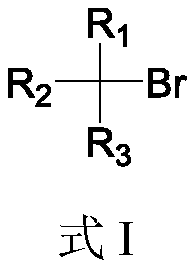
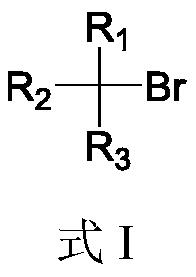
Boron esterification reaction method of alkyl bromide without transition metal catalysis
A technology of alkyl bromide and alkyl boronate, applied in the field of boron esterification reaction without transition metal catalysis, can solve the problems of artificial synthesis, high cost, sensitivity, etc. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
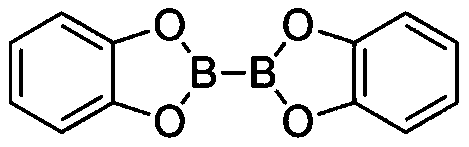
[0035]In a glove box under a nitrogen atmosphere, sequentially add B to a 10 mL Schlenk bottle equipped with a stir bar 2 cat 2 (0.45mmol, 1.5eq, 107.0mg), AIBN (0.33mmol, 1.1eq, 54.2mg), 2mL solvent N,N-dimethylacetamide, TTMSS (0.33mmol, 1.1eq, 101.8μL) and Octane (0.3 mmol, 52.3 μL). The capped Schlenk bottle was removed from the glove box, and the reaction mixture was stirred at 80 °C for 6 hours. After cooling to room temperature, pinacol (141.8 mg, 4.0 equivalents) was added to the reaction flask, 1.0 mL of triethylamine was added, and stirred at room temperature for 1 hour. Subsequently, the reaction mixture was transferred to a 125mL separatory funnel by 30mL ethyl acetate, then 30mL water was added thereto for washing, and then the aqueous phase was extracted 3 times with 30mL ethyl acetate, and the organic phases were combined and transferred to a 250mL separatory funnel, Washed with 100 mL of saturated brine, the organic phase was dried with anhydrous sodium sulf...
Embodiment 2
[0037] In a glove box under a nitrogen atmosphere, sequentially add B to a 10 mL Schlenk bottle equipped with a stir bar 2 cat 2 (0.75mmol, 1.5eq, 178.4mg), AIBN (0.55mmol, 1.1eq, 90.3mg), 3mL solvent N,N-dimethylacetamide, TTMSS (0.55mmol, 1.1eq, 169.7μL) and 6-bromo Methyl hexanoate (0.5 mmol, 79.4 μL). The capped Schlenk bottle was removed from the glove box, and the reaction mixture was stirred at 80 °C for 6 hours. After cooling to room temperature, pinacol (236.3 mg, 4.0 equivalents) was added to the reaction flask, 1.5 mL of triethylamine was added, and stirred at room temperature for 1 hour. Subsequently, the reaction mixture was transferred to a 125mL separatory funnel by 30mL ethyl acetate, then 30mL water was added thereto for washing, and then the aqueous phase was extracted 3 times with 30mL ethyl acetate, and the organic phases were combined and transferred to a 250mL separatory funnel, Washed with 100 mL of saturated brine, the organic phase was dried over an...
Embodiment 3
[0039] In a glove box under a nitrogen atmosphere, sequentially add B to a 10 mL Schlenk bottle equipped with a stir bar 2 cat 2 (0.75mmol, 1.5eq, 178.4mg), AIBN (0.55mmol, 1.1eq, 90.3mg), 3mL solvent N,N-dimethylacetamide, TTMSS (0.55mmol, 1.1eq, 169.7μL) and bromocyclic Heptane (0.5 mmol, 68.7 μL). The capped Schlenk bottle was removed from the glove box, and the reaction mixture was stirred at 80 °C for 6 hours. After cooling to room temperature, pinacol (236.3 mg, 4.0 equivalents) was added to the reaction flask, 1.5 mL of triethylamine was added, and stirred at room temperature for 1 hour. Subsequently, the reaction mixture was transferred to a 125mL separatory funnel by 30mL ethyl acetate, then 30mL water was added thereto for washing, and then the aqueous phase was extracted 3 times with 30mL ethyl acetate, and the organic phases were combined and transferred to a 250mL separatory funnel, Washed with 100 mL of saturated brine, the organic phase was dried with anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com