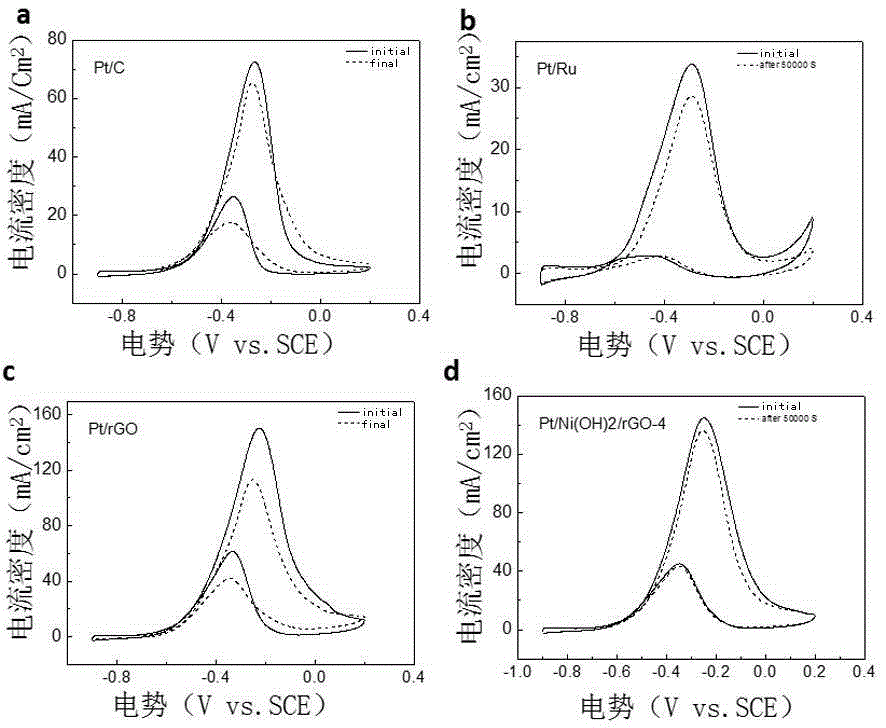
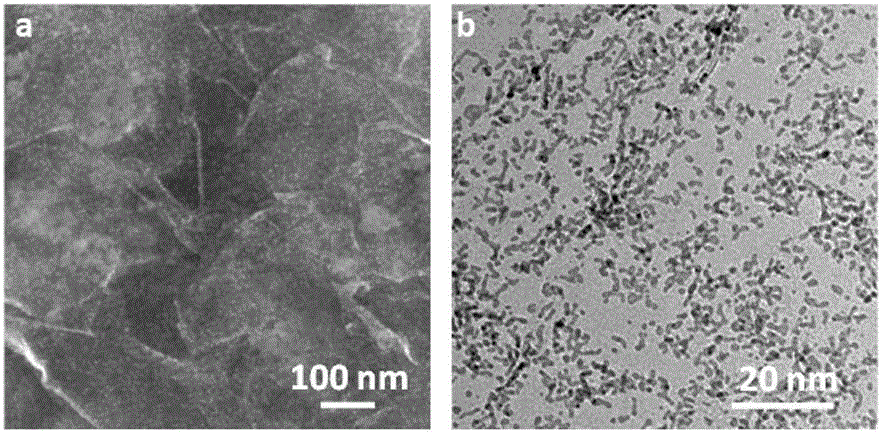
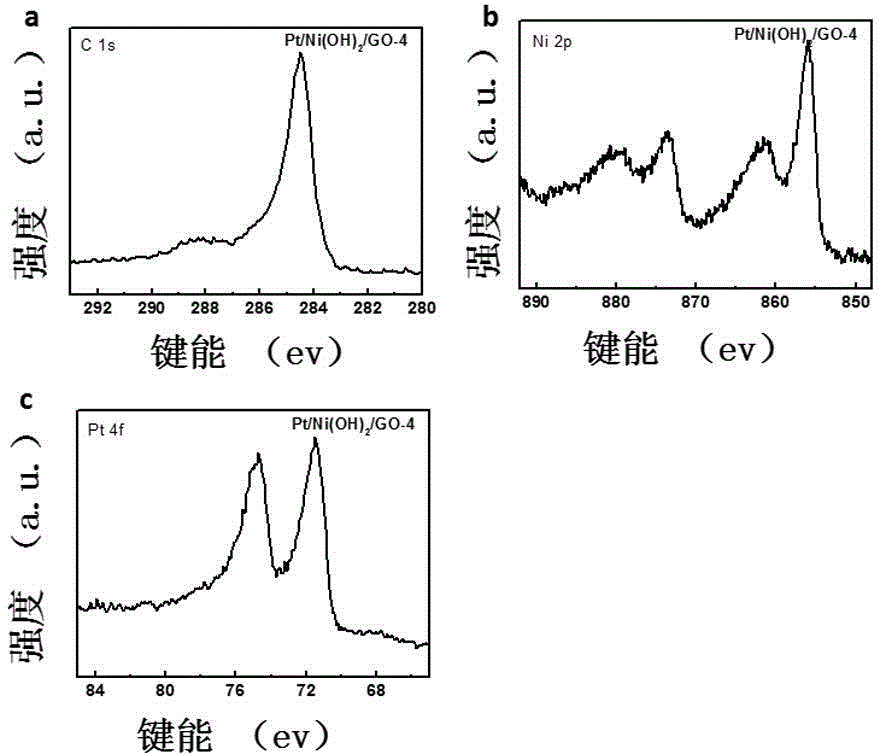
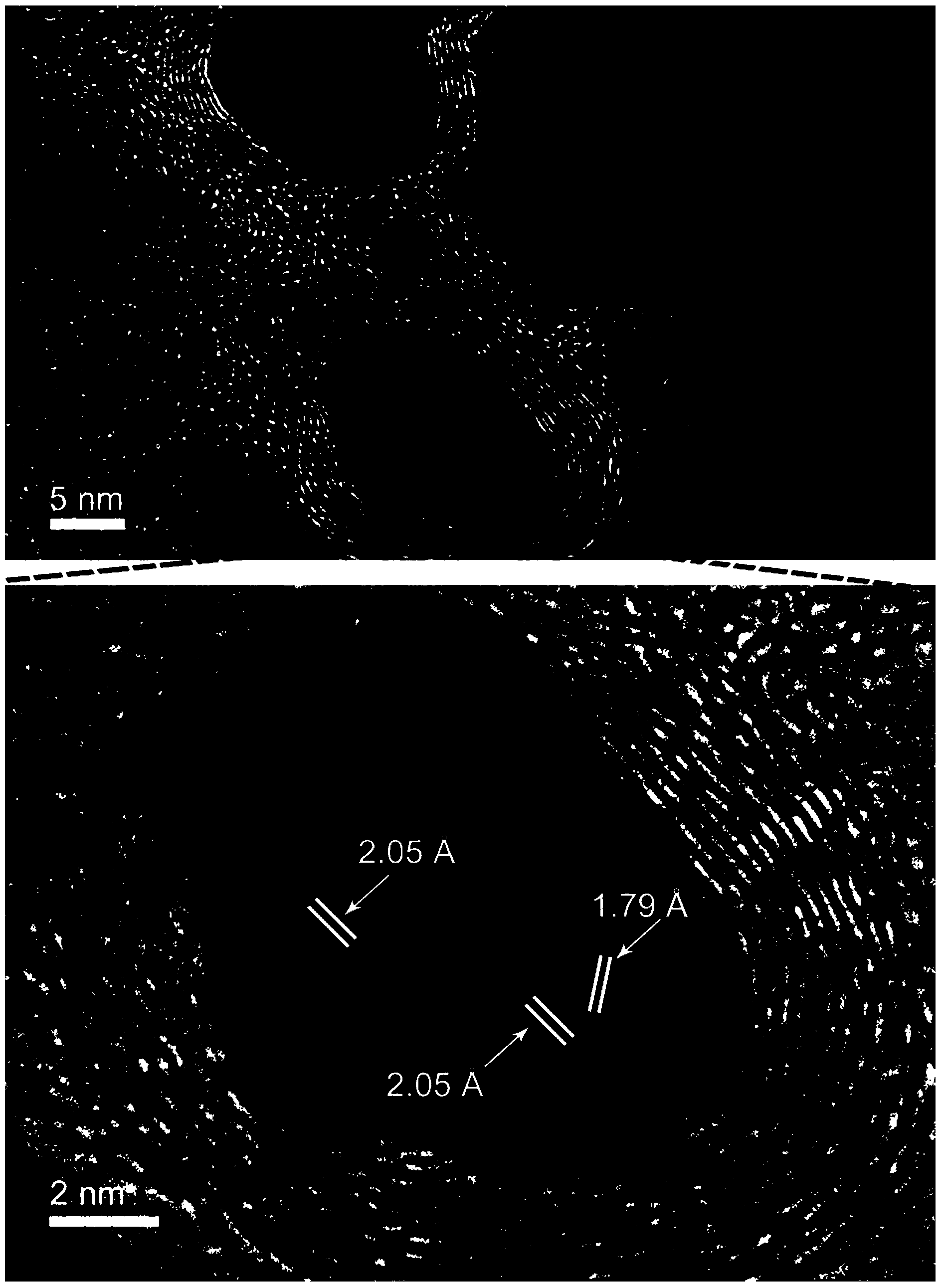
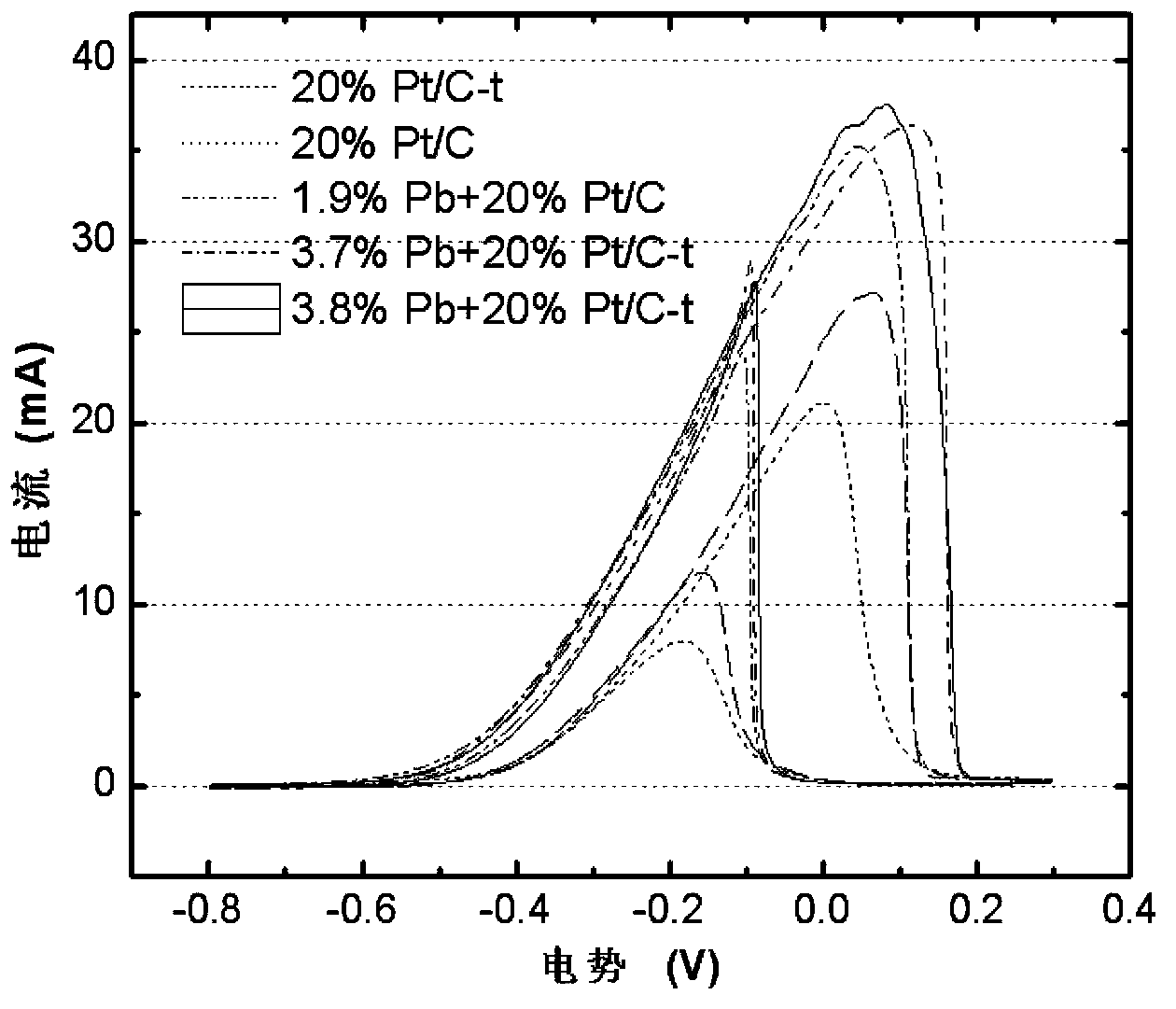
