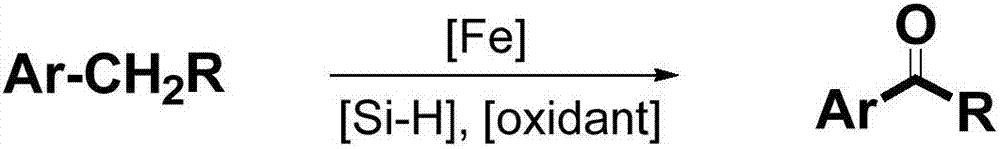Method for synthesizing aromatic aldehyde, aromatic ketone and aromatic ester through catalytically oxidizing alkyl aromatic compound by iron
A technology for aromatic compounds and alkylene oxides, which is applied in the field of catalytic synthesis, can solve the problems of poor compatibility of functional groups, high reaction costs, and low reaction efficiency, and achieve the effects of high selectivity, good reaction selectivity, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
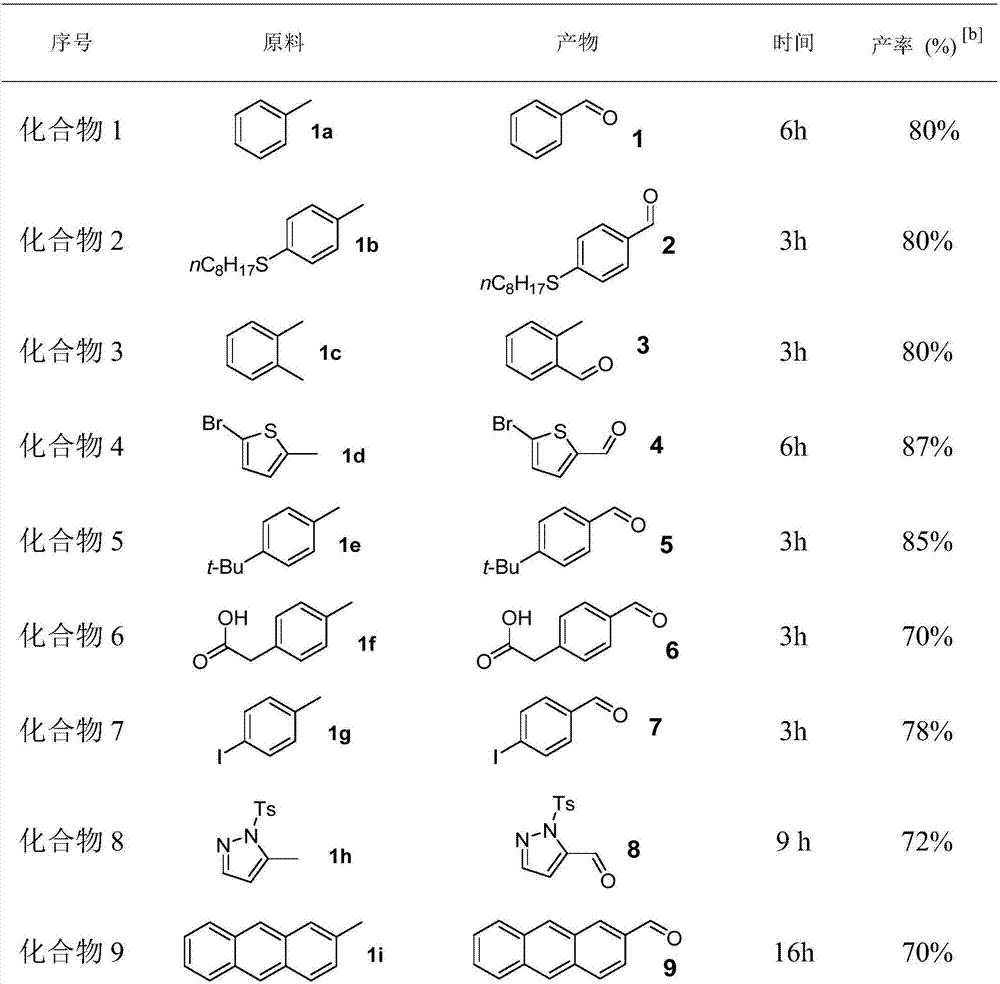
[0027] Compound 1: Add ferrous acetylacetonate (0.025mmol), polymethylhydrogensiloxane (0.75mmol), potassium persulfate (0.25mmol), 1a (0.25mmol), acetonitrile (1mL), water to a 25mL reaction flask in sequence (1 mL), the reaction mixture was reacted at 80°C for 6h. After the reaction, add ammonia water (2 mL) to remove polymethylhydrogen siloxane, add 10 mL of saturated saline, and extract with ether (10 mL×3), combine the organic phases, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 80%. .
Embodiment 2
[0029] Compound 2: Iron acetylacetonate (0.025mmol), polymethylhydrogensiloxane (0.75mmol), potassium persulfate (0.25mmol), 1b (0.25mmol), acetonitrile (1mL), water ( 1 mL), the reaction mixture was reacted at 80°C for 3h. After the reaction, add ammonia water (2 mL) to remove polymethylhydrogen siloxane, add 10 mL of saturated saline, and extract with ether (10 mL×3), combine the organic phases, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 80%. .
Embodiment 3
[0031] Compound 3: Add ferrous acetylacetonate (0.025mmol), polymethylhydrogensiloxane (0.75mmol), potassium persulfate (0.25mmol), 1c (0.25mmol), acetonitrile (1mL), water to a 25mL reaction flask in sequence (1 mL), the reaction mixture was reacted at 80° C. for 3 h. After the reaction, add ammonia water (2 mL) to remove polymethylhydrogen siloxane, add 10 mL of saturated saline, and extract with ether (10 mL×3), combine the organic phases, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 80%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com