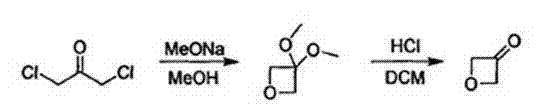
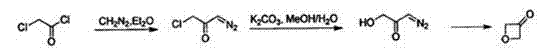Synthesis method of oxetanone
A technology of oxetanone and a synthesis method, applied in the formation/introduction of carbonyl group, organic chemistry and other directions, can solve the problems of less 3-oxetanone, hidden danger, high price and the like, and achieves improved reaction yield , the effect of reducing production costs and cheap materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Add 3 L of dichloromethane, 1.24 kg of TCCA, 1.28 kg of sodium bicarbonate, 33 g of potassium bromide and 8.7 g of TEMPO into a 5 L dry three-necked flask, stir and cool down to -5°C under nitrogen protection. Then 400 g of oxetanone was added dropwise. After the dropwise addition was complete, stirring was continued at 0°C for 2 hours. After filtering the reaction mixture, it was distilled under reduced pressure at room temperature to obtain 350 g of the product (purity greater than 95%), and the yield was 89%.
Embodiment 2
[0050] Add 2.0 L of dichloromethane, 770 kg of NCS, 710 kg of potassium bicarbonate, 150 g of tetrabutylammonium bromide and 15 g of TEMPO into a 5 L dry three-necked flask, stir and cool down to -5°C under nitrogen protection. Then 400 g of oxetanone was added dropwise. After the dropwise addition was complete, stirring was continued at 25°C for 4 hours. After the reaction mixture was filtered, it was distilled under reduced pressure at room temperature to obtain 320 g of the product (purity greater than 95%), with a yield of 82%.
Embodiment 3
[0052] Add 2.5 L of dichloromethane, 1.92 kg of NBS, 1.48 kg of potassium carbonate, 25 g of sodium bromide and 25 g of TEMPO into a 5 L dry three-necked flask, stir and cool down to 0 °C under nitrogen protection. Then 400 g of oxetanone was added dropwise. After the dropwise addition was complete, stirring was continued at 25°C for 4 hours. After the reaction mixture was filtered, it was distilled under reduced pressure at room temperature to obtain 335 g of the product (purity greater than 95%), with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com