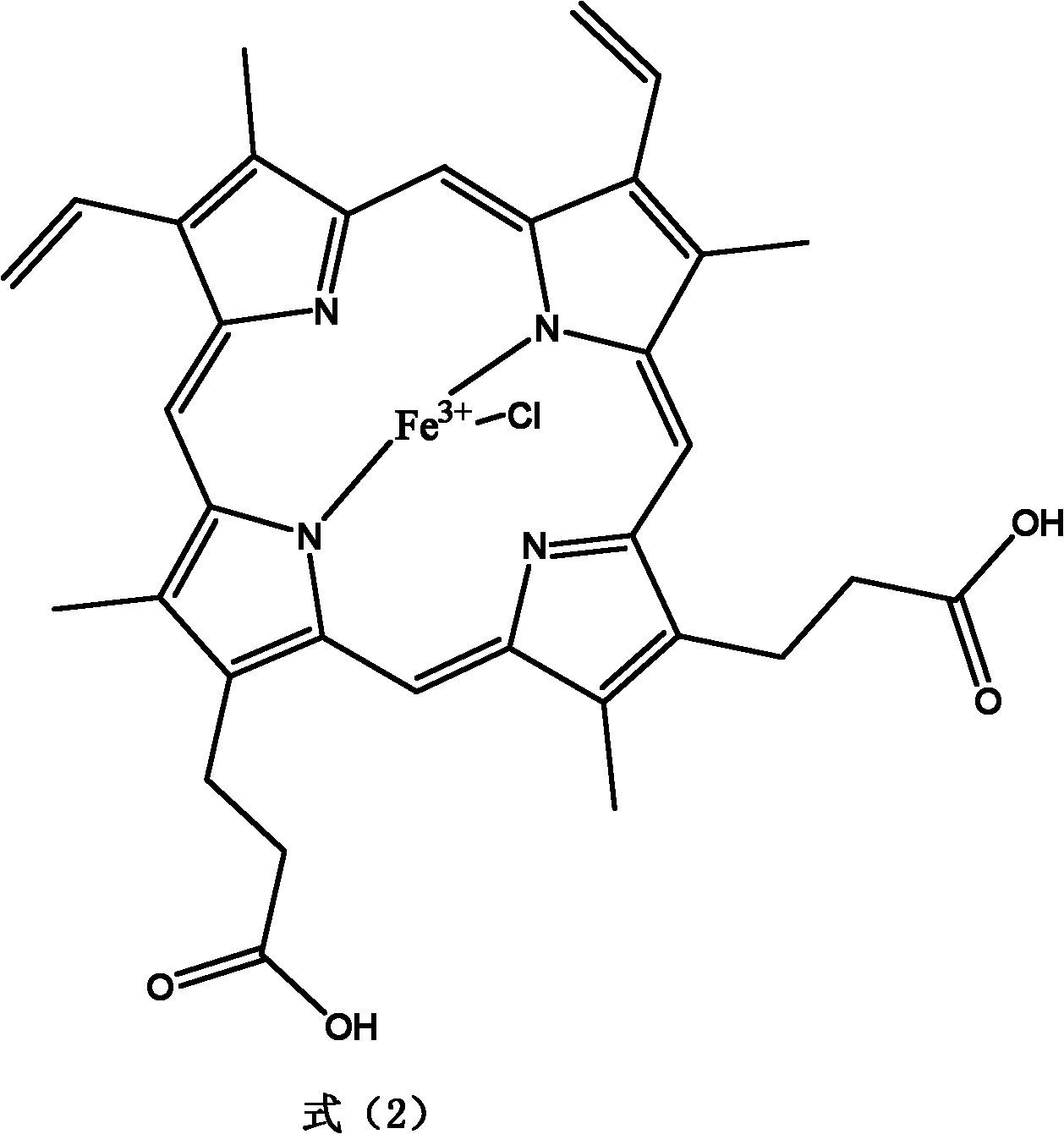
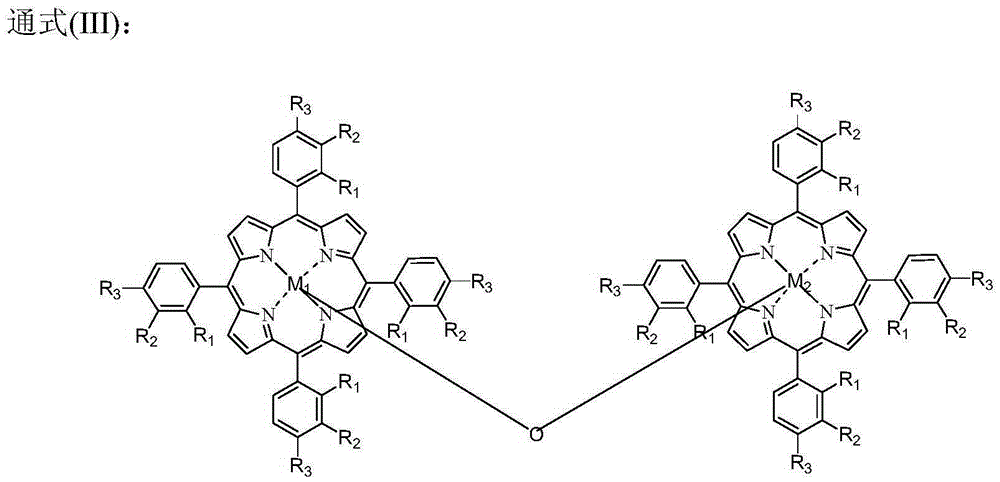
The invention provides a method for preparing benzaldehyde or substituted benzaldehyde by catalytically oxidizing methylbenzene or substituted methylbenzene and relates to the field of chemical industrial material intermediates. The benzaldehyde or substituted benzaldehyde of a general formula (1) and relevant side products are prepared by using a substance containing ferroporphyrin, derivatives of the ferroporphyrin or relevant load as a catalyst, adding a proper cocatalyst according to requirements, and oxidizing methylbenzene or substituted methylbenzene by using oxygen-containing gases such as oxygen, air and the like. In the method, the reaction is performed at a certain temperature of between 25 and 250 DEG C and pressure of 0.1 to 2 MPa; the using amount of the catalyst is small, and is 1 ppm to 1 mass percent under a non-load condition and 0.1 to 10 mass percent under a load condition; and other solvents are not added, and if necessary, a certain amount of cocatalyst is added. The method has the characteristics of environmental friendliness and simple process.
Owner:SHANDONG YINGYANG FLAVORS & FRAGRANCES
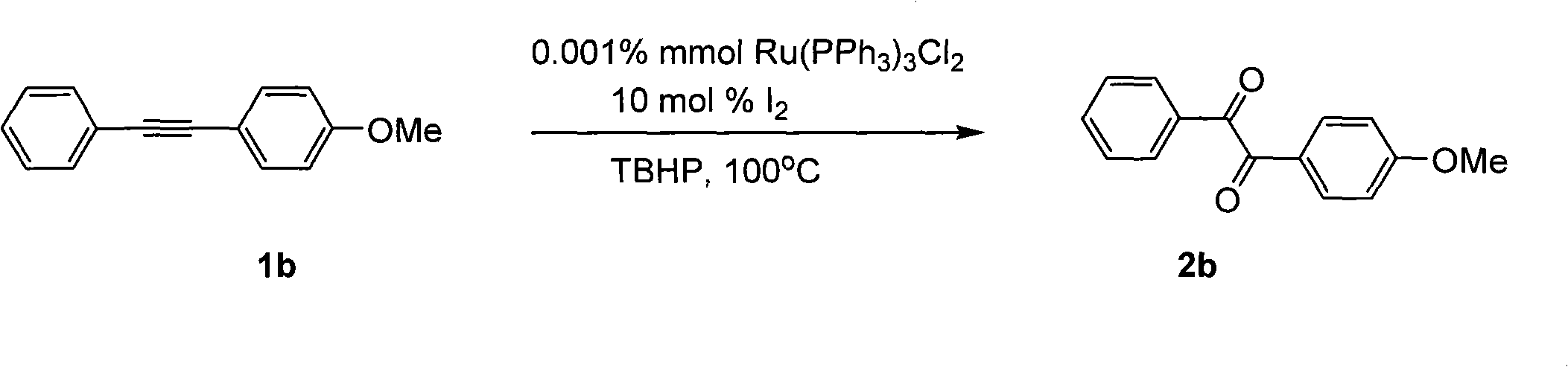
Method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes
InactiveCN101624322AMild reaction conditionsMeet the requirementsCarboxylic acid nitrile preparationOrganic compound preparationCatalytic oxidationKetone
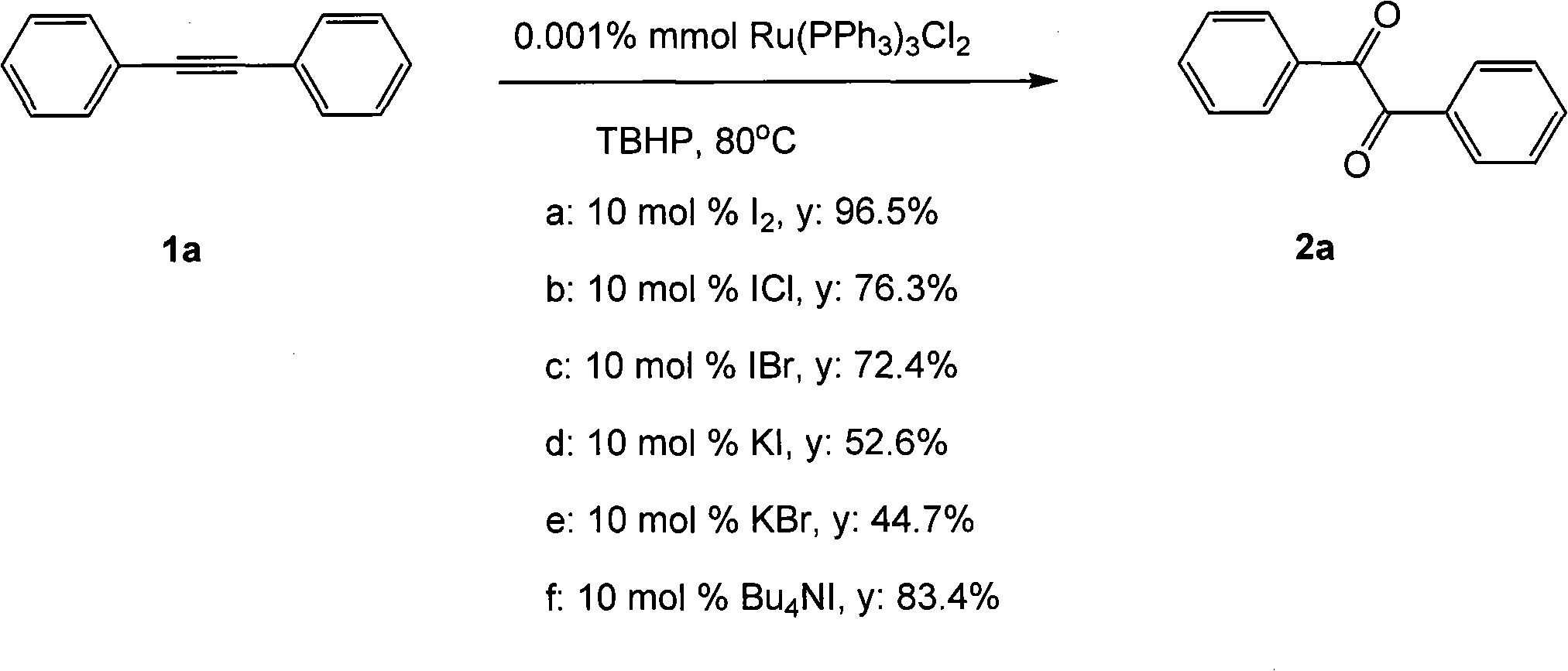
The invention belongs to the field of catalysis and oxidization, and particularly discloses a method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes. The method comprises the following steps: taking alkynes R1-C-C-R2 (acetylenic link exists between C and C) as a reaction substrate, taking one of TBHP, m-chloroperoxybenzoic acid and p-benzoquinone as a oxidant, taking one of dichloro (p-cymene) ruthenium (II) dimer, tri (triphenylphosphine) ruthenous chloride, ruthenium acetate, ruthenium dichlorophenyl (II) dimer, ruthenium trichloride, BINAP ruthenous chloride, dodecacarbonyltriruthenium and tricarbonyldichlororuthenium (II) dimer as a catalyst, taking one of iodine, iodine chloride, iodine bromide, potassium iodide, tetrabutyl ammonium iodide and potassium bromide as a cocatalyst, and taking 1, 4-dioxane as a solvent to react under 40 DEG C to 100 DEG C for 4 to 24 h to prepare the 1, 2-diketone. The method is economic, environmental-friendly and mild.
