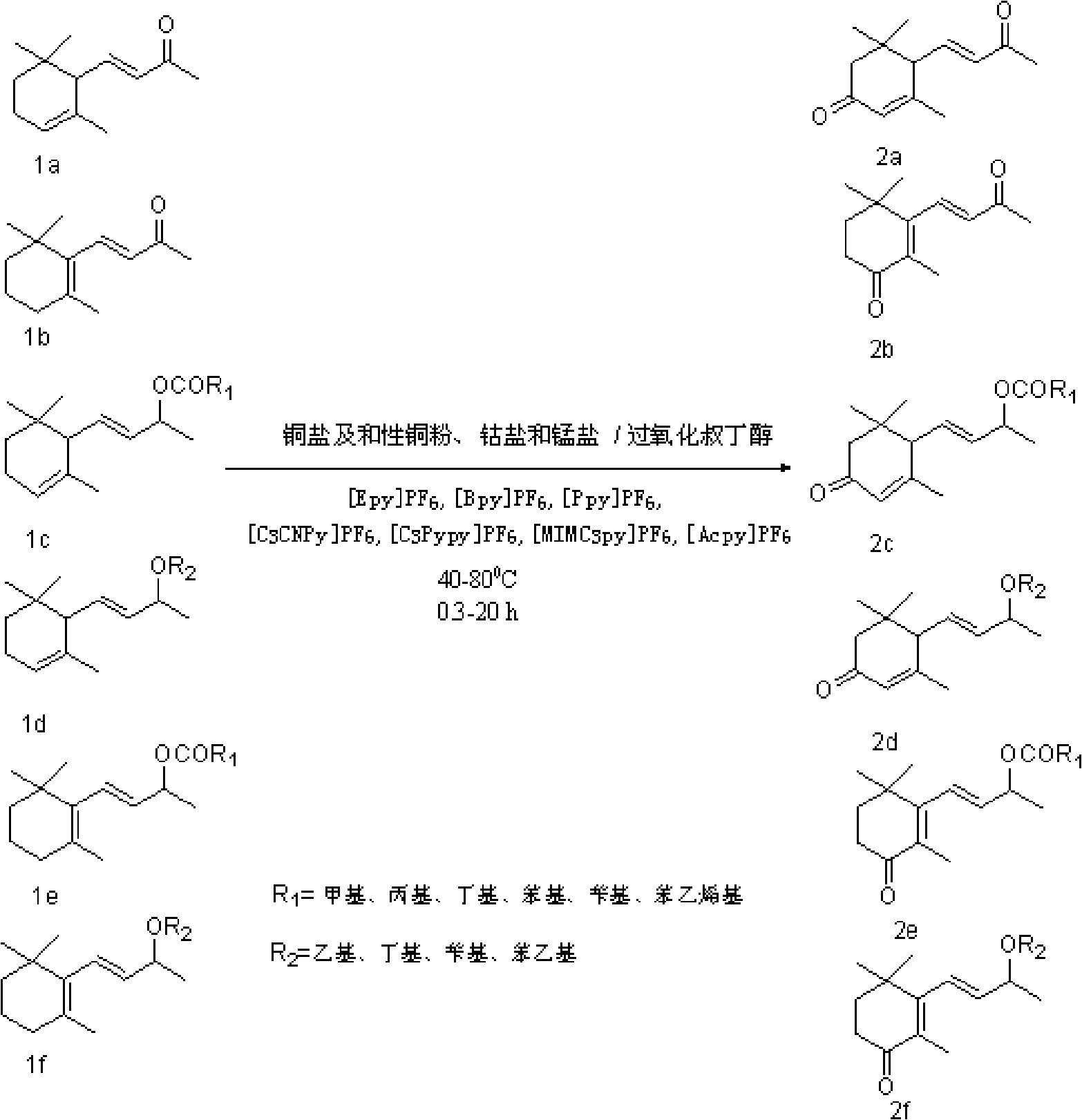
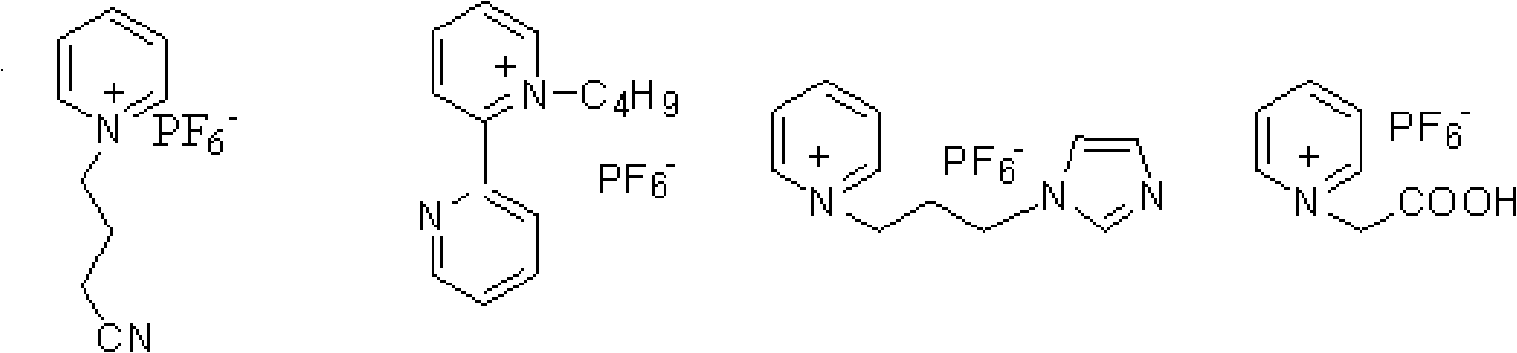Method for synthesizing violet dienone with homogeneous phase reaction and heterogeneous phase separation
A technology of ionodienone and ionone, which is applied in the synthesis of ester derivatives and ketone-substituted α-ionone, can solve the problems of unfavorable economy and environment, the catalyst cannot be recovered and can only be treated as waste liquid, etc. The effect of recycling, quantity reduction, and volume reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
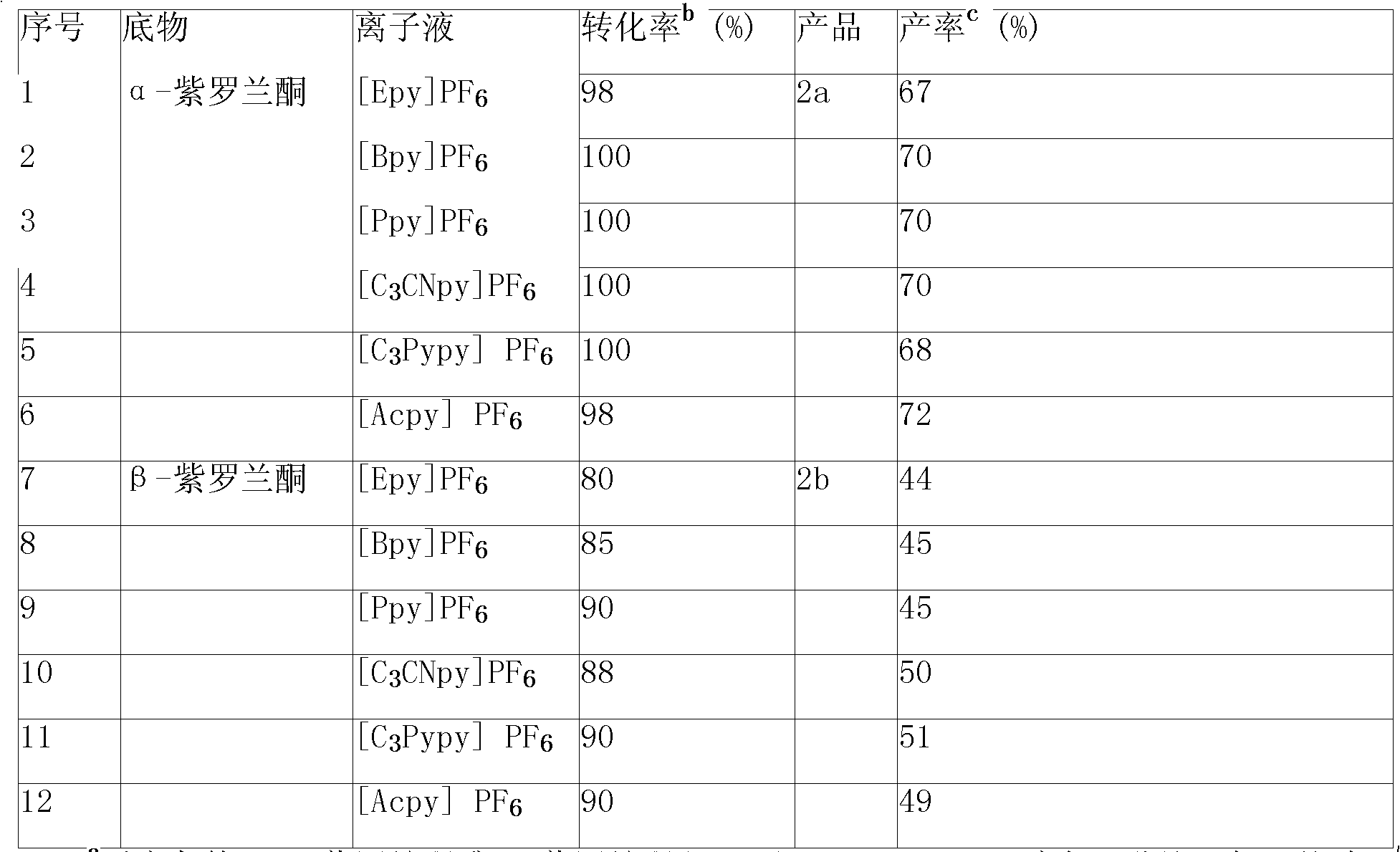
[0098] Example 1, add 5mol α-ionone and its ester, g pyridinium ionic liquid, 0.01mol copper chloride in a 25ml reactor equipped with a stirrer and a condenser, keep the temperature at 40°C, and add tert-butyl dropwise under stirring After 20 hours of reaction, butanol and unconsumed tert-butyl hydroperoxide were distilled off under reduced pressure, and diethyl ether was added for solid-liquid extraction. Extraction was carried out for ~10 times with 5ml each time, filtered, combined extracts, and reduced Concentrate under reduced pressure to remove the solvent, and then use silica gel column chromatography