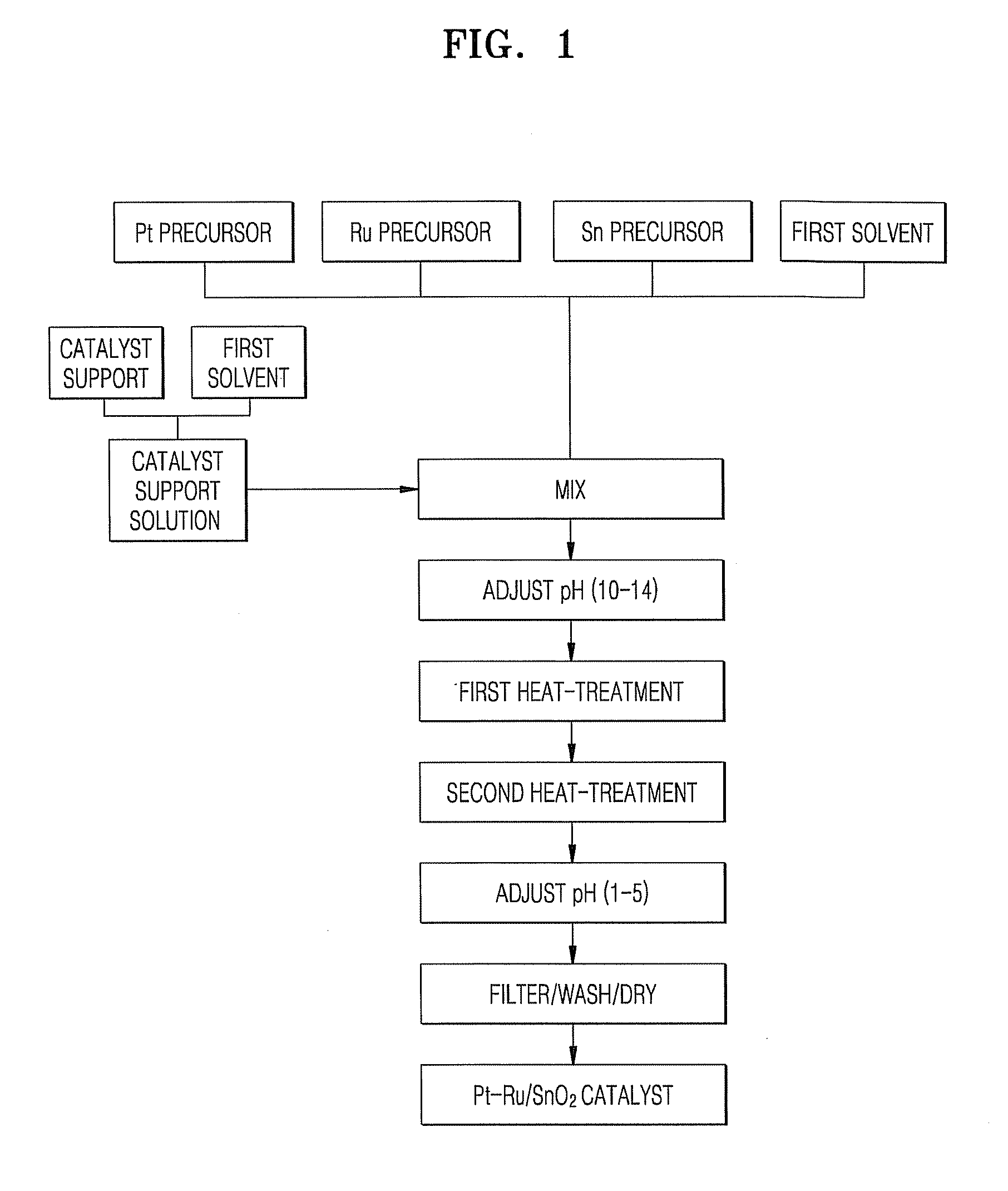
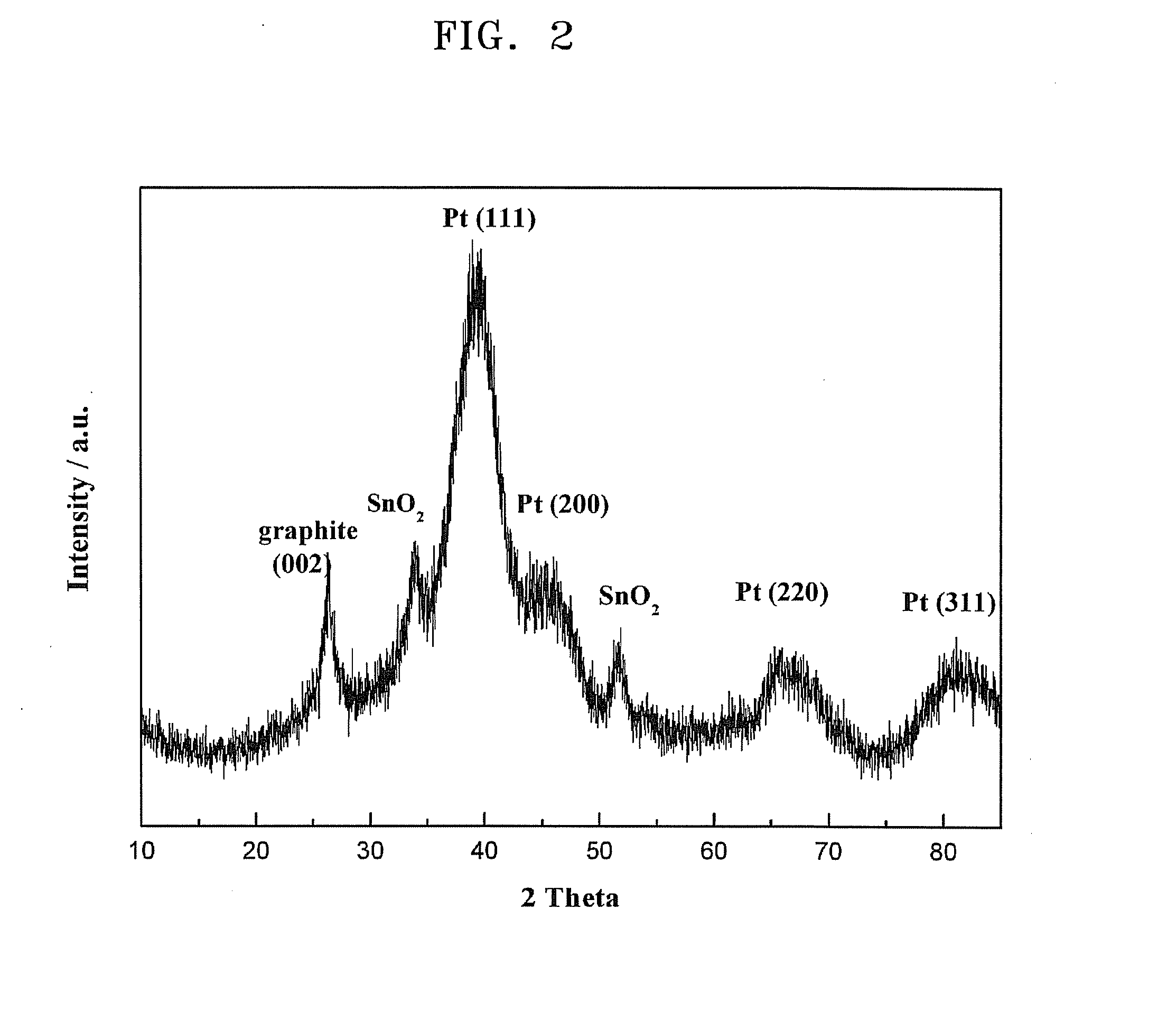
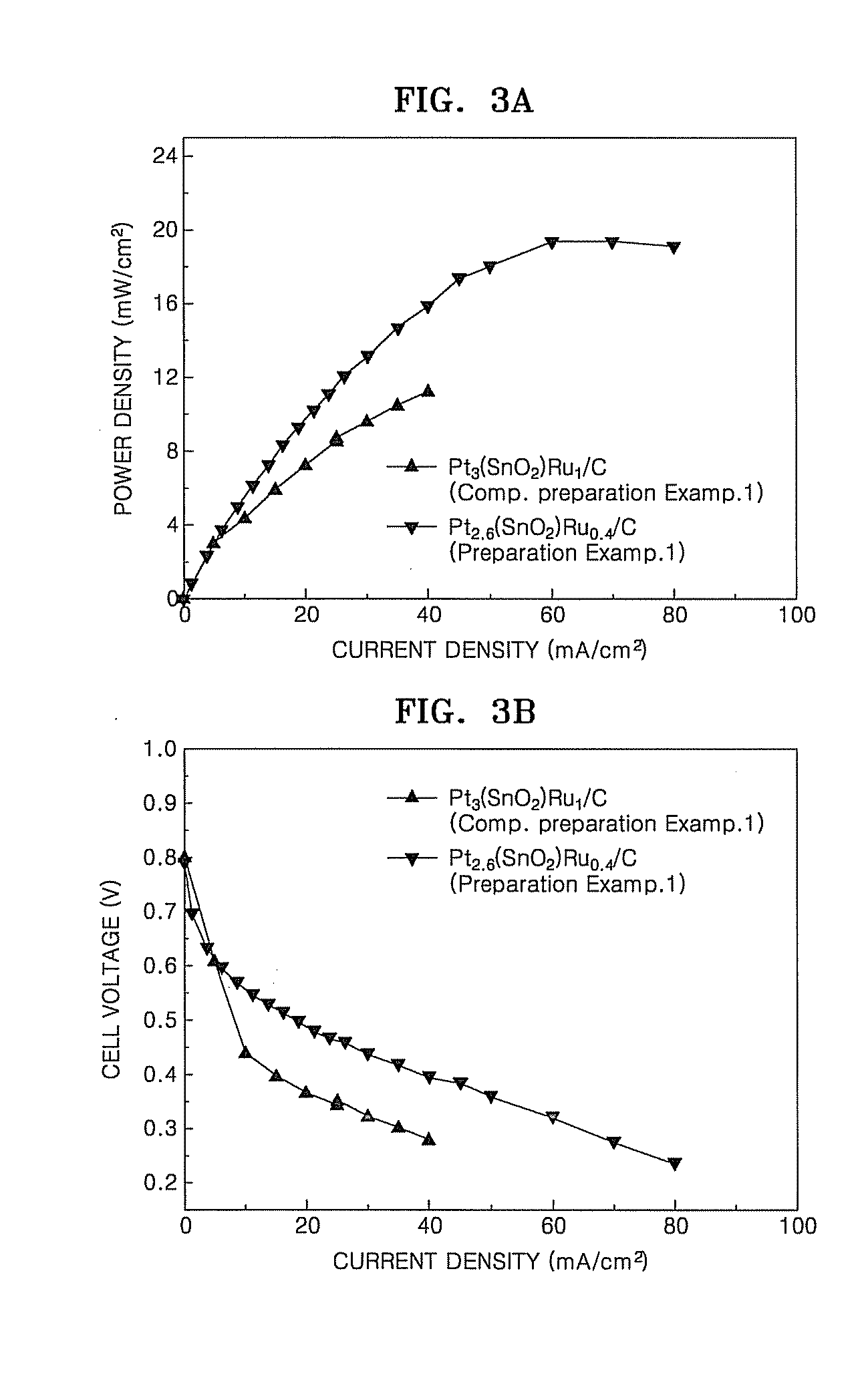
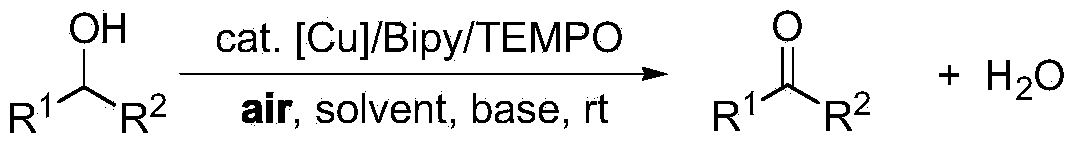