Owner:SUZHOU UNIV
Method for preparing aldehyde or ketone by catalyzing air and oxidizing alcohol
InactiveCN101486621AEasy to separateWide substrate applicabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkyl nitritesKetone
The invention provides a method for preparing aldehyde or ketone through the catalysed air oxidation of alcohol. Calculated by 5mmol of a reaction substrate, 1 percent to 8 percent of 2, 2, 6, 6-tetramethylpiperidine-oxygen free radical (TEMPO) or derivatives thereof, 4 percent to 20 percent of halide-containing compounds, 4 percent to 10 percent of nitrite or nitrite esters are taken as catalysts, 0.1MPa to 0.8MPa of oxygen or air is taken as an oxidizer, the reaction lasts for 1 hour to 36 hours at temperature of 0 DEG C to 80 DEG C and a series of alcohols can be oxidized into aldehyde or ketone with high selectivity. The method has the advantages of fairly cheap and safe reagent adopted, wide applicability of the reaction substrate, mild reaction conditions, convenient separation of products, no pollution to the environment, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydroformylation reaction method by using rhodium-ruthenium bimetal and quadridentate phosphine ligand and catalyst
ActiveCN106824282APositive and lowPromote generationRhodium organic compoundsOrganic compound preparationIsomerizationRuthenium
The invention discloses a long-chain internal olefin isomerization / hydroformylation homogeneous catalytic reaction method and a catalyst. According to the method, a rhodium-ruthenium bimetal complex is used as the catalyst, and the ligand adopts a quadridentate phosphine ligand. The catalytic system can be used for homogeneous internal olefin isomerization and hydroformylation reaction under the conditions of certain temperature and pressure. The method is suitable for long-chain internal olefins (>=C8) and internal olefins (<C8), and is a high-normal / isomeric ratio homogeneous bimetal-catalyzed reaction method.
Owner:WUHAN CATALYS TECH CO LTD
Method for preparing aldehyde or alkone by oxygen catalysis and alcohol oxidation under mild condition
InactiveCN101565344AEasy to separateWide substrate applicabilityOrganic oxidationOrganic compound preparationNitrateHalogen
The invention relates to a preparation method for aldehyde or alkone, in particular to a method for preparing aldehyde or alkone by oxygen catalysis and alcohol oxidation under the mild condition. Themethod comprises the following steps: according to the 5 mmol of reaction substrate, 1-8 percent of 2,2,6,6-tetramethylpiperidine-oxygen radical (TEMPO) or a derivative thereof, 4-20 percent of halogen-containing compound and 4-20 percent of nitric acid or nitrate are taken as the catalysts, 0.1-0.8 MPa oxygen or air is taken as the oxidant, then the reaction is carried out for 1-36 h at the temperature of 0-80 DEG C, a series of alcohols can be oxidized into aldehyde or alkone with high selectivity, a catalyst TEMPO and the derivative thereof can be circularly and alternately used, the turn-over number (TON) is up to 800 and the cost is greatly reduced. The invention has the advantages of safer reagent with lower price, wider applicability of the substrate, mild reaction conditions, convenient product separation, no pollution to the environment, easy industrialization, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for hydroformylation reaction and preparation method of catalyst
The invention relates to a catalyst for hydroformylation reaction and a preparation method of the catalyst. The catalyst comprises a rhodium complex and a novel bis-phosphoramidite ligand and is prepared by the rhodium complex and the novel bis-phosphoramidite ligand with molar ratio of 2:1-1:10 through mixed reaction. The catalyst can be applied to the hydroformylation reactions of compounds with unsaturated C-C double-bonded structure at any end position and can improve the yield of linear-chain aldehyde in a product, and the service life of the catalyst is remarkably prolonged.
Owner:WANHUA CHEM GRP CO LTD +1
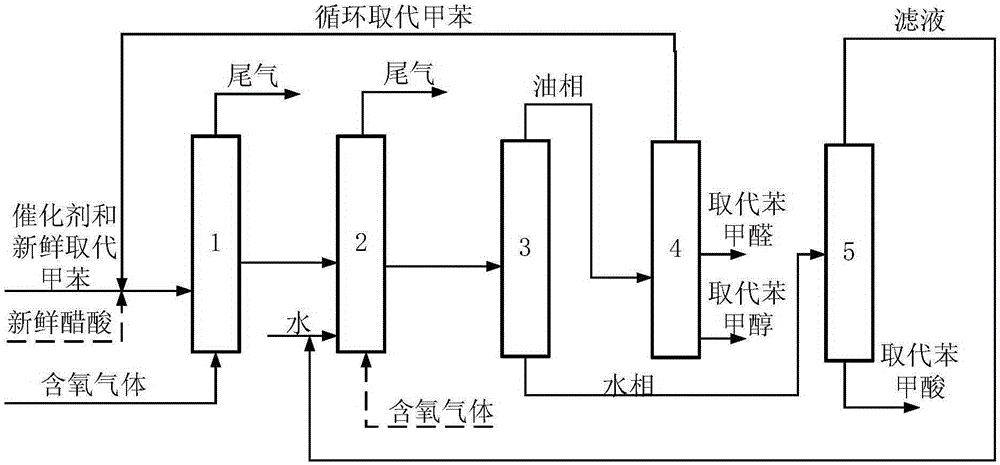
Combined production method for substituted benzaldehyde, substituted benzyl alcohol and substituted benzoic acid
ActiveCN105237317AAvoid the major pitfalls of rectification separationsIncrease profitPreparation by oxidation reactionsCarboxylic acid nitrile preparationBenzoic acidDistillation
The invention discloses a combined production method for substituted benzaldehyde, substituted benzyl alcohol and substituted benzoic acid. The method comprises the following steps: (1) oxidation: a step of continuously introducing substituted toluene, a catalyst and oxygen-contained gas into an oxidation reactor and carrying out reaction so as to obtain oxidation reaction liquid; (2) hydrolyzation: a step of allowing the oxidation reaction liquid to continuously enter a hydrolysis reactor, and continuously adding water into the hydrolysis reactor and carrying out reaction so as to obtain a hydrolysis reaction mixture; (3) liquid-liquid layering: a step of layering the hydrolysis reaction mixture so as to obtain an oil phase and an aqueous phase; and (4) separation of products: a step of subjecting the oil phase to distillation so as to respectively obtain incompletely-reacted substituted toluene, substituted benzyl alcohol and substituted benzaldehyde, and subjecting the aqueous phase to cooling, crystallizing and filtering so as to obtain filtrate and substituted benzoic acid. The combined production method provided by the invention has the advantages of high raw material conversion rate, few by-products, good selectivity of target products, greenness and environmental protection.
Owner:山东友道化学有限公司
Preparation and application of mercapto modified metal-organic frameworks catalyst
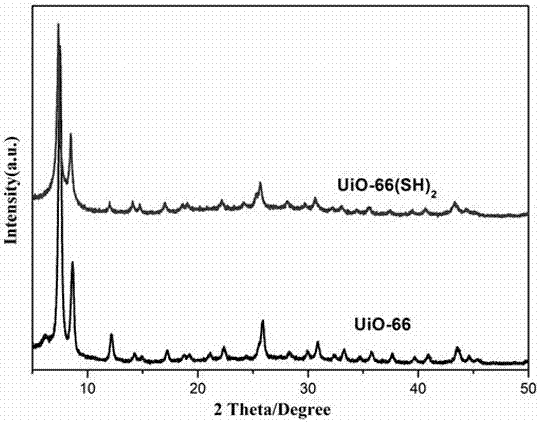
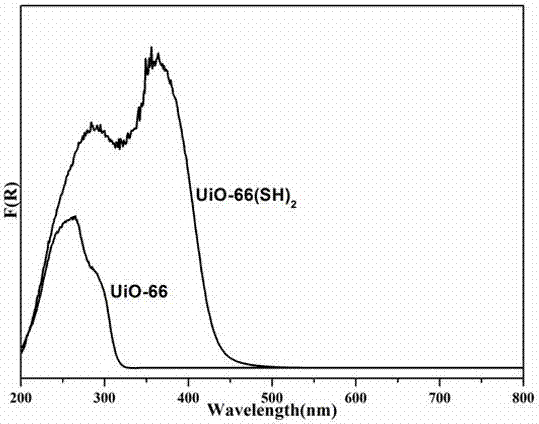
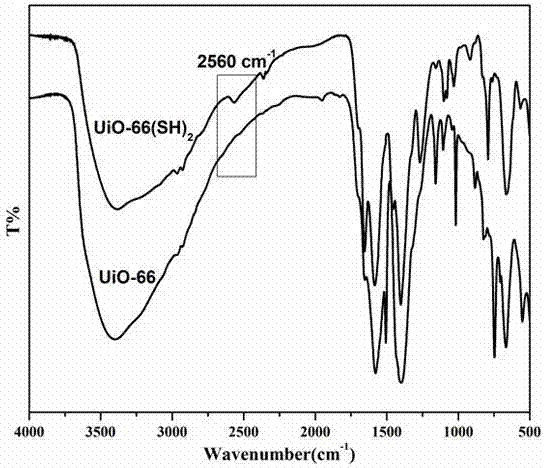
ActiveCN107233924AConfiguration indestructibleEnhanced light absorptionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrachlorideSynthesis methods
The invention discloses preparation methods and application of a mercapto MOFs (Metal-Organic Frameworks) photocatalyst UiO-66(SH)2 and a series of noble metal mercapto MOFs photocatalysts M / UiO-66(SH)2 (M=Au, Pd and Pt). The UiO-66(SH)2 is prepared based on a pre-functionalization strategy by using zirconium tetrachloride and 2,5-dimercapto-1,4-terephthalic acid as precursors and combining with a solvent thermal synthesis method. By utilizing the metal affine effect of a mercapto functional group on a ligand skeleton, a noble metal ion is anchored on the UiO-66(SH)2, so that an M / UiO-66(SH)2 composite photocatalytic material is successfully prepared. In a loading process, an inert atmosphere, the addition of a strong reducing agent or a heat treatment process is not needed; highly dispersed Au, Pd and Pt ions can be loaded on a UiO-66(SH)2 material through a simple mixing process only. The UiO-66(SH)2 and the M / UiO-66(SH)2 both show favorable activity in an experiment of photocatalytically selectively oxidizing benzyl alcohol.
Owner:FUZHOU UNIV
Titanate nanotube composite type photocatalyst as well as preparation method and application thereof
InactiveCN103599772AImproved visible light catalytic activityPromote sustainable developmentMaterial nanotechnologyOrganic compound preparationIon exchangeOxygen
The invention discloses a titanate nanotube composite type photocatalyst as well as a preparation method and application thereof. A metal ion doped titanate nanotube photocatalyst with the one-dimensiional tubular structure is obtained by using anatase TiO2 and metal ion acetate as raw materials through hydrothermal and an ion exchange method; in addition, the CdS / titanate nanotube composite type photocatalyst with the one-dimensiional tubular structure is obtained by using the anatase TiO2, Cd(NO3)2.4H2O and thioacetamide as raw materials through one-step hydrothermal. The titanate nanotube composite type photocatalyst is used for the photocatalytic selective oxidation of alcohols for the first time and has high catalytic efficiency. The titanate nanotube composite type photocatalyst is simple to prepare, is used for the selective oxidation of the alcohols by using visible light as driving energy and oxygen as an oxidizing agent and is beneficial to the sustainable development of environments and energy sources.
