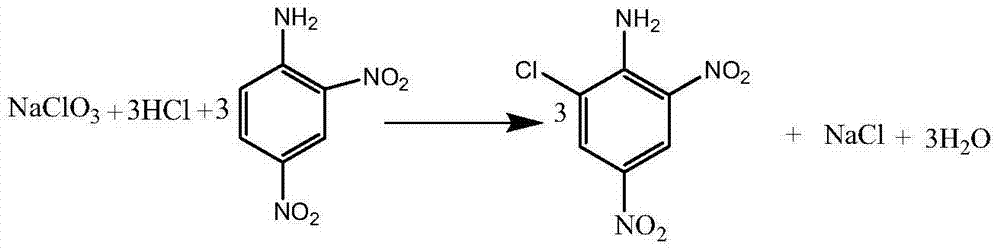
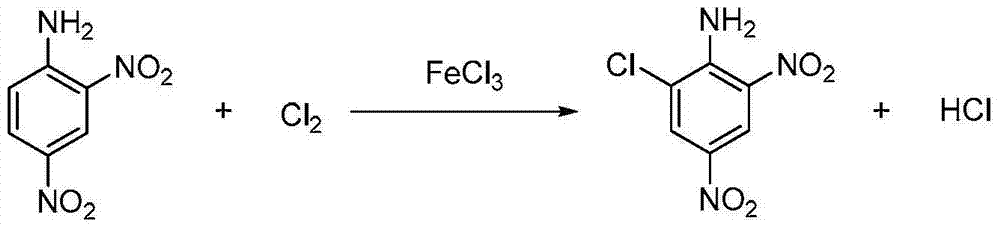
Clean production process of 6-chloro-2,4-dinitroaniline
A dinitroaniline, clean production technology, applied in the preparation of amino compounds, the preparation of organic compounds, organic chemistry and other directions, can solve the problems of long chlorination time, troublesome post-processing, low equipment productivity, etc., and achieves fast reaction speed, Less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 500ml flask equipped with a stirrer and a thermometer, 350g of hydrochloric acid with a mass percentage concentration of 12% was added, and 54.9g of 2,4-dinitroaniline was added. After beating at 20°C for half an hour, slowly introduce 10.65g of chlorine gas, the time of chlorine flow is 2 hours, the temperature of chlorine flow is 20-40°C, after the completion of heat preservation for half an hour, the temperature is slowly raised to 40°C, and the concentration of 30% by mass percentage is added dropwise. 21.3 g of sodium chlorate solution, the dropwise addition time is 1 hour, and the reaction temperature is 40-55°C. After dripping, keep it warm for half an hour, filter, and wash with water to obtain 6-chloro-2,4-dinitroaniline with a purity of 98.4% and a yield of 96.5%. The mother liquor was collected and used in the next batch. The mother liquor was applied continuously for 10 batches, with an average purity of 97.2% and a yield of 96.9%.
Embodiment 2
[0039] In a 500ml flask equipped with a stirrer and a thermometer, 350g of hydrochloric acid with a mass percentage concentration of 18% was added, and 54.9g of 2,4-dinitroaniline was added. After beating at 30°C for half an hour, slowly introduce 6.39g of chlorine gas, the time for chlorine flow is 1 hour, the temperature of chlorine flow is 20-40°C, and after half an hour of heat preservation, the temperature is slowly raised to 40°C, and 30% sodium chlorate solution is added dropwise 29.8g, the dropping time is 1.5 hours, and the reaction temperature is 40-55°C. After dripping, keep it warm for half an hour, filter and wash with water to obtain 6-chloro-2,4-dinitroaniline with a purity of 97.8% and a yield of 96.2%. The mother liquor was collected and used in the next batch. The mother liquor was applied continuously for 10 batches, with an average purity of 96.8% and a yield of 96.5%.
Embodiment 3
[0041] In a 1000ml flask equipped with a stirrer and a thermometer, 490g of hydrochloric acid with a mass percentage concentration of 15% was added, and 54.9g of 2,4-dinitroaniline was added. After beating at 30°C for half an hour, slowly introduce 8.52g of chlorine gas for 2 hours and the temperature of 20-40°C. After half an hour of heat preservation, slowly raise the temperature to 40°C, and add 30% sodium chlorate solution dropwise 25.56g, the dropping time is 1.5 hours, and the reaction temperature is 40-55°C. After dripping, keep it warm for half an hour, filter, and wash with water to obtain 6-chloro-2,4-dinitroaniline with a purity of 98.5% and a yield of 96.1%. The mother liquor was collected and used in the next batch. The mother liquor was applied continuously for 10 batches, with an average purity of 97.1% and a yield of 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com