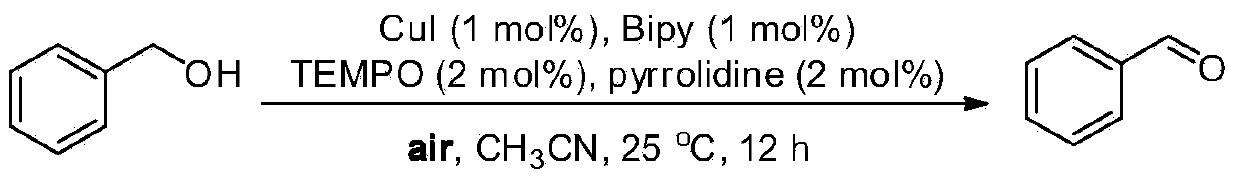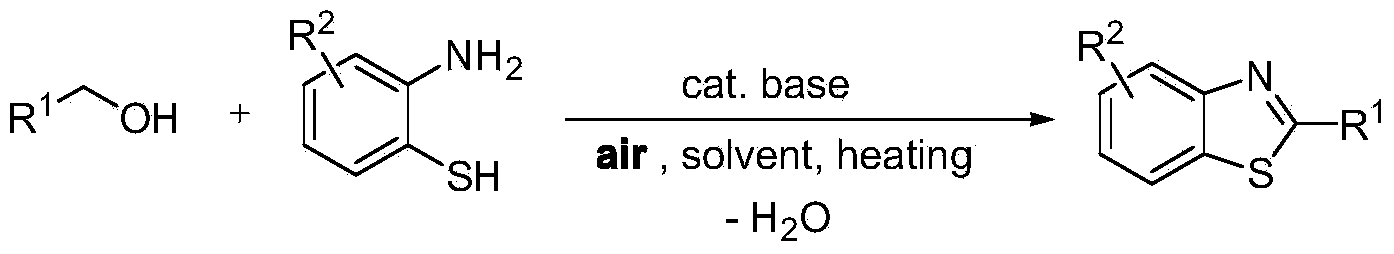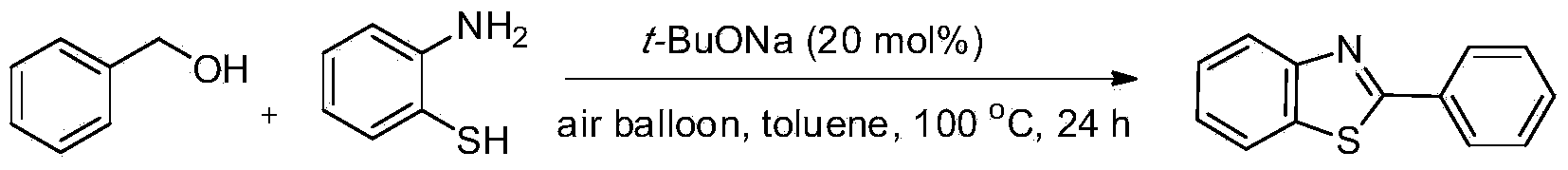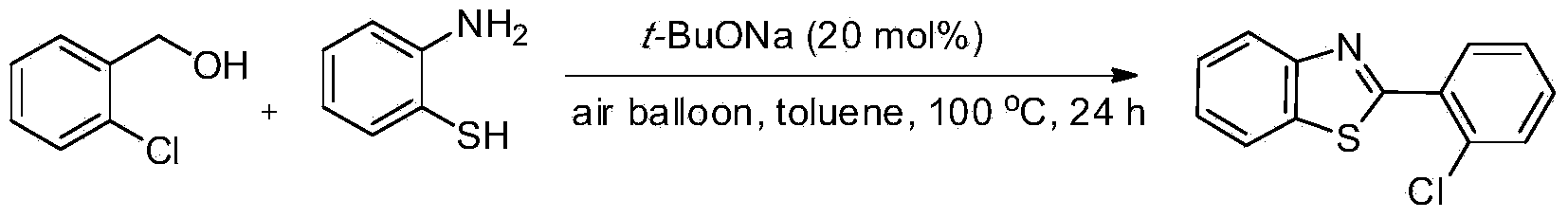Patents
Literature