Owner:FUZHOU UNIV
Electrochemical deblocking solution for electrochemical oligomer synthesis on an electrode array
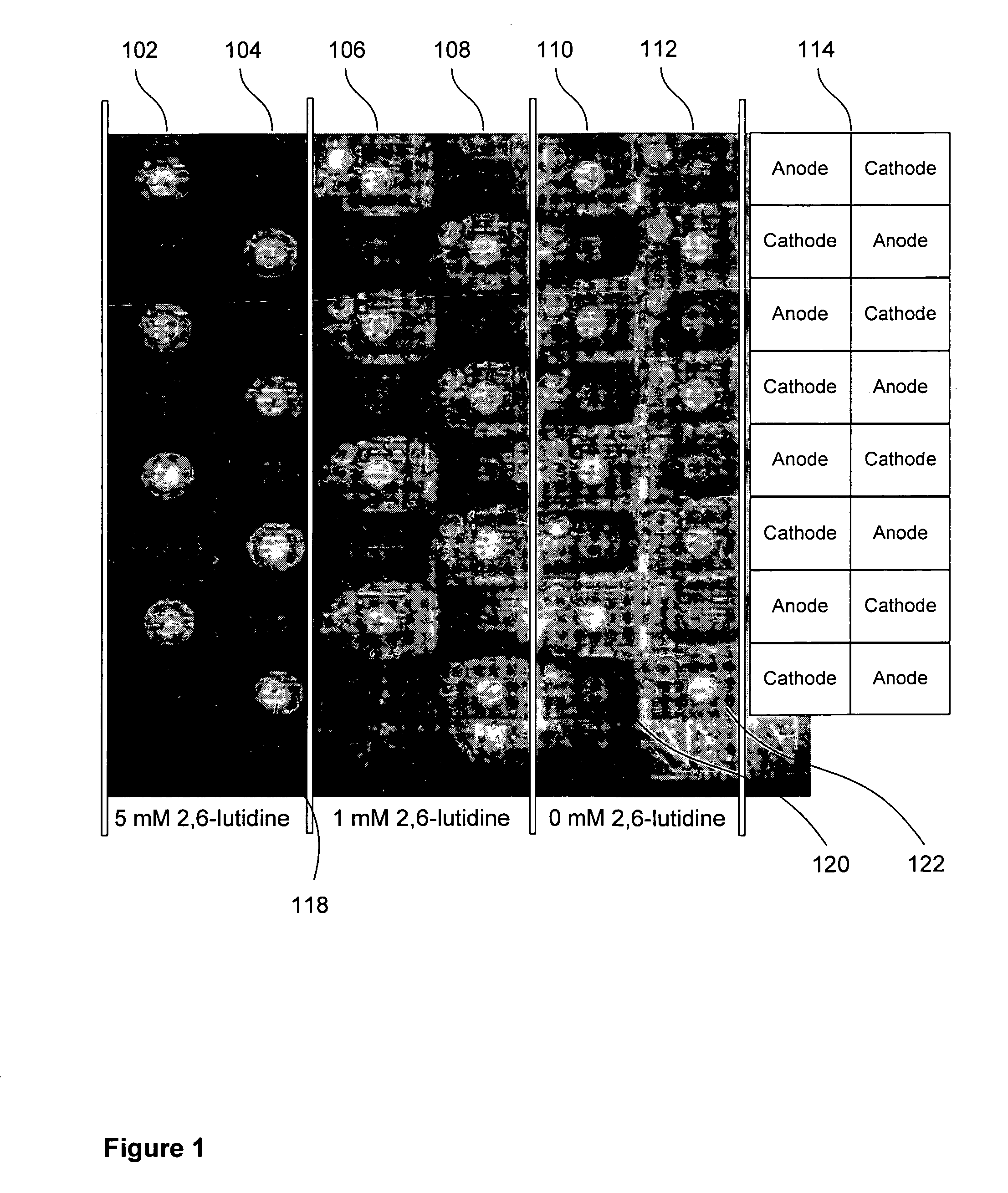
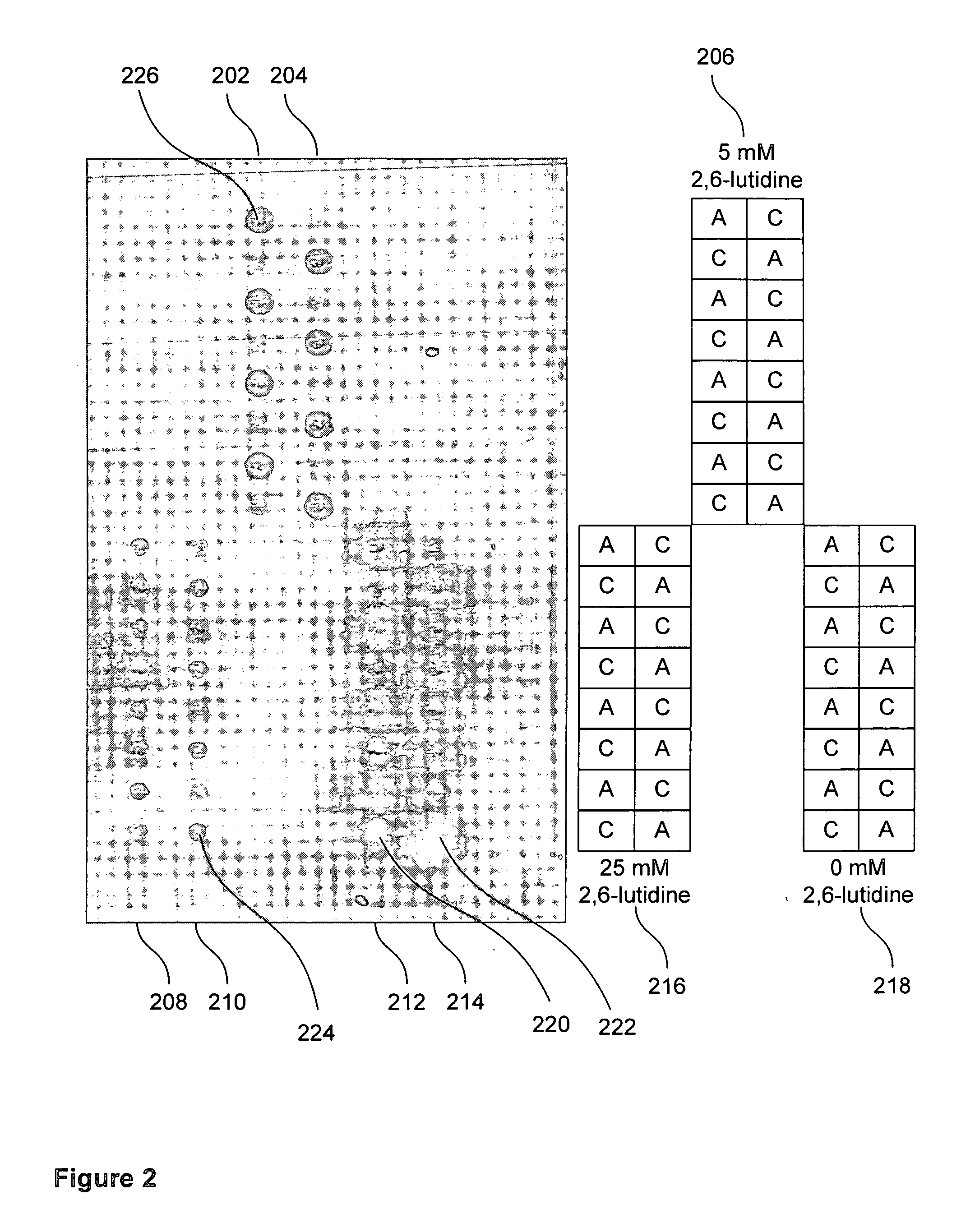
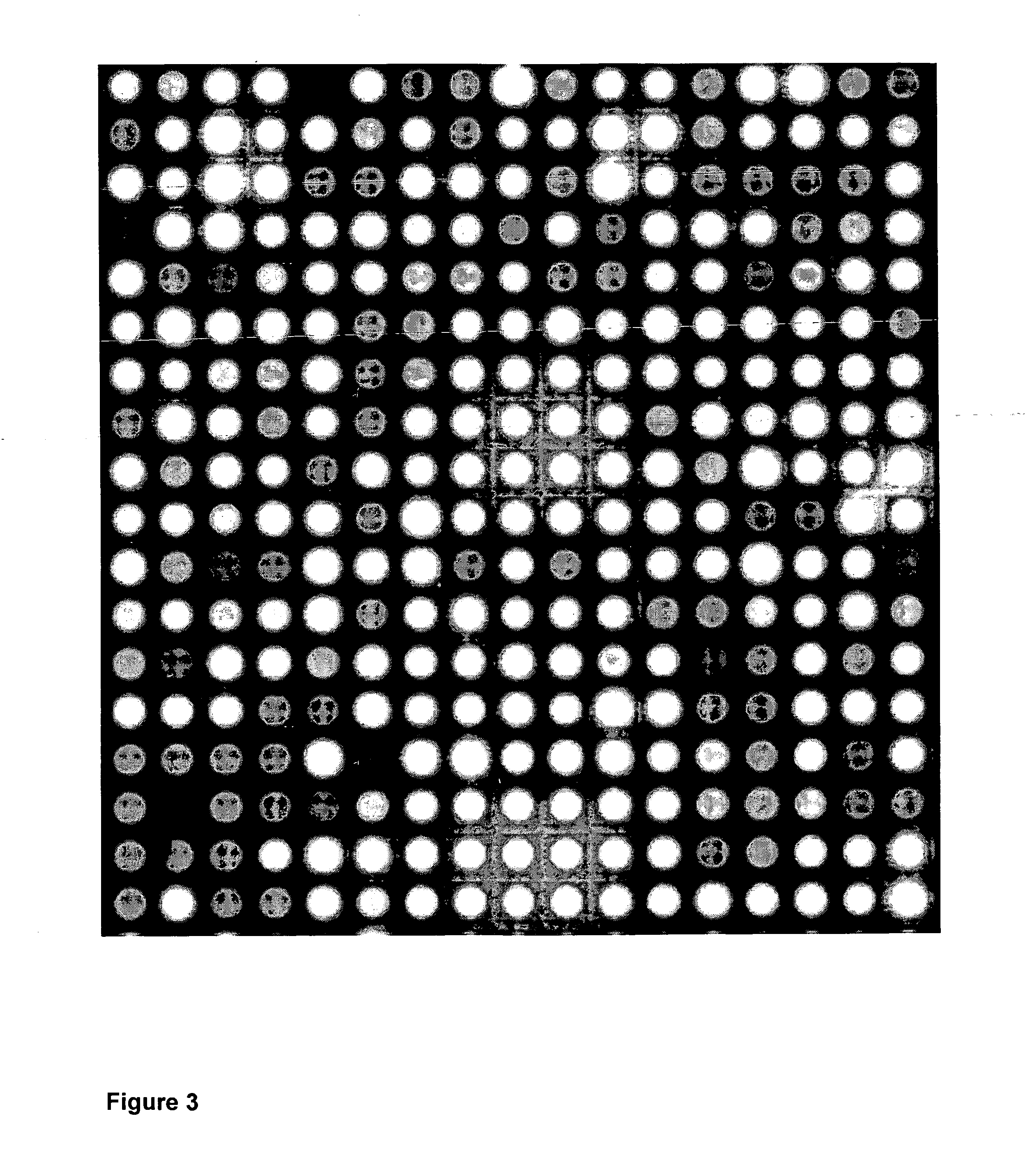
InactiveUS20070034513A1Sequential/parallel process reactionsElectrolysis componentsOligomerElectrochemistry
There is disclosed an electrochemical deblocking solution for use on an electrode microarray. There is further disclosed a method for electrochemical synthesis on an electrode array using the electrochemical deblocking solution. The solution and method are for removing acid-labile protecting groups for synthesis of oligonucleotides, peptides, small molecules, or polymers on a microarray of electrodes while substantially improving isolation of deblocking to active electrodes. The method comprises applying a voltage or a current to at least one electrode of an array of electrodes. The array of electrodes is covered by the electrochemical deblocking solution.
