Monatomic rhodium catalyst, and preparation and applications thereof
A catalyst and atomic technology, applied in the field of single-atom rhodium-based catalysts and their preparation, can solve the problems of difficult recovery and reuse of homogeneous catalysts, large phosphorus-containing ligands, and low actual utilization of rhodium, and achieve excellent recycling performance , the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
[0035] 1. Preparation of catalyst:
[0036] Weigh 200mg of carrier, disperse in 100ml of ultrapure water, stir at room temperature for 0.5h, then add 0.1mg Rh / mL rhodium chloride solution 0.2-40mL, continue stirring at room temperature for 3h. After suction filtration, it was washed with 50 mL of ultrapure water, dried at 60°C for 12 h, and reduced with hydrogen (hydrogen flow rate: 40 mL / min) at 200°C for 0.5 h. A monoatomic rhodium catalyst with a loading of 0.01-2 wt% is available. The obtained catalysts are listed in Table 1.
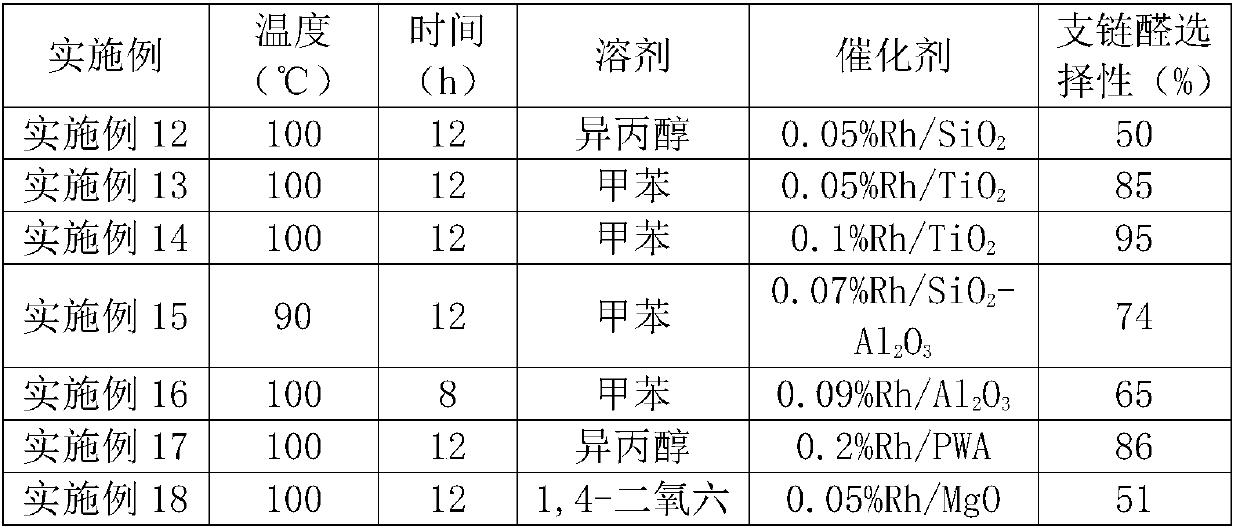
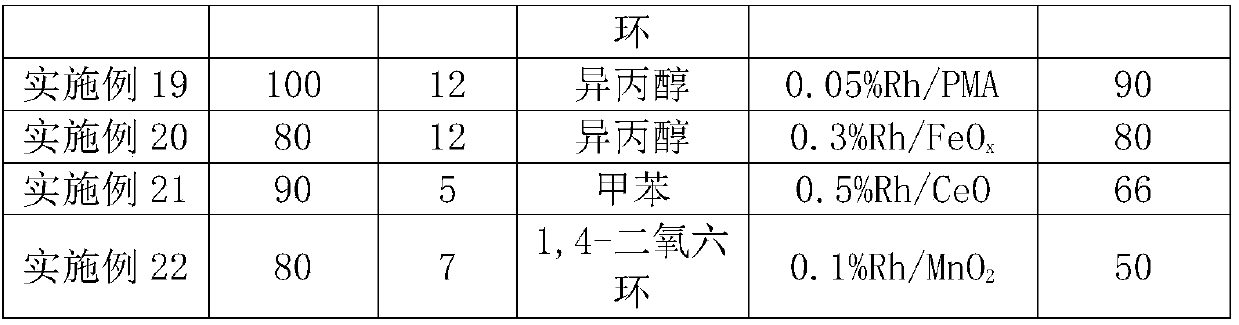
[0037] Table 1 Supported Rh-based catalysts
[0038] Example
Embodiment 12-22
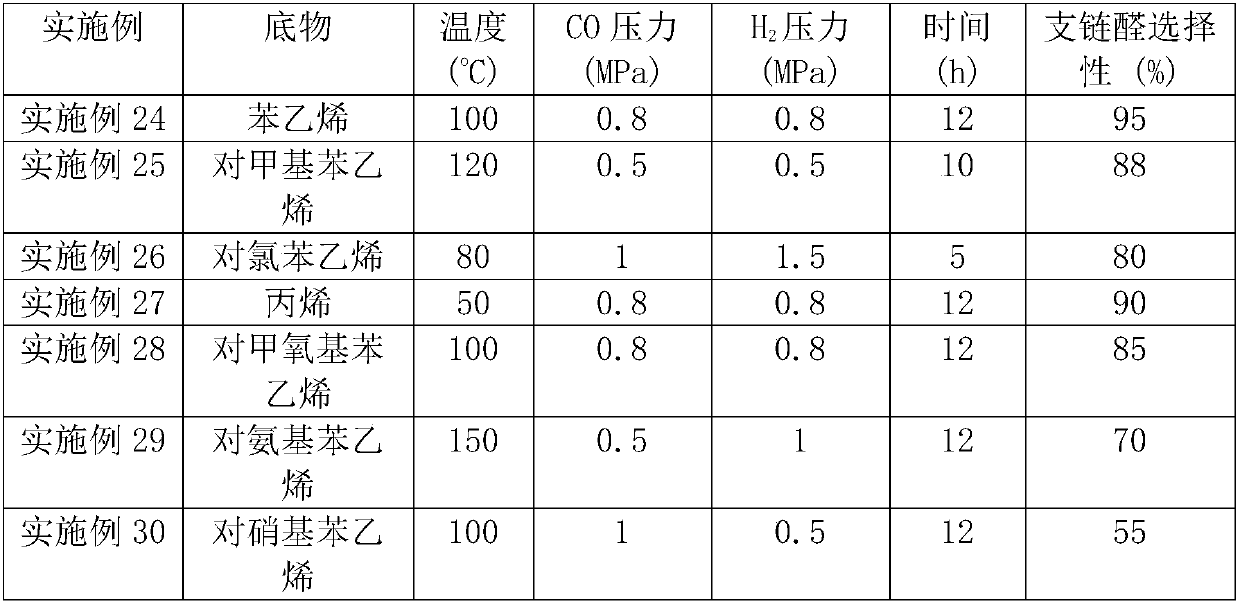
[0040] 2. Hydroformylation reaction: After adding 20mg of catalyst, 0.3mL of styrene, and 4mL of organic solvent into the autoclave, fill it with 0.8MPa carbon monoxide and 0.8MPa hydrogen, and stir at 80-100°C for 5-12h. After the reaction system was cooled to room temperature, the remaining gas was released, and the reaction solution was analyzed by GC. See Table 2 for detailed results.
[0041] Table 2 hydroformylation reaction results
[0042]
[0043]
[0044] The catalyst preparation process that embodiment 13 and 14 uses is with embodiment 2, and difference is that the add-on of rhodium chloride solution is respectively 1ml and 2ml;
Embodiment 17
[0045] The catalyst preparation process that embodiment 17 uses is with embodiment 5, and difference is that the add-on of rhodium chloride solution is 4ml;
[0046] The catalyst preparation process used in Example 19 is the same as in Example 6, except that the addition of rhodium chloride solution is 1ml.
[0047] It can be seen from Table 2 that in different rhodium-based catalysts, when titanium dioxide, phosphotungstic acid, phosphomolybdic acid and iron oxide are respectively used as carriers, the selectivity of branched-chain aldehydes in the product is greater than 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com