172 results about "Alcohol oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
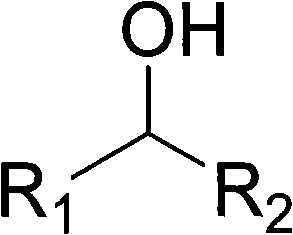
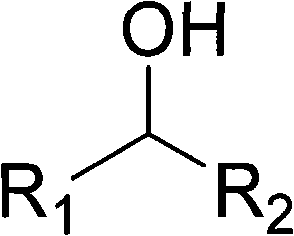
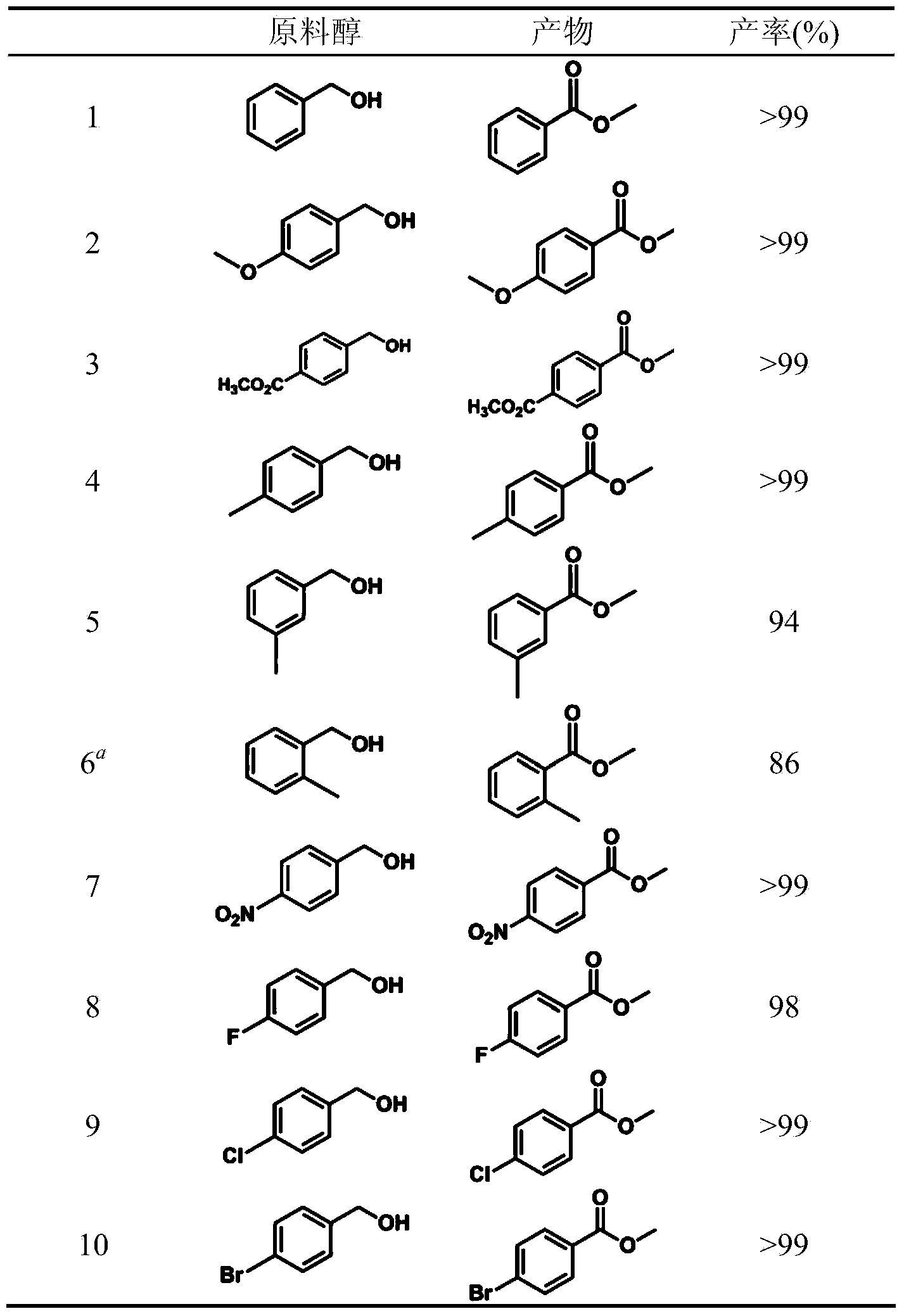
