Novel method for synthesizing 3-oxacyclobutanol
A technology for oxetanol and epichlorohydrin, which is applied in the field of synthesizing 3-oxetanol, can solve the problems of high price of 3-oxetanol, hinder application and development, etc. Environmentally friendly and easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
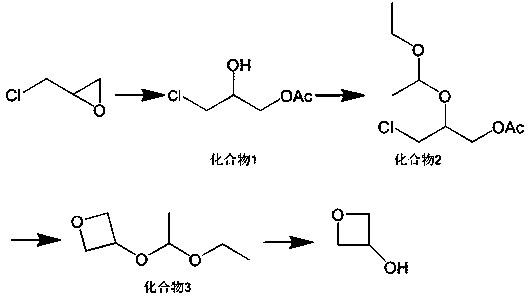
[0026] (1) Add 139g of epichlorohydrin and 90g of glacial acetic acid into a 500mL reaction bottle, then add 0.6g of ferric chloride, raise the temperature to 70°C, and stir for 7 hours.
[0027] (2) Add 1.0g of p-toluenesulfonic acid to the reaction solution, slowly add 80g of vinyl ethyl ether dropwise, and control the dropping temperature at 40°C. After the dropwise addition, continue to stir and react at 40°C for 2 hours, then cool down to 0 0.9 g of p-toluenesulfonic acid was added, and the reaction was stirred at 40° C. for 4 hours to obtain a reaction solution.
[0028] (3) Add 345g of 50% NaOH solution into another 1000mL reaction bottle, raise the temperature to 110°C, slowly add the reaction liquid obtained in step (2) dropwise, and control the temperature during the dropping process at 110°C. Stir the reaction at 110°C for 7 hours, lower the temperature to 20°C, add 145g of water, 383g of dichloromethane, and 190g of saturated saline into the reaction bottle, stir a...
Embodiment 2
[0031] (1) Add 13.9kg of epichlorohydrin and 9kg of glacial acetic acid into a 50L reactor, and then add 60g of ferric chloride. The temperature was raised to 70°C, and the reaction was stirred for 7 hours.
[0032] (2) Add 100g of p-toluenesulfonic acid to the reaction solution, slowly add 8kg of vinyl ethyl ether dropwise, and control the dropping temperature at 40°C. After the dropwise addition, continue to stir and react at 40°C for 2 hours, then cool down to 0°C , continue to add 90g of p-toluenesulfonic acid, and continue to stir and react at 40°C for 4 hours to obtain a reaction solution.
[0033] (3) Add 34.5kg of 50% NaOH solution into another 100L reaction bottle, raise the temperature to 110°C, slowly add the reaction solution in step (2), and control the temperature during the dropping process at 110°C. Stir and react at 110°C for 7 hours, cool down to 20°C, add 14.5kg of water, 38.3kg of dichloromethane, and 19kg of saturated saline into the reaction bottle, stir...
Embodiment 3
[0036] (1) Add 139g of epichlorohydrin and 90g of glacial acetic acid into a 500mL reaction bottle, then add 1.1g of ferric bromide, raise the temperature to 70°C, and stir for 7 hours.
[0037] (2) Add 1.2g of 2-naphthalenesulfonic acid to the reaction solution, slowly add 80g of vinyl ethyl ether dropwise, and control the dropping temperature at 40°C. After the dropwise addition, continue to stir and react at 40°C for 2 hours, then cool down to 0°C , continue to add 1.1 g of 2-naphthalenesulfonic acid, and continue to stir and react at 40° C. for 4 hours to obtain a reaction solution.
[0038] (3) Add 483g of 50% KOH solution into another 1000mL reaction bottle, raise the temperature to 110°C, slowly add the reaction solution obtained in step (2) dropwise, and control the temperature during the dropping process at 110°C. Stir the reaction at 110°C for 7 hours, lower the temperature to 20°C, add 145g of water, 383g of dichloromethane, and 190g of saturated saline into the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com