to separate (eluent V 石油醚 :V 乙酸乙酯 =4:1), 2.50~2.6mol of 3-keto-α-ionone and its ester were obtained, and the yield was 50~52%. Or combine the extracts, concentrate under reduced pressure to remove the solvent, and rectify under reduced pressure to obtain 2.40-2.50 mol of the product 3-keto-α-ionone and its ester, with a yield of 48-50%. The filter cake was distilled at 5...
Embodiment 2
[0099] Example 2, add 5mol α-ionone and its ester, 5g pyridinium ionic liquid, 0.01mol copper chloride into a 25ml reactor equipped with a stirrer and a condenser, keep the temperature at 80°C, and add tert-butyl dropwise under stirring After 20 hours of reaction, distill out butanol and unconsumed tert-butyl hydroperoxide under reduced pressure, add ether for solid-liquid extraction, extract 6-10 times with 5ml each time, filter, and combine the extracts, Concentrate under reduced pressure to remove solvent, and then use silica gel column chromatography to separate (eluent V 石油醚 :V 乙酸乙酯 =4:1), to obtain 3.05~3.15mol of 3-keto-α-ionone and its ester, and the yield was 61~63%. Or combine the extracts, concentrate under reduced pressure to remove the solvent, and rectify under reduced pressure to obtain 2.95-3.05 mol of the product 3-keto-α-ionone and its ester, with a yield of 59-61%. The filter cake was distilled at 50°C to remove the residual extractant ether, and dried in ...
Embodiment 3
[0100] Embodiment 3, in the 25ml reactor that stirrer and condenser tube are equipped with, add 5mol with the ether of α-ionone and the ether of β-ionone or its ester, 5g pyridinium ionic liquid, 0.2mol cobalt chloride at 50 ℃, add 1-2 mol of tert-butyl hydroperoxide dropwise under stirring, react for 0.3h-1h, distill off butanol and unconsumed tert-butyl hydroperoxide under reduced pressure, add diethyl ether for solid-liquid extraction, extract 6-10 Use 5ml each time, filter, combine the extracts, concentrate under reduced pressure to remove the solvent, and then separate with silica gel column chromatography (eluent V 石油醚 :V 乙酸乙酯 =4:1), to obtain 2.15-2.25mol of the ether of 3-keto α-ionone and the ether of β-ionone or its ester, with a yield of 43-45%. Or combine the extracts, concentrate under reduced pressure to remove the solvent, and rectify under reduced pressure to obtain 2.05-2.15 mol of the product 3-keto-α-ionone and its ester, with a yield of 41-43%. The filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com