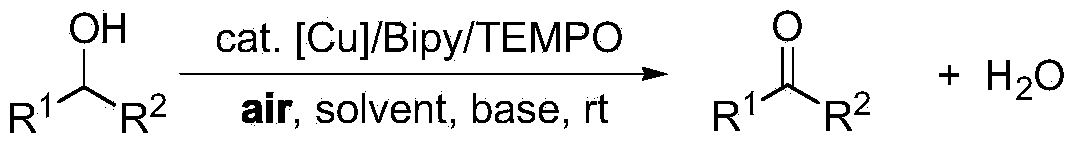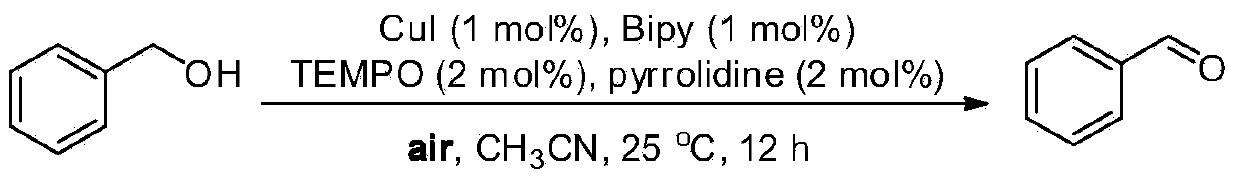Greening method for preparing aldehydes and ketones through alcohol oxidation of copper catalyst
A catalytic alcohol, green technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as high toxicity, limited application potential, and impact on applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Oxidation of benzyl alcohol to benzaldehyde
[0024]
[0025] Weigh CuI (0.0038g, 1mol%), 2,2'-bipyridine (0.0031g, 1mol%), TEMPO (0.0063g, 2mol%), pyrrolidine (0.0033mL, 2mol%), benzyl alcohol (0.2163g , 2mmol) into an ordinary reactor, add acetonitrile (0.5mL) as a solvent, open the reaction at room temperature (about 25°C), and then track and detect the reaction with GC-MS or TLC. After about 12 hours, the reaction is over, and the mixture is concentrated Afterwards, use petroleum ether and ethyl acetate as developing solvents for flash column chromatography (silica gel as stationary phase) for separation to obtain benzaldehyde with a separation yield of 94%. 1 HNMR (500MHz, CDCl 3 ):δ9.98(s,1H),7.87-7.84(m,2H),7.61-7.57(m,1H),7.50-7.47(t,J=7.5Hz,2H). 13 C NMR (125.4MHz, CDCl 3 ):δ192.5,136.3,134.5,129.7,129.0.MS(EI):m / z(%)107(7),106(100),105(94),78(13),77(87),76( 4),75(3),74(6),52(7),51(28),50(14).
Embodiment 2
[0027] Oxidation of 4-chlorobenzyl alcohol to 4-chlorobenzaldehyde
[0028]
[0029] Sequentially weigh CuI (0.0190g, 5mol%), 2,2'-bipyridine (0.0156g, 5mol%), TEMPO (0.0315g, 10mol%), pyrrolidine (0.0165mL, 10mol%), 4-chlorobenzyl alcohol (0.2852g, 2mmol) into a common reactor, add acetonitrile (0.5mL) as a solvent, open the reaction at room temperature (about 25°C), then track and detect the reaction with GC-MS or TLC, and the reaction is over after about 24 hours , the mixture was concentrated and separated by flash column chromatography using petroleum ether and ethyl acetate as the developing solvent (silica gel as the stationary phase) to obtain 4-chlorobenzaldehyde with a separation yield of 70%. 1 HNMR (500MHz, CDCl 3 ):δ9.99(s,1H),7.84-7.81(dt, 1 J=8.5Hz, 2 J=2.0Hz,2H),7.53-7.50(dt, 1 J=8.5Hz, 2 J=2.0Hz,2H). 13 CNMR (125.4MHz, CDCl 3 ):δ190.8,141.0,134.8,130.9,129.5.MS(EI):m / z(%)142(2),141(24),140(36),139(75),138(100),112( 16), 111(7), 110(49), 84(3), 76(13...
Embodiment 3
[0031] 4-Fluorobenzyl alcohol as 4-fluorobenzaldehyde
[0032]
[0033] Sequentially weigh CuI (0.0038g, 1mol%), 2,2'-bipyridine (0.0031g, 1mol%), TEMPO (0.0063g, 2mol%), pyrrolidine (0.0033mL, 2mol%), 4-fluorobenzyl alcohol (0.2482g, 2mmol) into a common reactor, add acetonitrile (0.5mL) as a solvent, open the reaction at room temperature (about 25°C), then track and detect the reaction with GC-MS or TLC, and the reaction ends after about 12 hours , the mixture was concentrated and separated by flash column chromatography using petroleum ether and ethyl acetate as the developing solvent (silica gel as the stationary phase) to obtain 4-fluorobenzaldehyde with a separation yield of 75%. 1 HNMR (500MHz, CDCl 3 ):δ9.93(s,1H),7.74(d,J=8Hz,2H),7.29(d,J=8Hz,2H),2.40(s,3H). 13 CNMR (125.4MHz, CDCl 3 ):δ191.9,145.5,134.3,129.8,129.7,21.8.MS(EI):m / z(%)125(8),124(94),123(100),96(13),95(83), 94(5),76(3),75(22),74(7),70(6),69(6),68(4),63(2),57(3),51(6), 50(12).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com