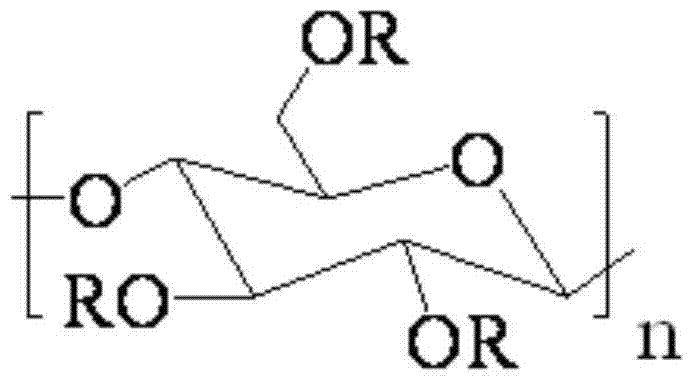
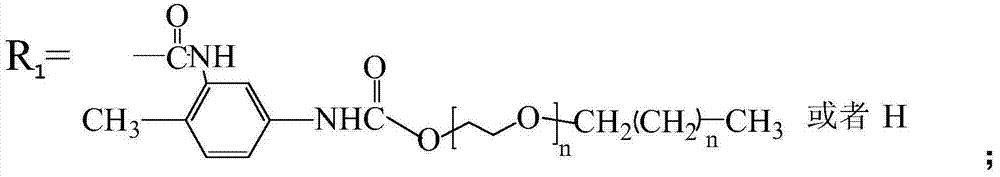
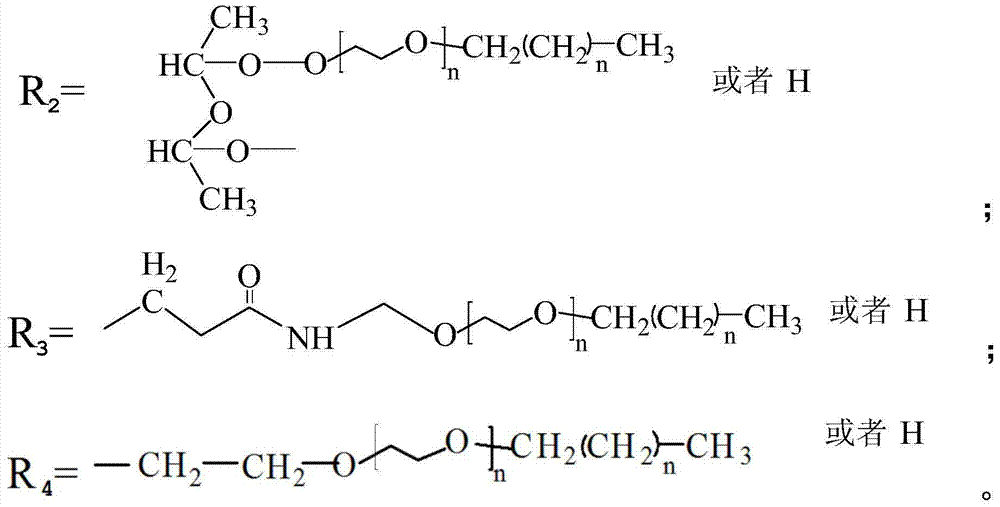
Thermoplastic cellulose-based solid-solid phase transition material and preparation method thereof
A cellulose-based, phase-change material technology, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of high cost of raw materials, cellulose does not have the function of heat energy storage and release, etc., to achieve fast reaction speed, reliable Good melt processability and good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The microcrystalline cellulose was dried in a vacuum oven at 40 °C for 36 h.
[0038] Toluene-2,4-diisocyanate and polyethylene glycol n-tetradecyl ether HO (CH 2 CH 2 O) 20 C 14 h 29 Dissolve in N'N-dimethylformamide respectively, prepare toluene-2,4-diisocyanate solution with a concentration of 6mol% and HO(CH with a concentration of 12mol%) 2 CH 2 O) 20 C 14 h 29 solution; then HO(CH 2 CH 2 O) 20 C 14 h 29 The solution was added into the toluene-2,4-diisocyanate solution in 3 times, toluene-2,4-diisocyanate and polyethylene glycol n-tetradecyl ether HO(CH 2 CH 2 O) 20 C 14 h 29 The molar ratio is 1:1, add the crosslinking agent (toluene-2,4-diisocyanate) mass 0.01% catalyst dioctyltin mercaptide, and react for 6h at 70°C with a stirring speed of 500r / min to obtain the pre- polymer solution.
[0039] Dissolve microcrystalline cellulose in ionic liquid 1-allyl-3-methylimidazolium chloride salt at 80°C to obtain A solution with a microcrystalline cell...
Embodiment 2
[0043] The cellulose acetate was dried in a vacuum oven at 30 °C for 48 h.
[0044] Diphenylmethane diisocyanate and polyethylene glycol n-octadecyl ether HO (CH 2 CH 2 O) 100 C 18 h 37 Diphenylmethane diisocyanate solution and 9mol% polyethylene glycol n-stearyl ether solution were dissolved in dimethyl sulfoxide to prepare a concentration of 2mol%; then HO(CH 2 CH 2 O) 100 C 18 h 37 The solution is added dropwise to the diphenylmethane diisocyanate solution, the molar ratio of diphenylmethane diisocyanate to polyethylene glycol n-octadecyl ether is 1:1, and the mass of crosslinking agent (diphenylmethane diisocyanate) is added at 2.0% The catalyst dioctyltin mercaptide was reacted for 8 hours at 60°C and the stirring speed was 600r / min to obtain a prepolymer solution.
[0045] Dissolve cellulose acetate in ionic liquid propylpyridinium chlorate at 65°C to obtain A solution with a cellulose acetate concentration of 1 wt%, and then add the prepolymer solution to A sol...
Embodiment 3
[0048] The cyanoethyl cellulose was dried in a vacuum oven at room temperature for 48 h.
[0049] Methylenebisacrylamide and polyethylene glycol n-behenyl ether (HO(CH 2 CH 2 O) 2 C 22 h 45 ) was dissolved in ethylene glycol monoethyl ether to prepare a solution of methylenebisacrylamide with a concentration of 2mol% and a solution of polyethylene glycol n-docosyl ether with a concentration of 8mol%, and then the polyethylene glycol n-behenyl ether The alkyl ether solution was added into the methylene bisacrylamide solution in 10 times, the molar ratio of methylene bisacrylamide to polyethylene glycol n-docosyl ether was 1:1, and a crosslinking agent (methylene bisacrylamide Bisacrylamide) with 0.05% by weight of the catalyst dibutyltin dilaurate was reacted for 8 hours at 35°C with a stirring rate of 600r / min to obtain a prepolymer solution.
[0050] Dissolve cyanoethylcellulose into the ionic liquid 1-butyl-3-methyl-imidazolium chloride salt at 65°C to obtain a solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Endothermic temperature | aaaaa | aaaaa |
| Exothermic temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com