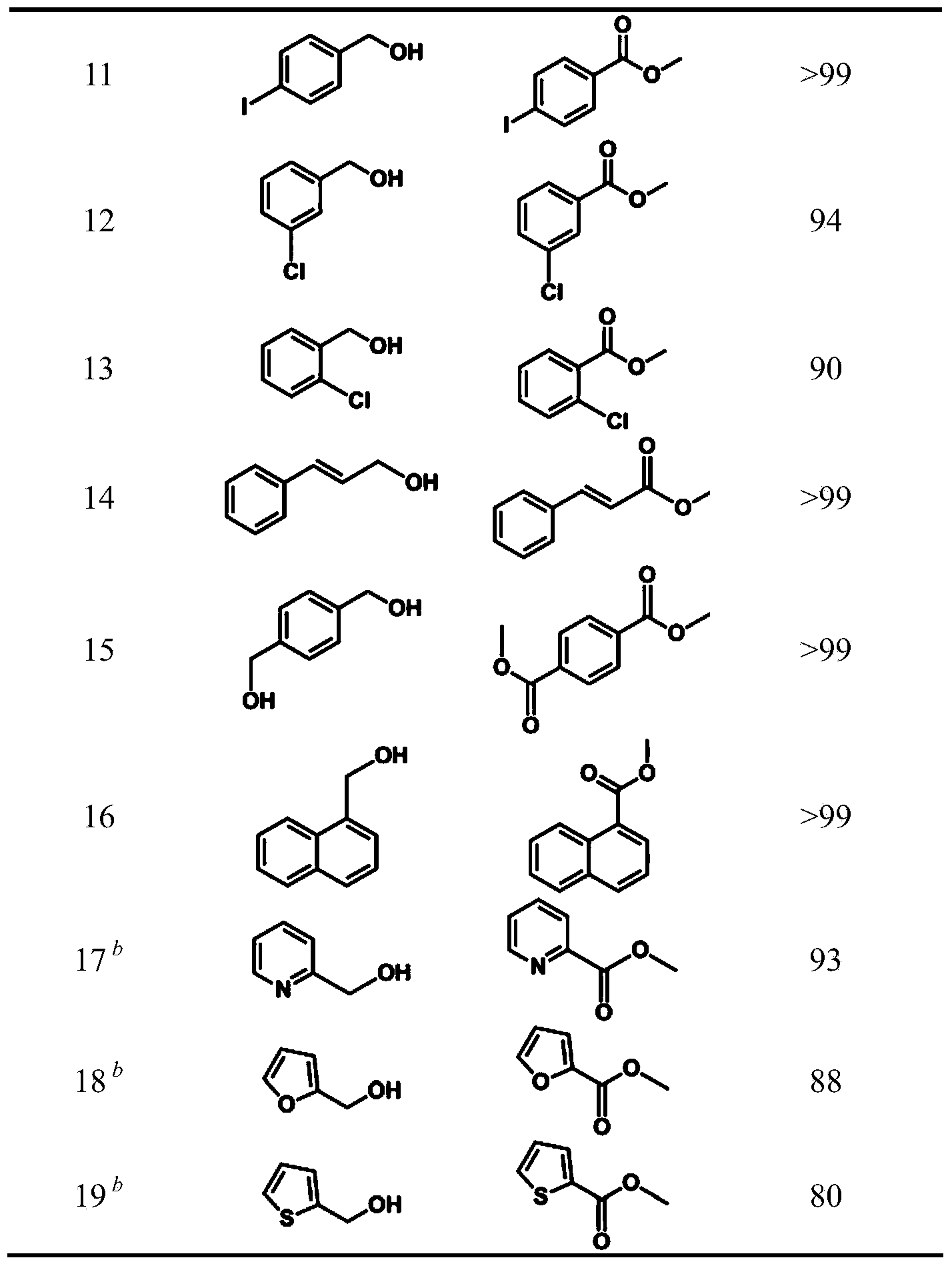
Alcohol oxidation is an important organic reaction. Primary alcohols (R-CH₂-OH) can be oxidized either to aldehydes (R-CHO) or to carboxylic acids (R-CO₂H), while the oxidation of secondary alcohols (R¹R²CH-OH) normally terminates at the ketone (R¹R²C=O) stage. Tertiary alcohols (R¹R²R³C-OH) are resistant to oxidation.