Owner:CUSTOMARRAY
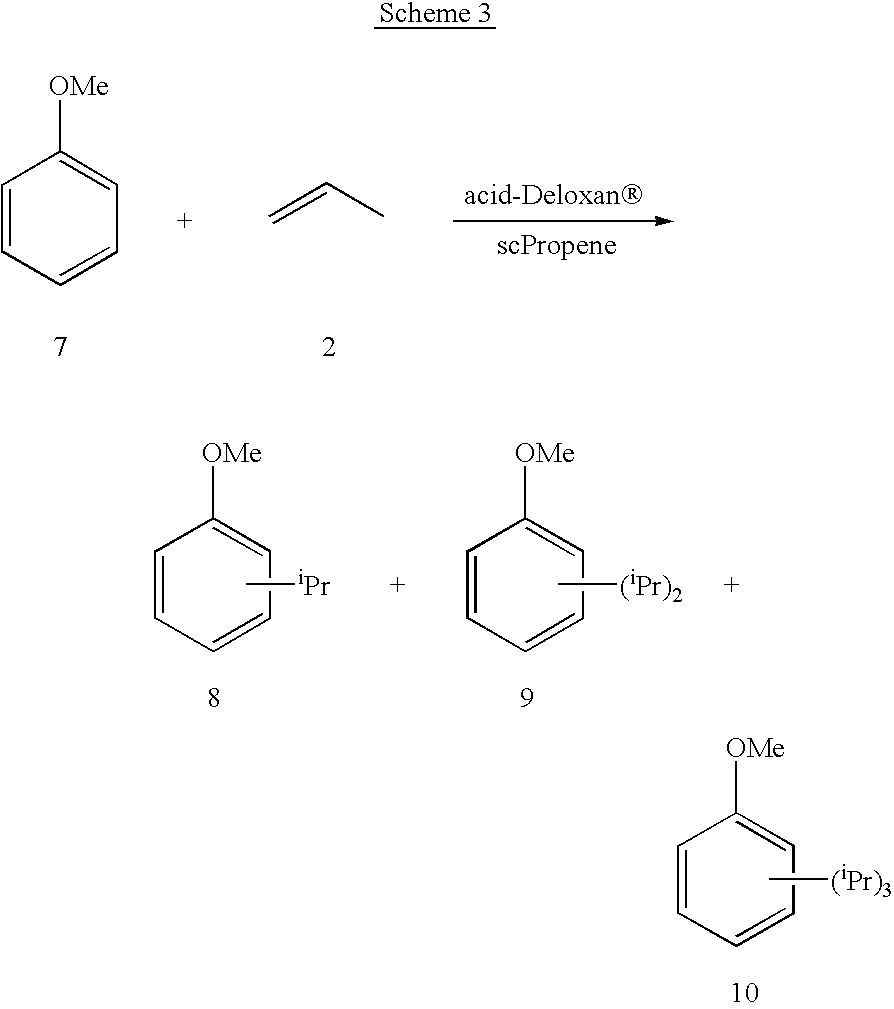
Acylation reactions of aromatic substrates
InactiveUS6630606B2Increase ratingsHigh selectivityCarbonyl compound preparation by condensationHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferProduct formation
Method of performing alkylation or acylation reactions of aromatic substrates under supercritical or near-critical reaction conditions. In particular, a method of performing Friedel-Crafts alkylation or acylation reactions is disclosed under those conditions. Friedel-Crafts reactions may be effected using a heterogeneous catalyst in a continuous flow reactor containing a super-critical or near-critical reaction medium. Selectivity of product formation can be achieved by varying one or more of the temperature, pressure, catalyst, flow rates and also by varying the ratios of aromatic substrate to acylating or alkylating agent.
Owner:THOMAS SWAN & CO LTD +1
Rhodium/carbon nanotube catalyst and preparation method and application
InactiveCN104056622AEasy to operateEvenly dispersedOrganic compound preparationCarboxylic acid esters preparationFormylation reactionCarbon nanotube
The invention relates to a rhodium / carbon nanotube catalyst of rhodium nanoparticle supported in a tube chamber of a carbon nanotube used for an olefin hydrogen formylation reaction, the catalyst takes the carbon nanotube as a carrier, the rhodium nanoparticle can be dispersed in the tube chamber, the size is uniform and the particle size is 1-3nm; the rhodium / carbon nanotube catalyst appears higher activity by comparing with other carrier supported catalyst in the hydrogen formylation reaction of styrene, the highest TOF value can reach more than 300h<-1>, when an appropriate phosphite ester ligand is added in the reaction system, activity is increased, the highest TOF value can reach more than 1000h<-1> which is the highest intrinsic reaction activity obtained in the styrene hydrogen formylation reaction of a heterogeneous catalyst; the selectivity of product aldehyde generated by catalysis of the catalyst is high and can reach more than 99%, the selectivity for producing branched chain aldehyde can reach 94%; and 42% of enantioselectivity can be obtained in the presence of the chiral ligand.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing 2-iodine amyl -2-ene-1,4-diketone derivative by adopting visible light catalysis
ActiveCN107011145AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationIodidePollution
Owner:ZHEJIANG UNIV OF TECH
Monatomic rhodium catalyst, and preparation and applications thereof
InactiveCN109847741AEvenly dispersedHigh catalytic activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFormylation reactionChemistry
The invention relates to a preparation method of a monoatomic rhodium catalyst used for catalyzing alkene hydroformylation reaction to generate branched chain aldehydes and olefine aldehydes. Applications of the monoatomic rhodium catalyst are capable of realizing a whole new reaction approach, tandem of alkene hydroformylation reaction and aldol condensation reaction is realized without adding ofadditional acid or base, and one-step of production of branched chain aldehydes and olefine aldehydes from alkenes and synthetic gas is realized. The main active component of the monoatomic rhodium catalyst is rhodium; rhodium is dispersed on a carrier in atom grade; the mass amount of rhodium accounts for 0.005 to 2wt% of the total mass amount of the monatomic rhodium catalyst. The monoatomic rhodium catalyst is relatively high in catalytic activity and stability in alkene hydroformylation reaction; under the optimum reaction conditions, branched chain aldehyde product selectivity is 95% orhigher. The monoatomic rhodium catalyst is low in economical and environment cost; production separation is convenient; post-treatment is simple; and the application prospect is promising.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for carrying out catalytic conversion with high efficiency on cellulose at low temperature by utilizing compound ion liquid system
InactiveCN102060642AAchieve homogeneous transformationReduce dosageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIce waterPhysical chemistry
The invention discloses a method for carrying out catalytic conversion with high efficiency on cellulose at low temperature by utilizing a compound ion liquid system, comprising the following steps of: (1) preparation of a compound ion liquid: fully mixing an ion liquid solvent and an acid ion liquid catalyst in ice water bath under the protection of N2 to obtain the compound ion liquid; and (2) catalytic conversion of the cellulose: mixing the compound ion liquid and microcrystalline cellulose, adding a nonpolar solvent, and after N2 replacement, heating, stirring and reacting for 5-75 minutes to obtain a biochemical product. The cellulose is one of main components of biomass, and due to the adoption of the method provided by the invention, the cellulose in the biomass can be converted and prepared into bio-oil and biochemical products so that high-efficiency recycling of the cellulose is realized. The method has the advantages of simple process, mild conditions, small amount of ion liquid and realization of recycling, and the conversion rate of the cellulose under the optimized condition can be up to 100%.
