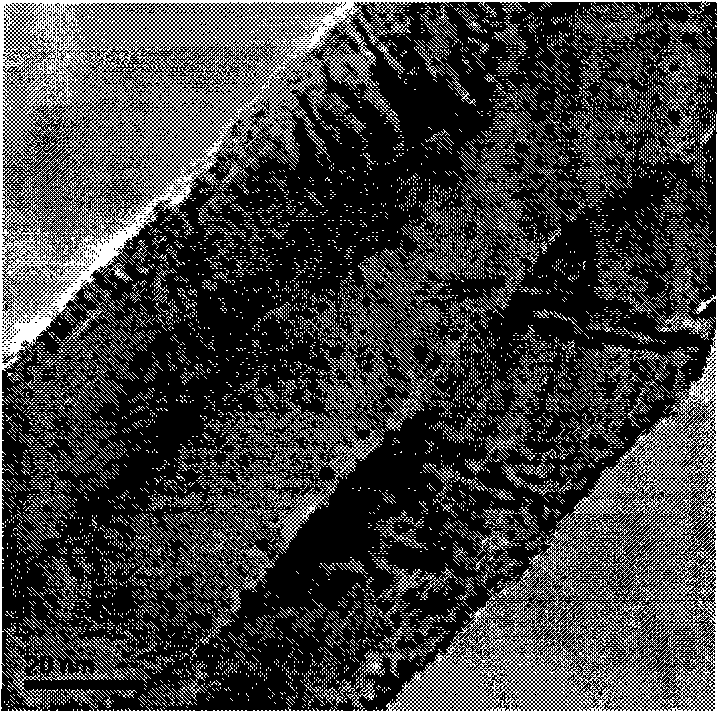Method for preparing aldehyde or ketone compounds with catalytic oxidation of alcohol compounds
A ketone compound, catalytic oxidation technology, applied in the direction of carbon-based compound preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problem of expensive catalysts, achieve easy separation and recycling, low cost, and wide application range wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1. Preparation of carbon nanotube-supported ruthenium catalyst
[0016] The carbon nanotubes used in the experiment were purchased from Shenzhen Nano Harbor Co., Ltd., and their specific surface area is about 160 m 2 / g. Use the modulated H before using 2 SO 4 -HNO 3 The oxidation treatment method introduces oxygen-containing functional groups on CNTs. The experimental method is as follows: 10 g of carbon nanotubes were placed in a 250 mL round bottom flask, and 50 mL of H 2 O, 100mL HNO 3 , 50mL H 2 SO 4 , ultrasonically dispersed for 10 minutes, and then refluxed at 120°C for 4 hours under magnetic stirring conditions. After reflux, filter and wash with distilled water until neutral, and dry under vacuum at 130°C; grind for later use, and store in a desiccator.
[0017] with RuCl 3 Ru / CNTs catalyst was prepared by impregnation method as the precursor of ruthenium, mixed acid-functionalized carbon nanotubes as the catalyst carrier. The experimental method is...
Embodiment 2
[0023] 1. Preparation of activated carbon-supported ruthenium catalyst
[0024] The activated carbon used in the experiment is coconut shell activated carbon, purchased from Beijing Yuanda Integrated Activated Carbon Company, and its specific surface area is about 1000m 2 / g. Grind the commercial coconut shell activated carbon before use, and then use a standard sieve to sieve the activated carbon with the required mesh (200-320 mesh) for later use.
[0025] Modulated H 2 SO 4 -HNO 3 Oxidation treatment method introduces oxygen-containing functional groups on activated carbon. The experimental method is as follows: Weigh 10 g of activated carbon with the required mesh number (200-320 mesh) and place it in a 250 mL round bottom flask, add 50 mL H 2 O, 100mL HNO 3 , 50mL H 2 SO 4 , ultrasonically dispersed for 10 minutes, and then refluxed at 120°C for 4 hours under magnetic stirring conditions. After reflux, filter and wash with distilled water until neutral, and dry u...
Embodiment 3
[0030] 1. Preparation of titanium dioxide supported ruthenium catalyst
[0031] Titanium dioxide (P25) used in the experiment was purchased from Shanghai Haiyi Science and Trade Co., Ltd., and its specific surface area is about 50m 2 / g.
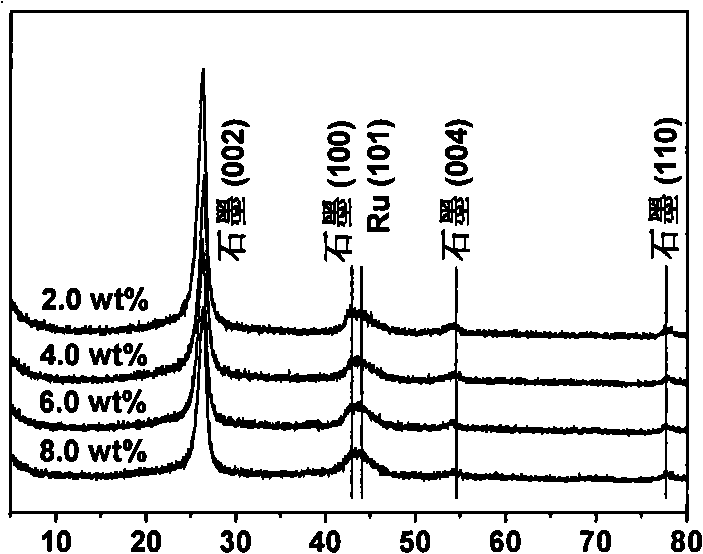
[0032] with RuCl 3 As the precursor of ruthenium, mixed acid functionalized activated carbon was used as the catalyst carrier, and the Ru / CNTs catalyst was prepared by impregnation method. The experimental method is as follows: measure 6mL RuCl 3 (0.10M) into a 100mL small beaker, add 1g of titanium dioxide (P25); ultrasonically disperse for 30min; age at room temperature for 24h; vacuum dry at 110°C for 12h; grind to obtain RuCl 3 / TiO 2 Sample; 400°C, hydrogen atmosphere activation for 2h, heating rate of 3°C / min, and finally passivation in argon atmosphere to room temperature to obtain 6.0wt% Ru / TiO 2 catalyst.
[0033] 2. Oxidation of Benzyl Alcohol Catalyzed by Titanium Dioxide Supported Ru Catalyst
[0034] Weigh 0.2g 6.0wt%Ru / T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com