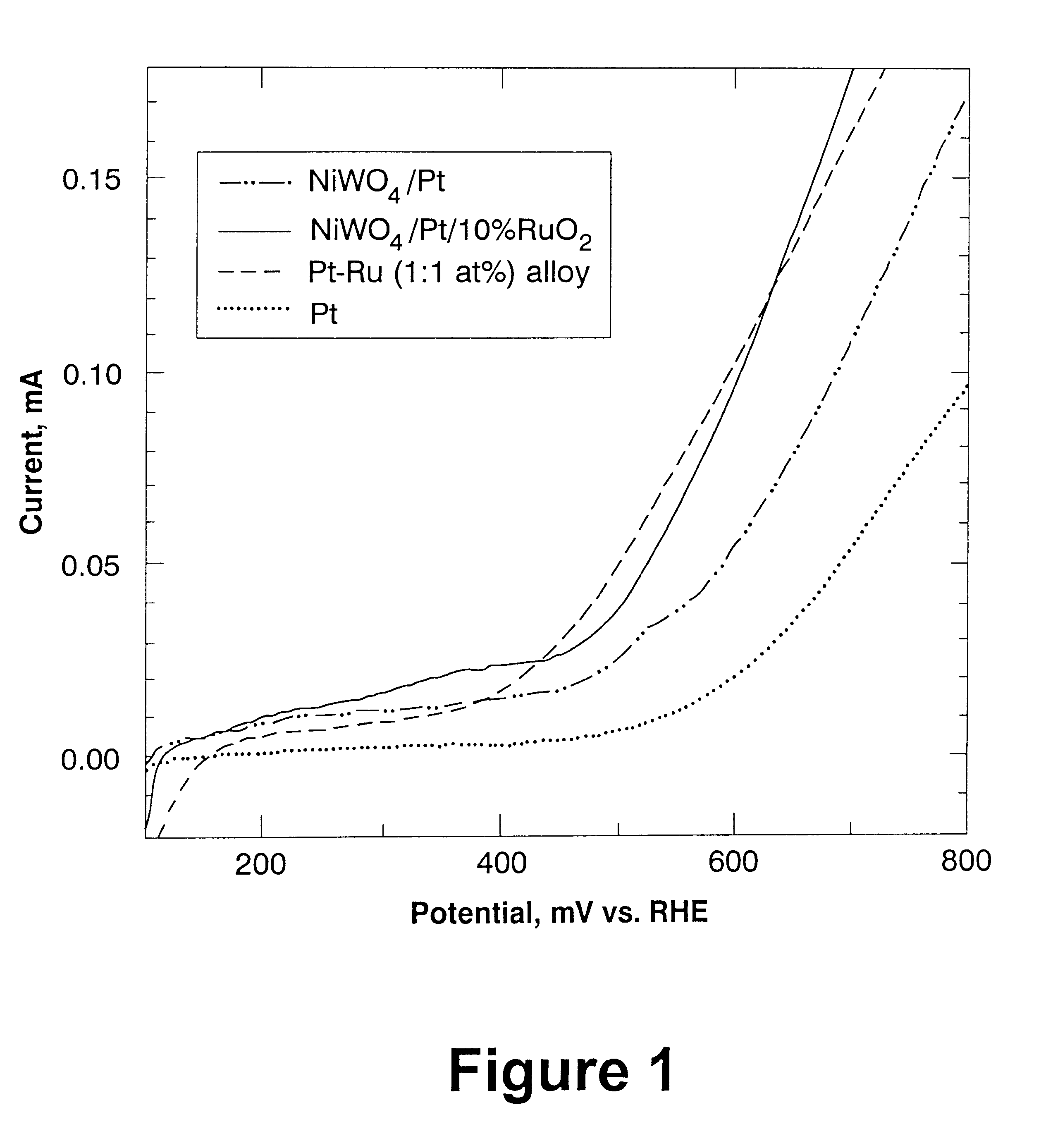
Electrocatalyst for alcohol oxidation in fuel cells
InactiveUS6183894B1Low costImprove stabilityElectrolysis componentsBreech mechanismsPtru catalystFuel cells
Binary and ternary electrocatalysts are provided for oxidizing alcohol in a fuel cell. The binary electrocatalyst includes 1) a substrate selected from the group consisting of NiWO4 or CoWO4 or a combination thereof, and 2) Group VIII noble metal catalyst supported on the substrate. The ternary electrocatalyst includes 1) a substrate as described above, and 2) a catalyst comprising Group VIII noble metal, and ruthenium oxide or molybdenum oxide or a combination thereof, said catalyst being supported on said substrate.
Owner:BROOKHAVEN SCI ASSOCS
Preparation method of benzaldehydes compound and novel double-metal catalyst loaded by mesoporous carbon for preparation method
ActiveCN102513104AHigh catalytic activityGood synergyCatalyst carriersOrganic compound preparationBenzeneBenzaldehyde
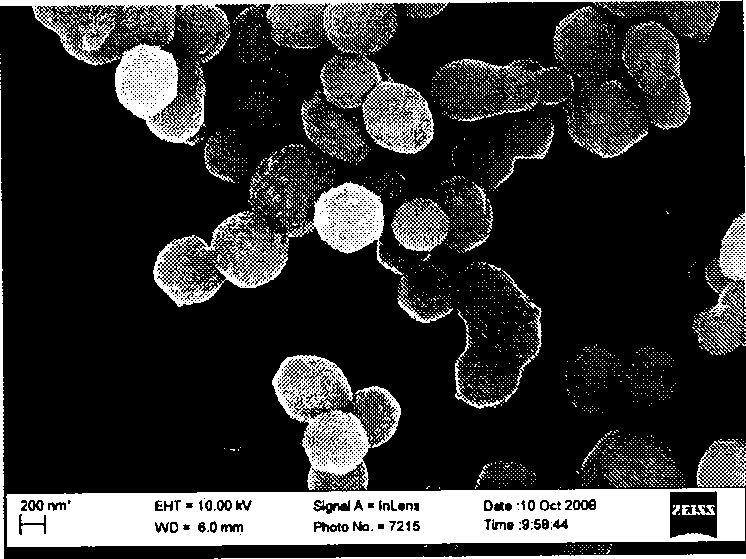
The invention relates to a preparation method of a benzaldehydes compound and a catalyst for the preparation method. The catalyst is composed of 0.01-90 wt% of metal grains and 10-99.99 wt% of mesoporous carbon carrier; the metal grains are selected from any two of Pd, Au, Ag, Pt, Ru, Rh, Ni, Cu, Fe, Co, Cr, W, Mo, Ti and Ta; the weight ratio of two types of the metal is 1: (0.01-100) and the average grain diameter of the metal grains is 1-100 nm; and the mesoporous carbon carrier is prepared from a heteroatom-doped mesoporous carbon material. The content of heteroatoms in the mesoporous carbon material is 0.01-80 wt%. The catalyst provided by the invention is stable for water, air and heat and has an excellent catalytic activity; particularly, the catalyst has a high selectivity when being used for catalyzing an alcohol oxidation reaction to prepare aldehyde or ketone. The preparation method of the benzaldehydes compound provided by the invention has the advantages of high conversion rate of raw materials and good selectivity of a target product.