Owner:SOUTH CHINA UNIV OF TECH
Synthesis method of oxetanone
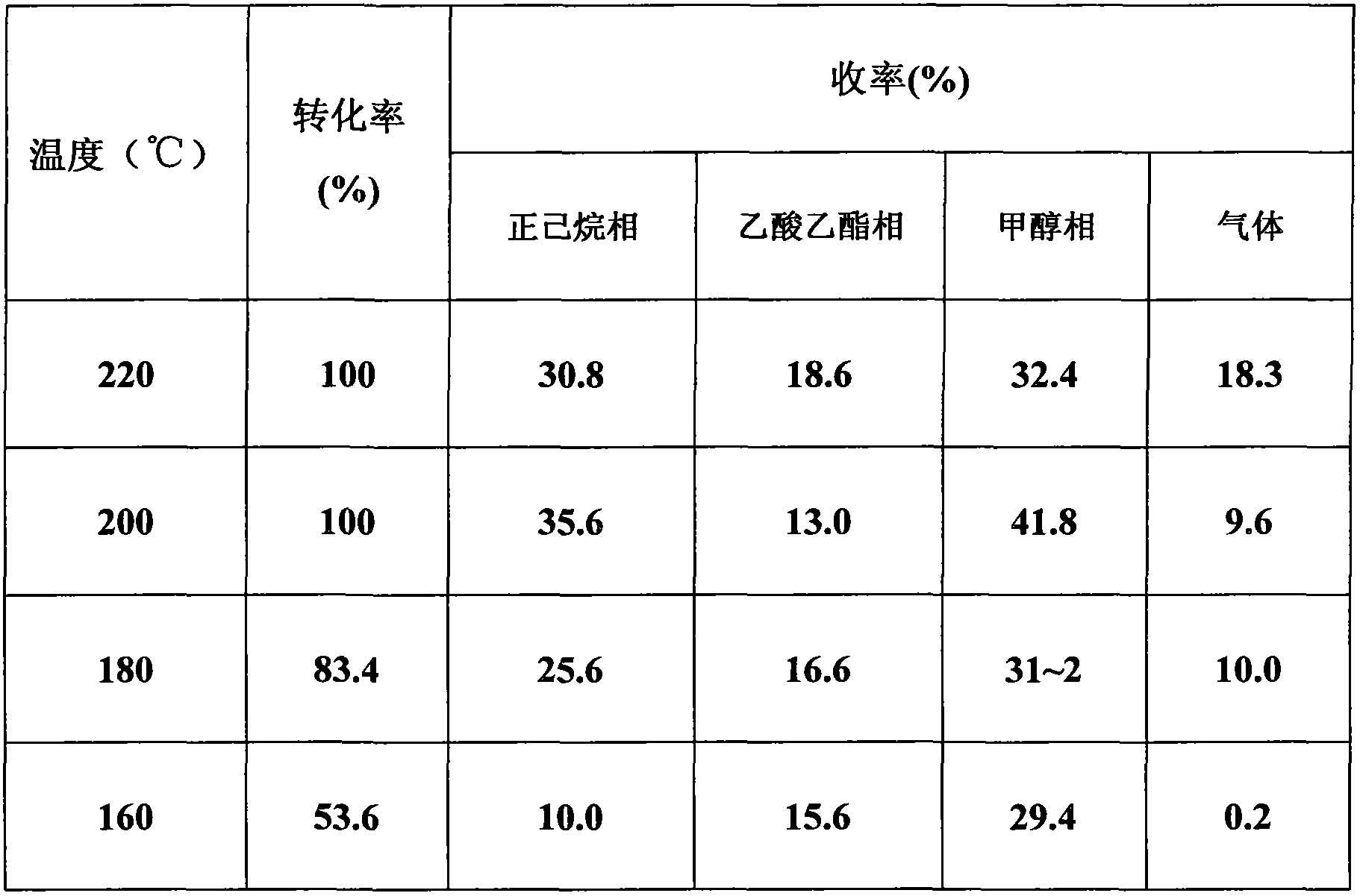
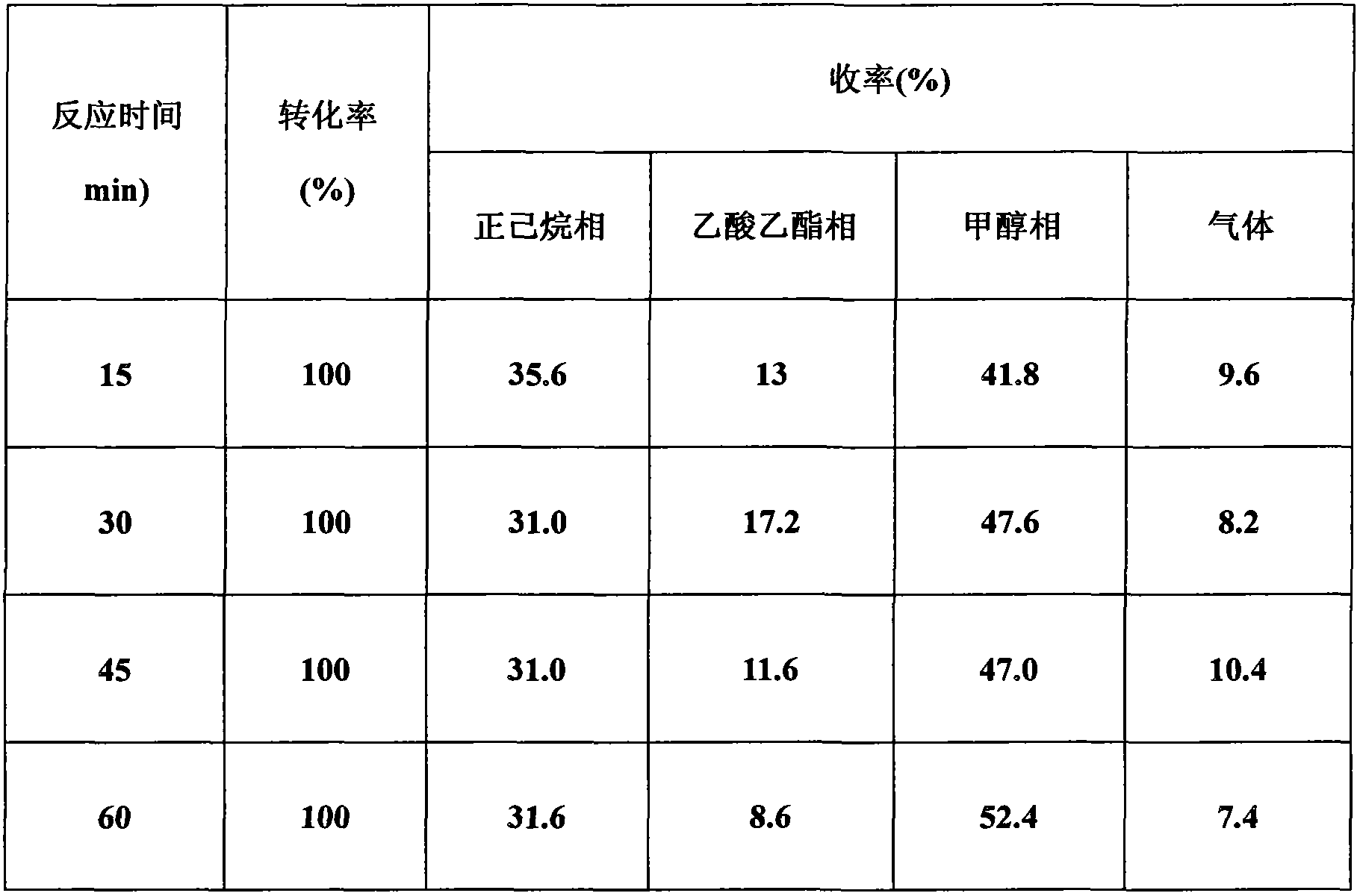
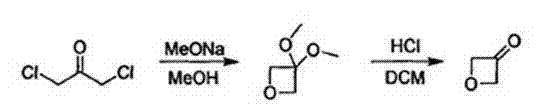
ActiveCN103694201ASynthetic method safe scale-upReduce usageCarbonyl group formation/introductionMethyl azidePtru catalyst
The invention discloses a synthesis method of oxetanone. The method comprises the steps: in an organic solvent and in the presence of a catalyst, a halide and an alkali, utilizing an oxidant to oxidize oxetanol, and then separating and purifying to obtain oxetanone. The method adopts the organic oxidation system for oxidation production of oxetanone for the first time, is cheap in adopted materials and simple in operation, avoids use of methyl azide, butyl lithium, or 1,3-dichloroacetone and other dangerous chemicals, enables the synthesis method of oxetanone to be safely amplified, and can be produced in large scale; the method is friendly to the environment and avoids use of phosphorus pentoxide and other reagents, so that the process meets the requirements of environmental protection; and moreover, the method increases the reaction yield which is increased from 50% to 80% or more, thereby greatly reducing the production cost, and being in favor of further application and development of oxetanone in organic chemistry and biological medicines.
Owner:赣州康瑞泰药业有限公司
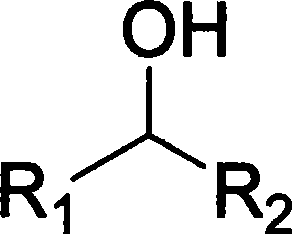
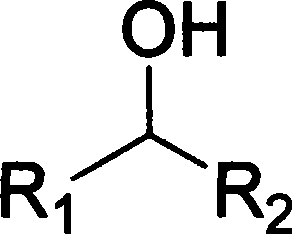
Catalytic conversion of alcohols and aldehydes
ActiveUS20130211146A1Reduce decreaseSuitable for preparationOxygen-containing compound preparationOrganic compound preparationActivated carbonCatalytic transformation
The invention provides a process for preparing higher alcohols and / or aldehydes and also mixtures thereof by catalytic reaction of ethanol, the reaction taking place in the presence of at least one catalyst, the catalyst comprising an activated-carbon substrate which is provided with at least one metal, and more particularly has at least one metal dope.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Method for preparing aldehyde or ketone compounds with catalytic oxidation of alcohol compounds
InactiveCN101293799AEasy to separateEasy to useOrganic compound preparationCarbonyl group formation/introductionOrganic solventAlcohol
The invention relates to a method for preparing aldehyde or ketone compound by catalytic oxidation. The method comprises adding high-activity Ru-supported catalyst, water, mixed organic solvent and alcohol compound into a reactor; reacting under normal pressure at 30-100 DEG C for 1-12 hours while introducing oxygen or air; standing after the reaction is finished to automatically demix two phases of water and oil, wherein the catalyst is in the water phase; and separating the upper organic phase to obtain the aldehyde or ketone compound. The conversion rate of the alcohol compound is 10-100%, and the selectivity of the aldehyde or ketone compound reaches 100%. The method is characterized by simple process, low production cost, environment friendliness and easy separation and reutilization of catalyst.
Owner:DALIAN UNIV OF TECH
Photocatalyst for selective oxidation of toluene and toluene derivatives
ActiveCN103357424AHigh catalytic efficiencyPromote sustainable developmentPhysical/chemical process catalystsOrganic compound preparationColloidToluene
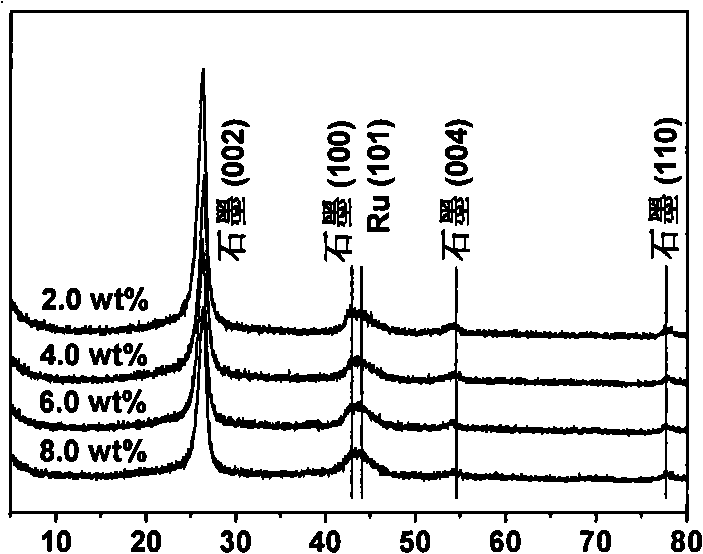
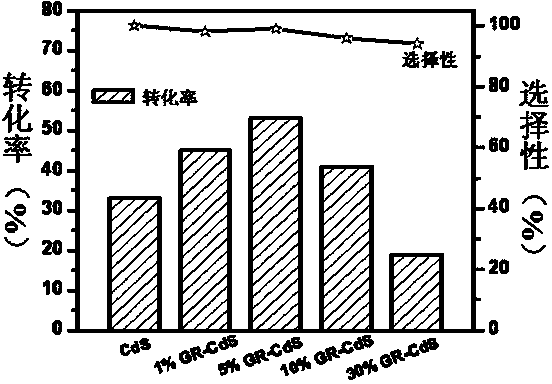
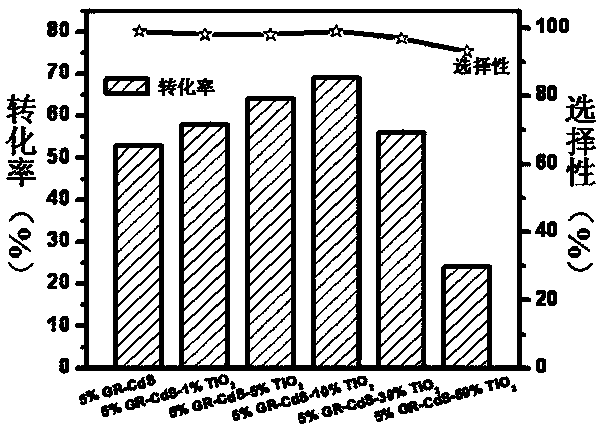
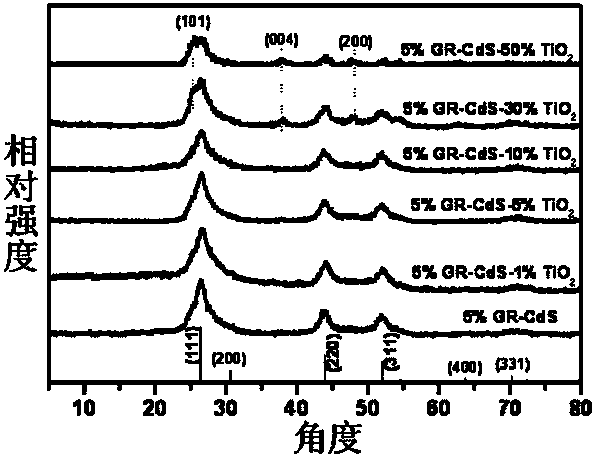
The invention discloses a photocatalyst for selective oxidation of toluene and toluene derivatives. By taking Cd(Ac)2-H2O(1 / 2), Na2S-H2O(1 / 9), tetrabutyl titanate and graphene as raw materials, nano particle combined sheet-shaped CdS, TiO2 colloid and an aqueous solution of oxide graphene are respectively obtained, then subjected to vacuum automatic assembly, hydrothermal processing at 120 DEG C, cooling, filtering, washing and drying for obtaining of the visible light photocatalyst GR-CdS-TiO2. The compound photocatalyst GR-CdS-TiO2 is firstly applied to photocatalyzed selective oxidation of toluene and the toluene derivatives, and has high catalytic efficiency and corresponding aldehyde selectivity of 98%. The photocatalyst is simple in preparation, is applied to selective oxidation of toluene and the toluene derivatives by taking visible light as driving energy and oxygen as an oxidizing agent, and is beneficial to sustainable development of environment and energy.
Owner:FUZHOU UNIV
Method for preparing aryl ketone
ActiveCN102153434AMild reaction conditionsReduce usageCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystRuthenium Compounds
The invention relates to the field of catalysis, in particular to a method for preparing aryl ketone through reacting aldehyde with aryl boric acid under the catalysis of a ruthenium catalyst. In the method, an organic phosphide is used as a ligand, potassium phosphate is used as alkali, pinacolone or acetone is used as an additive, toluene or / and water is (are) used as a solvent(s), aldehyde and aryl boric acid which are used as reaction substrates react at 95-100 DEG C for 10-24h in the presence of a ruthenium compound used as a catalyst to prepare aryl ketone, wherein the catalyst is one of [Ru(cymene)Cl2]2, [Ru(CO)3Cl2]2, RuH2(CO)PPh3, Ru2(OAc)4, [Ru(benzene)Cl2]2, Ru(S-BINAP)Cl2 or Ru3(CO)12. In the invention, the used catalyst has relatively low price and low toxicity, thereby reducing the preparation cost and being more environmentally friendly.
Owner:铜陵市官作文化有限公司
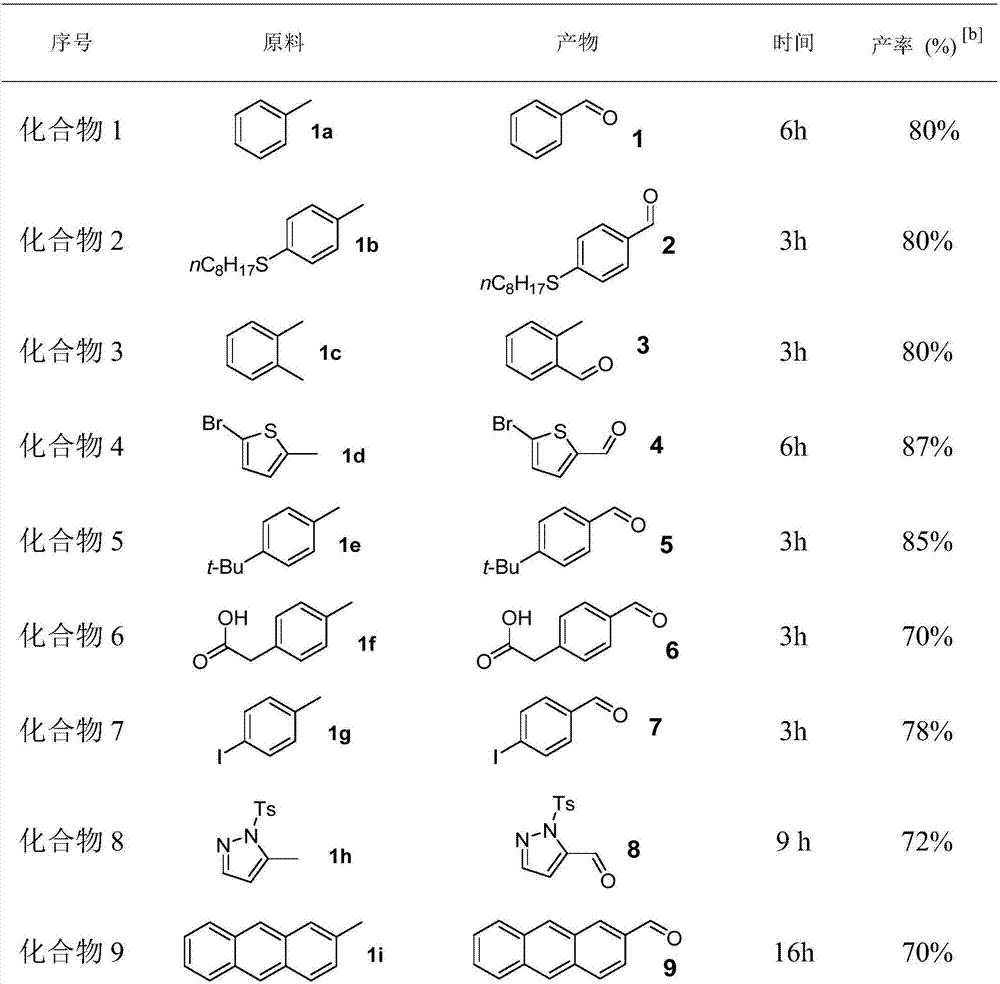
Method for synthesizing aromatic aldehyde, aromatic ketone and aromatic ester through catalytically oxidizing alkyl aromatic compound by iron
ActiveCN107216242ABroad compatibilityGood compatibilitySugar derivativesOrganic compound preparationSide chainCatalytic oxidation
The invention discloses a method for synthesizing aromatic aldehyde, aromatic ketone and aromatic ester through catalytically oxidizing an alkyl aromatic compound by iron, and belongs to the technical field of catalytic synthesis. According to the method, a low-cost and environment-friendly iron catalyst is used under a normal pressure; under the action of hydrogen and silicon reagents serving as an accelerant and an oxidant, a side chain of an aromatic hydrocarbon is oxidized into a carbonyl group for generating the corresponding aromatic aldehyde, aromatic ketone and aromatic ester. The method for preparing the aromatic aldehyde, the aromatic ketone and the aromatic ester through a catalytic oxidation reaction, which is provided by the invention, has numerous advantages that a catalyst, reaction raw materials, the oxidant and a silicon reagent are wide in sources and good in stability and is low-cost and environment-friendly; the alkyl aromatic compound is metered to participate in a reaction; the reaction condition is mild; the compatibility of functional groups is good; the scope of application is wide; the reaction selectivity is good; in an optimized reaction condition, the separation yield of a target product can be up to approximately 95 percent.
Owner:NANJING NORMAL UNIVERSITY
Greening method for preparing aldehydes and ketones through alcohol oxidation of copper catalyst
InactiveCN103420748ALow toxicityReduce pollutionOrganic compound preparationCarbonyl group formation/introductionOrganic solventDouble teeth
The invention provides a greening method for preparing aldehydes and ketones through the alcohol oxidation of a copper catalyst. The greening method comprises the following steps: taking copper salt as the catalyst, a single-tooth or double-teeth N ligand as the ligand and an organic nitric oxide compound as a co-catalyst, and performing the alcohol aerobic oxidation reaction in water-phase or organic solvent under the existence of alkali and air to prepare an aldehyde and Ketone compound. A copper catalyst system, which is low in cost, easy to obtain, low in toxicity and high in activity, is adopted in the greening method, air is used as an economic, safe and green oxidant, and under the mild condition of the indoor temperature air, the aldehyde and ketone compound is prepared through effective alcohol oxidation. The reaction condition is simple and mild, the operation is easy, the reaction conversion rate is high, the product separation and purification are simple, and the recovery rate is high. The requirement on the reaction condition is relatively low, and the greening method has a good research and industrial application prospect.
Owner:WENZHOU UNIVERSITY
Method for oxidizing alcohol into aldehyde, ketone or carboxylic acid
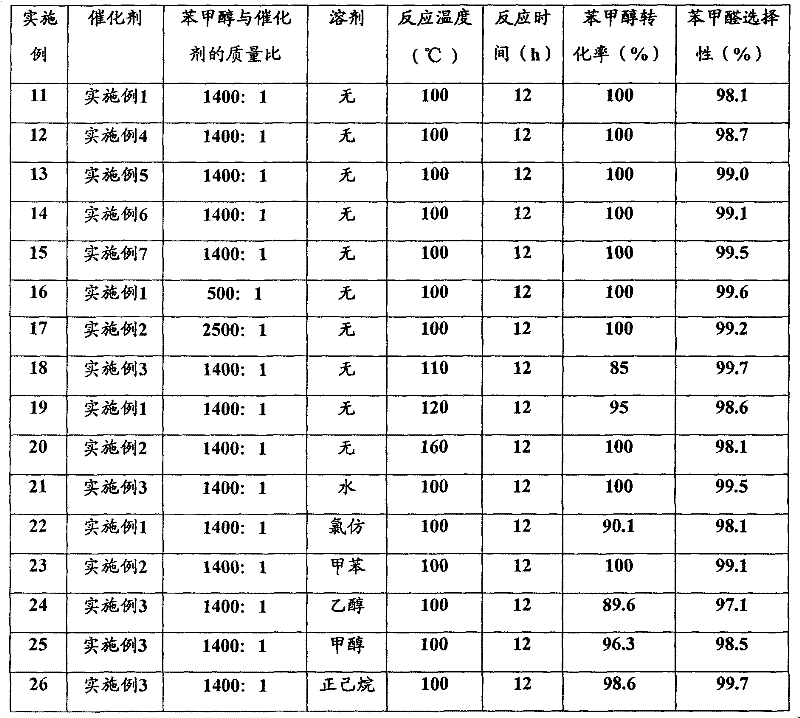
ActiveCN102924205AEasy to controlGood repeatabilityOrganic compound preparationCarbonyl group formation/introductionHydrogenAlcohol
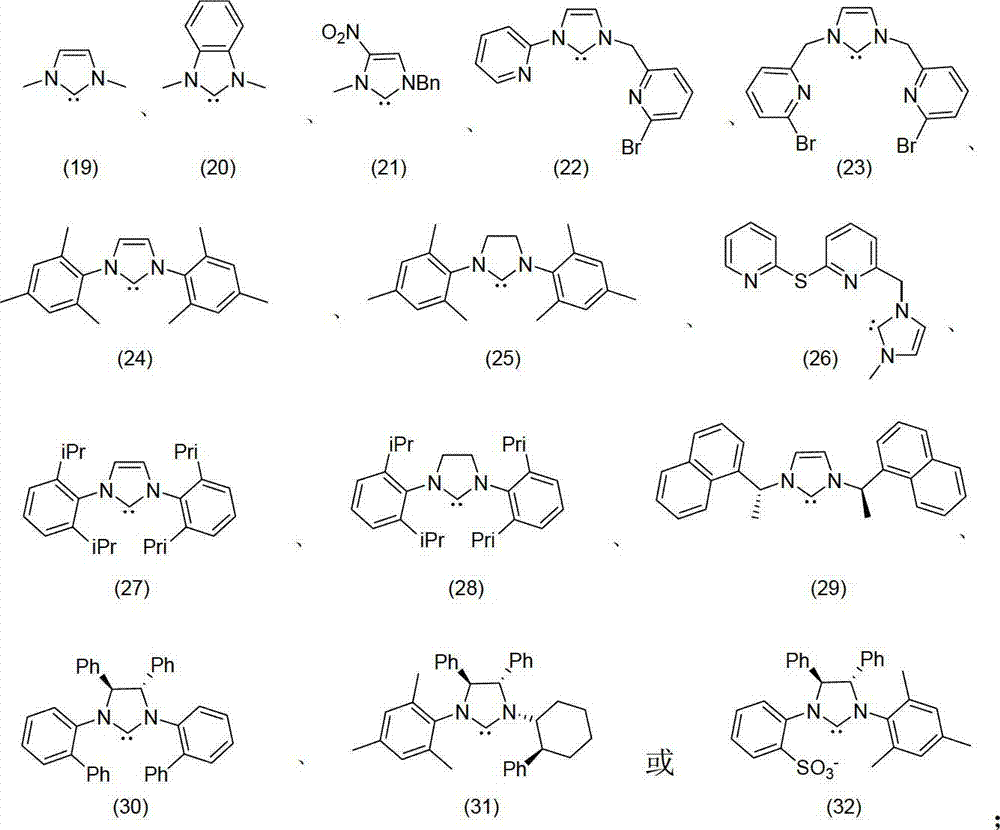
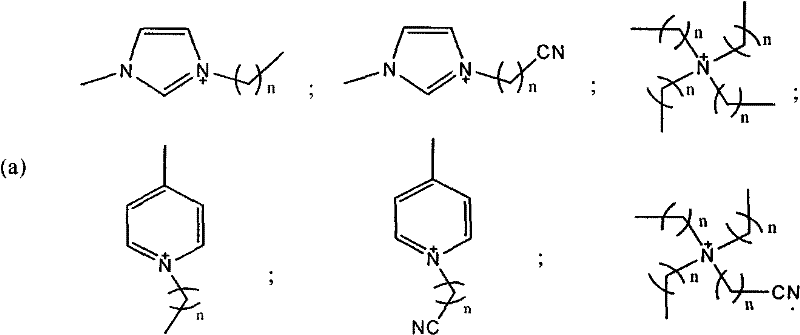
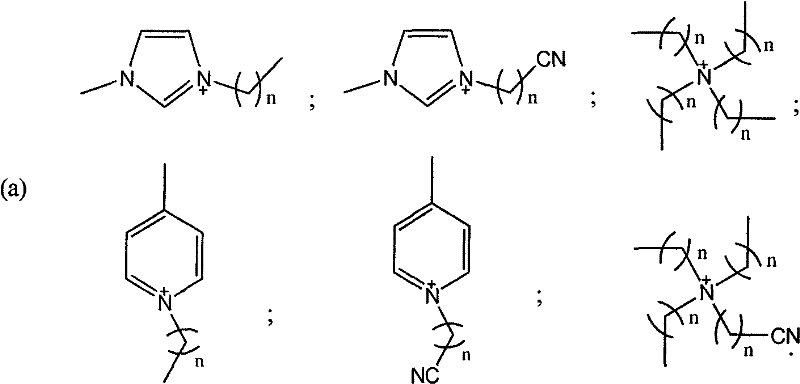
The invention discloses a method for oxidizing alcohol into aldehyde, ketone or carboxylic acid. The method utilizes a metal nitrogen heterocyclic carbine compound as the catalytic agent and oxygen in air as the oxidizing agent. The method comprises the steps of selectively oxidizing alcohol compounds into the aldehyde or the ketone efficiently and gently; and oxidizing alpha-hydrogen-free primary alcohol into corresponding carboxylic acid compounds efficiently under the heating condition.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for synthesizing violet dienone with homogeneous phase reaction and heterogeneous phase separation
InactiveCN101723769AGuaranteed yieldEnable recyclingOrganic compound preparationCarboxylic acid esters preparationPyridiniumManganese
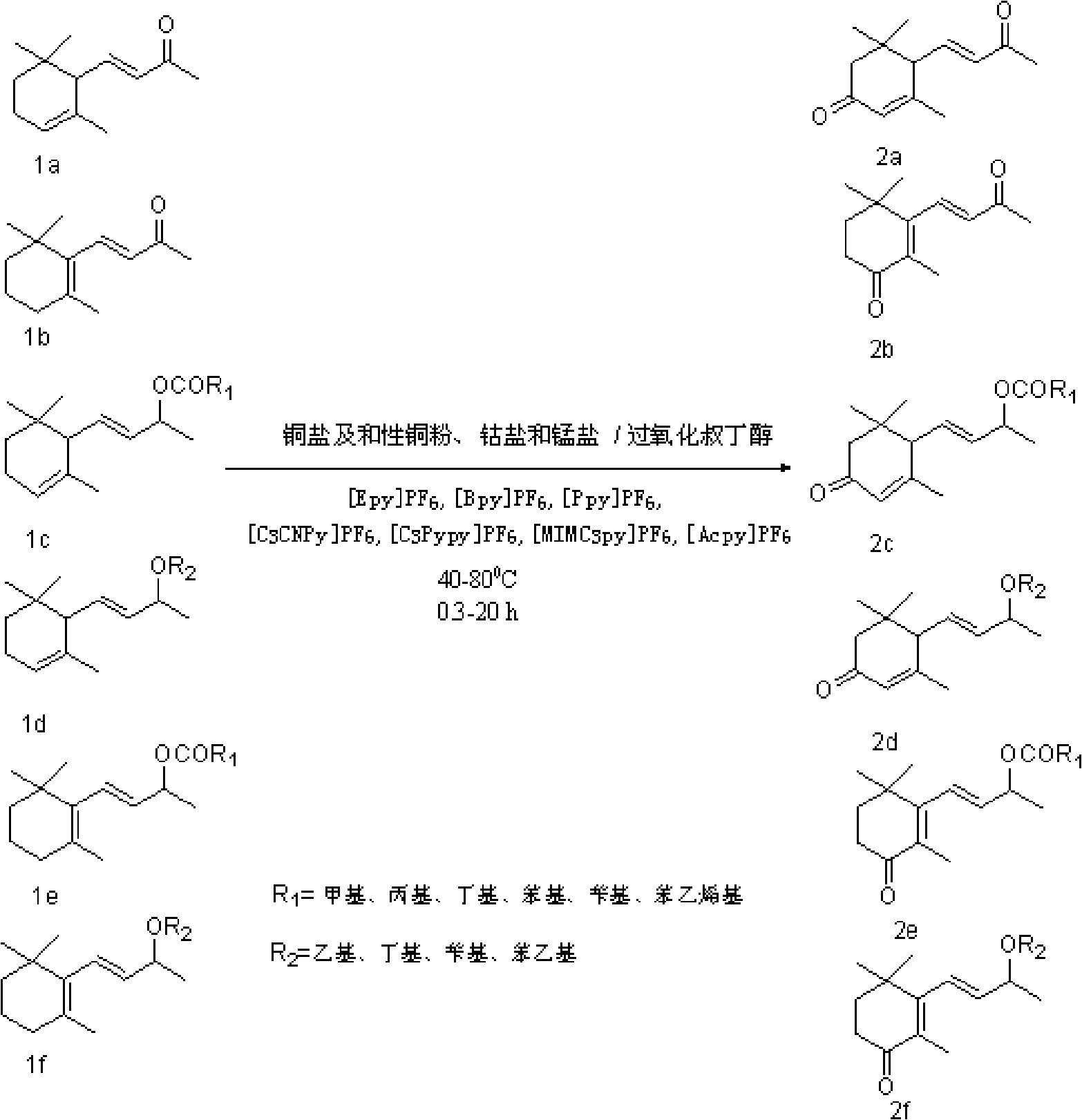
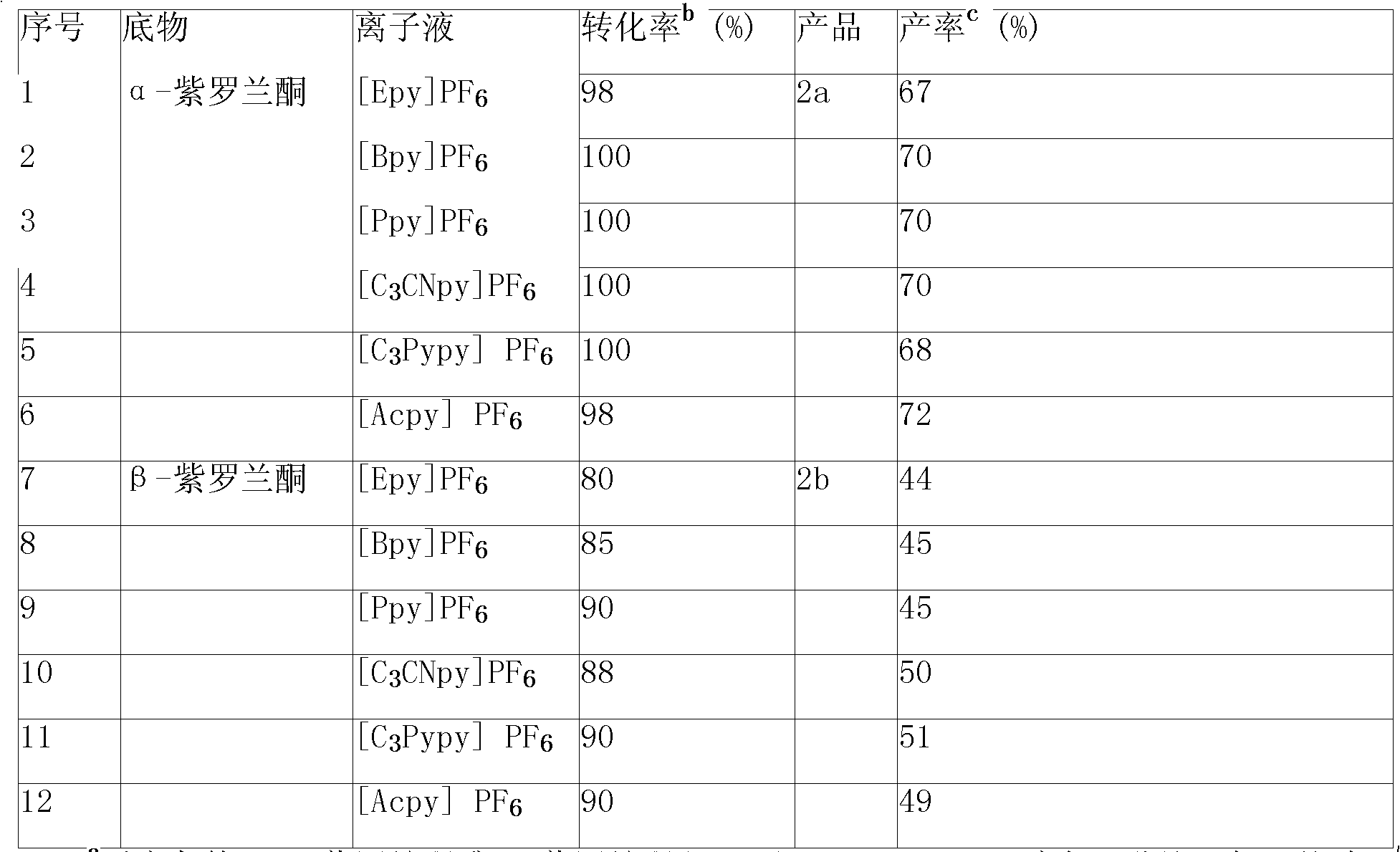
The invention provides a method for transforming alpha-irisone, beta-irisone and ether and ester derivatives thereof into relevant ketenes, and then forming keto-alpha-irisone, keto-beta-irisone and ether and ester derivatives thereof by using pyridinium ionic liquid as a reaction medium, a simple copper salt or manganese salt or cobalt salts as a catalyst and tert-butyl hydroperoxide as an oxidant,. The catalyst and the reaction medium in the method can be recycled, and the method has the characteristics that the unique phase changes are presented along with reaction and separation, and the method has good selection and high yield when the reaction is carried out in homogeneous phases, and is convenient for operation and the recycling of the catalyst and the reaction medium when the separation is carried out in heterogeneous phase. Therefore, the method for synthesizing the keto-alpha-irisone, the keto-beta-irisone and the ether and ester derivatives thereof is an efficient and environmental-friendly method having the characteristics of high-efficiency reaction of the homogeneous phases and convenient separation of the heterogeneous phase.
Owner:CHINA TOBACCO GUIZHOU IND +1
Method for preparing 2,3-dihydro-1-indanone and derivative thereof
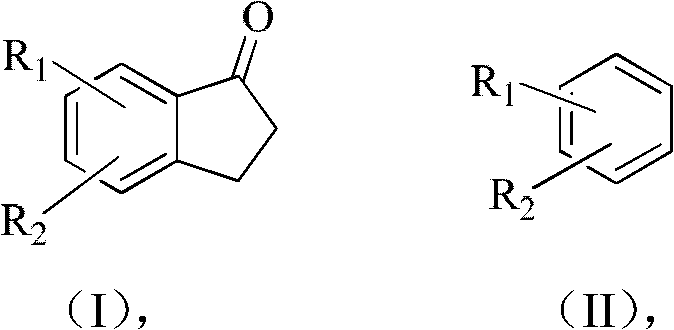
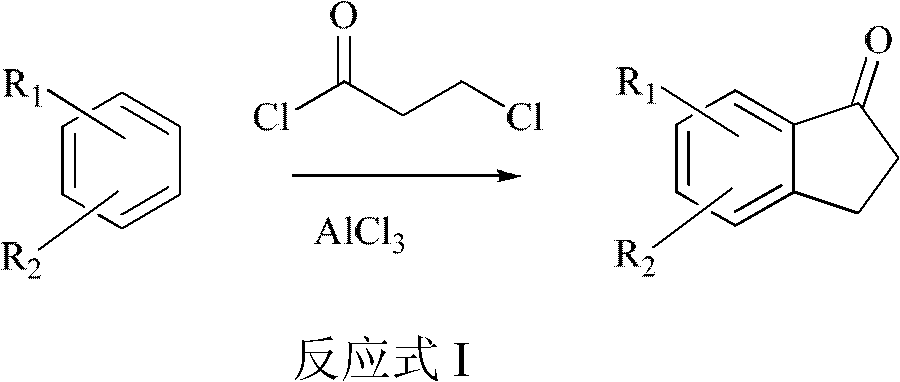
ActiveCN103012086ALow costReduce investmentCarbonyl group formation/introductionCarbonyl compound preparation by condensationHalogenHydrogen
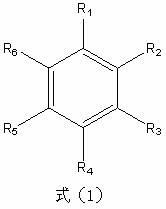
The invention relates to a synthetic method of indanone substances, and particularly relates to a method for preparing 2,3-dihydro-1-indanone and a derivative thereof. The method comprises the following steps of: mixing benzene substituendum of which the structure is shown in a formula (I) with AlCl3 / NaCl, dropwise adding 3-chloropropionylchloride under agitation, and reacting to prepare the 2,3-dihydro-1-indanone and the derivative thereof in one step, wherein R1 and R2 are H atoms, halogen atoms, C1-C10 alkyl, methoxyl, ethyoxyl or amino; dropwise adding 3-chloropropionylchloride at 0-40 DEG C, wherein the control temperature is 0-60 DEG C and the retention time is 0.5-3hours; carrying out Fuke acylation reaction, heating to 150-200 DEG C to keep for 0.5-4hours; and carrying out cyclization reaction, and hydrolyzing cyclization reaction products, wherein the hydrolysis temperature is 50-100 DEG C. Two steps of reaction are merged into a one-pot method. Thus, purifying, drying and packaging of a midbody are reduced; the allergy possibility is avoided; the usage amount of a catalyst is reduced; and the production cost is reduced, and the environmental pollution is also reduced.
Owner:JIANGXI ALPHA HI TECH PHARMA
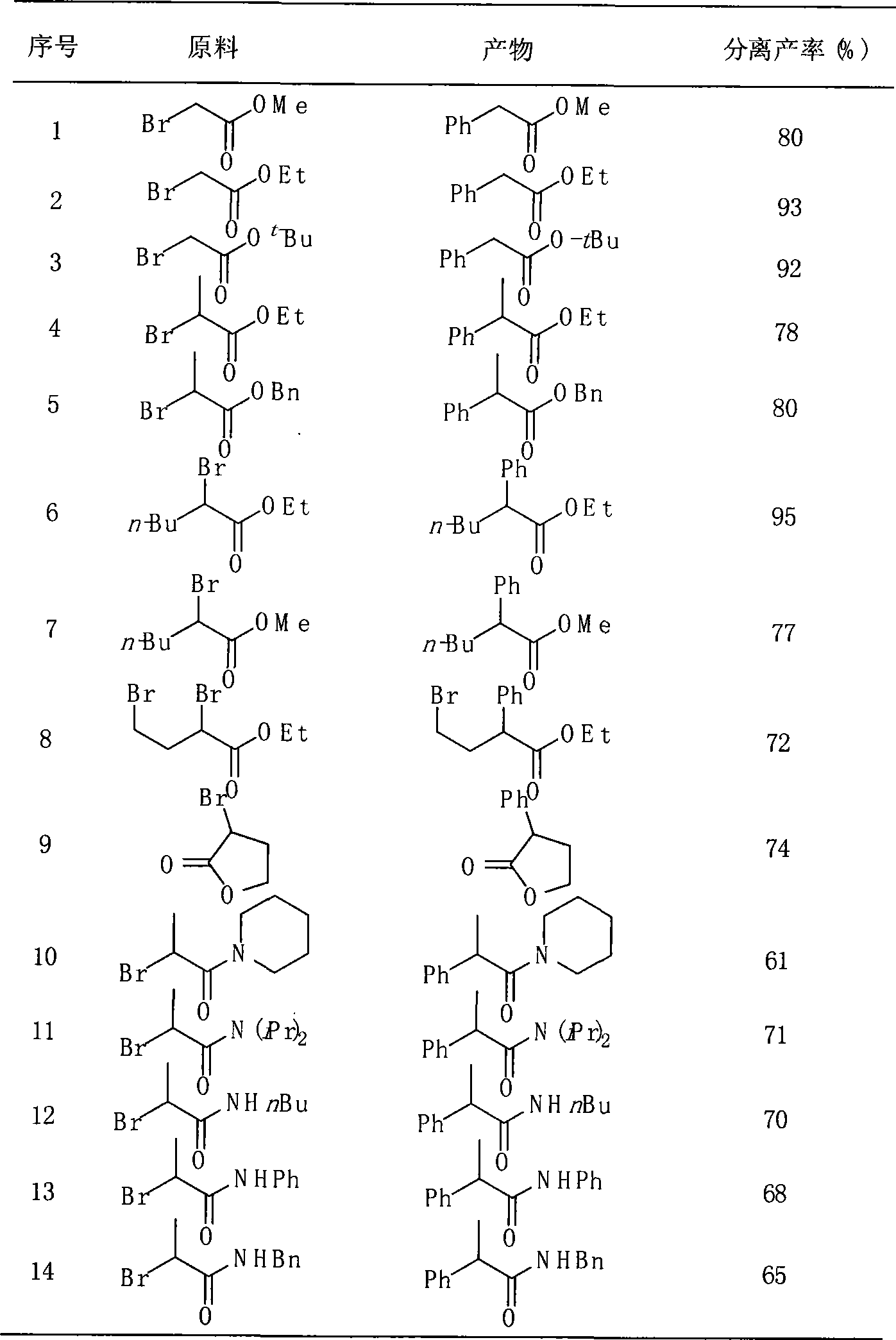
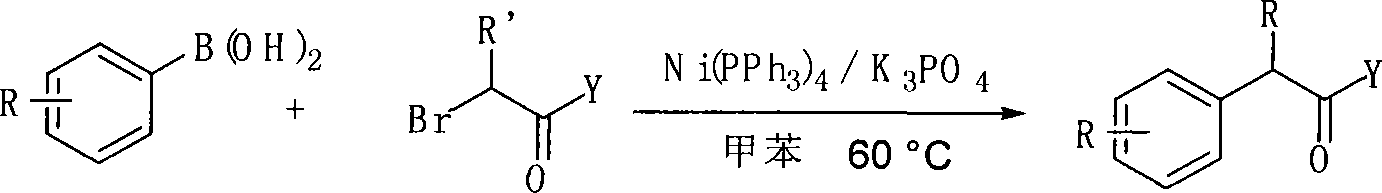
Method for preparing alpha-aryl carbonyl compound
InactiveCN101157590ASubstrate stabilizationCheap and easy to getOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl group formation/introductionKetonePhenylphosphine
The invention relates to a method of preparing alpha-aryl carbonyl compound. With the catalyst of four triphenylphosphine nickel or acetylacetone nickel ligand, the aryl boric acid, the alpha-halogenated carbonyl compound, the ligand and the additive are dissolved in toluene, and after reaction for 10 to 24 hours in 50 to 100 DEG C, the alpha-aryl carbonyl compound is obtained, wherein, the ligand is single-tooth phosphine ligand or double-tooth phosphine ligand; and the additive is absolute potassium phosphate or tri-potassium phosphate. The invention, which utilizes the catalyst with low cost, stable substrate, easy obtaining and gentle reaction conditions, can not only catalyze arylation of ester, amide and ketone, but also can be compatible with beta hydrogen, thereby greatly expanding the range of the substrate. Moreover, the invention possesses great application potential and can directly constitute the main framework of the steride.
Owner:WUHAN UNIV
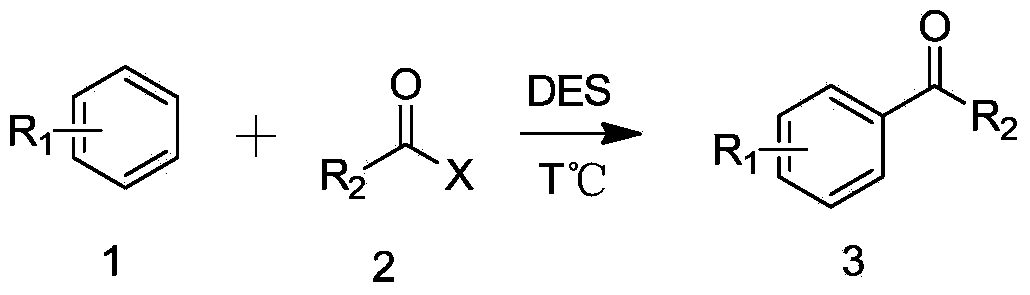
Method for synthesizing aromatic ketone compound
ActiveCN104045541ASimple processImprove stabilityCarbonyl group formation/introductionCarbonyl compound preparation by condensationSolventAromatic hydrocarbon
The invention relates to a method for synthesizing an aromatic ketone compound and particularly relates to DES (Deep Eutectic Solvents) catalyzed Friedel-Crafts (F-C) acylation reaction methods. The aromatic ketone compound is synthesized from an aromatic hydrocarbon compound and an acylating reagent through F-C acylation reaction, and a DES (wherein the ratio of ZnCl2 / ChCl is 2: 1) serves as a catalyst during reaction. According to the method, the DES (wherein the ratio of ZnCl2 / ChCl is 2:1) serves as the reaction catalyst while serving as a reaction solvent; the process is simple, the stability is strong, no volatilization exists, and the catalytic activity is high; during reaction aftertreatment, acid is not required to be added, so that the method is simple, convenient and environmental friendly.
Owner:大连海化医药科技有限公司
Popular searches
Reagents Educts Metal/metal-oxides/metal-hydroxide catalysts Preparation from carbon dioxide or inorganic carbonates Carbonyl compound preparation Carbonyl compound preparation by oxidation Group 5/15 element organic compounds Catalytic reactions Chemical recycling Preparation by carbon monoxide reaction
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
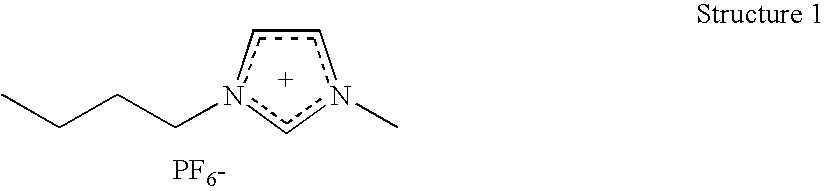
![Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole](https://images-eureka.patsnap.com/patent_img/f7ed2a5c-6e2a-42c2-84c3-4ab262ab0a99/US06268502-20010731-C00001.png)
![Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole Process of synthesis of 5-methoxy- 2-[(4-methoxy-3,5-dimethy-2-pyridyl)methyl]sulfiny-1h-benzimidazole](https://images-eureka.patsnap.com/patent_img/f7ed2a5c-6e2a-42c2-84c3-4ab262ab0a99/US06268502-20010731-C00002.png)