Owner:ZHEJIANG UNIV
Polymer photocatalyst, and method of water-phase photo-catalytic selective alcohol oxidation
InactiveCN103127948AImprove conversion rateGood choicePhysical/chemical process catalystsOrganic compound preparationPtru catalystUltraviolet
The invention discloses a polymer photocatalyst and a method of water-phase photo-catalytic selective alcohol oxidation. According to the invention, an organic polymer carbon nitride catalyst is prepared, and is applied in a water-phase photo-catalytic selective alcohol oxidation system. Compared with common ultraviolet catalyst TiO2, visible light catalyst TiO2-xNx, metal sulfide, metal nitride, metal nitrogen oxides, and the like, when alcohol is oxidized in water phase with a green oxidant air / oxygen under mild conditions, the carbon nitride catalyst is more stable and shows higher efficiency. The preparation method of the carbon nitride catalyst is simple and feasible, and the catalyst comprises no metal element, such that the catalyst is cheap. With the catalyst, alcohol oxidation aldehyde synthesis or ketone synthesis processes are simple and feasible, such that industrialized large-batch productions of aldehyde and ketone compounds can be facilitated.
Owner:FUZHOU UNIV
Noble-metal/composite metal oxide/ carbon nanometer tubular electro-catalyst and preparation method and application
InactiveCN101814604AUniform particle sizeHigh crystallinityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsAlcohol fuelCarbon nanotube
The invention provides a noble-metal / composite metal oxide / carbon nanometer tubular electro-catalyst and a preparation method and an application thereof, which belong to the technical field of the nanometer composite material, and are characterized in that: by utilizing the controllability of the hydrotalcite slab composition and the designability of the structure, Pt2+, Pd2+ and Ni2+, Co2+, Mg2+, Al3+ and Fe3+ ions are introduced into the slab so as to synthesize a layered dual-metal hydroxide precursor containing the noble metal elements. The Pt2+ and Pd2+ can be highly scattered and uniformly distributed on the molecule level, and the Pt2+ and Pd2+ can be used as a catalyst after being reduced to catalyze the growth of the carbon nanometer tube loading the noble metal particles and the composite electro-catalyst which is highly doped with the composite metal oxide. The method not only can effectively disperse the noble metal catalyst and controls the growth of the noble metal catalyst on the carbon nanometer tube and the composite metal oxide, but also strengthens the electro-catalyzing property of the noble metal which is loaded in the carbon nanometer tube and the composite metal oxide network matrix. The electro-catalyst is used as an electrode in the alcohol fuel battery, the specific activity of the electro-catalyst on the maximum peak value of the alcohol oxidation can reach 120 to 200 mA.mg-1. The preparation method realizes the integration, has simple operation, is free from the environmental pollution, and is applicable to the industrialization process.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing mesoporous titanium silicate molecular sieve
InactiveCN101456562ALarge specific surface areaHigh porosityCrystalline aluminosilicate zeolitesFiltrationMesoporous material
The invention relates to a method for preparing mesoporous titanium silicon molecular sieves, which belongs to the technical field of preparation of mesoporous molecular sieve catalytic materials. The method comprises: adopting a cationic surfactant as a template agent, dispersing the cationic surfactant into deionized water, adding ammonia water to adjust the pH value of the solution, and adding silicon sources after the template agent is completely dissolved; making the materials undergo filtration, washing, drying and roasting, removing the organic template agent, and obtaining silicon dioxide mesoporous materials; and dispersing the obtained silicon dioxide mesoporous materials into ethanol, slowly adding titanium sources, loading titanium ions after hydrolysis of the titanium sources on the silicon dioxide mesoporous molecular sieves to form an active center, and obtaining titanium-loaded mesoporous molecular sieves. The reaction is easy to control, and the product has good repeatability and is suitable for mass production. By adoption of the prepared titanium silicon molecular sieves as a catalyst and hydrogen peroxide as an oxygen source to catalyze olefin epoxidation and alcohol oxidation, the reaction conditions are mild and the reaction has good selectivity and conversion rate.
Owner:UNIV OF SCI & TECH BEIJING
Method for preparing aldehyde or alkone by oxygen catalysis and alcohol oxidation under mild condition
InactiveCN101565344AEasy to separateWide substrate applicabilityOrganic oxidationOrganic compound preparationNitrateHalogen
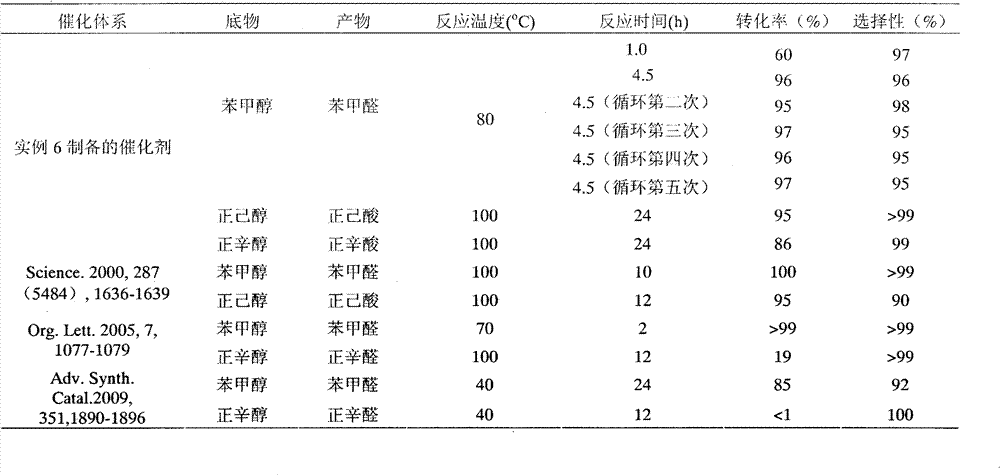
The invention relates to a preparation method for aldehyde or alkone, in particular to a method for preparing aldehyde or alkone by oxygen catalysis and alcohol oxidation under the mild condition. Themethod comprises the following steps: according to the 5 mmol of reaction substrate, 1-8 percent of 2,2,6,6-tetramethylpiperidine-oxygen radical (TEMPO) or a derivative thereof, 4-20 percent of halogen-containing compound and 4-20 percent of nitric acid or nitrate are taken as the catalysts, 0.1-0.8 MPa oxygen or air is taken as the oxidant, then the reaction is carried out for 1-36 h at the temperature of 0-80 DEG C, a series of alcohols can be oxidized into aldehyde or alkone with high selectivity, a catalyst TEMPO and the derivative thereof can be circularly and alternately used, the turn-over number (TON) is up to 800 and the cost is greatly reduced. The invention has the advantages of safer reagent with lower price, wider applicability of the substrate, mild reaction conditions, convenient product separation, no pollution to the environment, easy industrialization, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Small-molecular alcohol oxidation electro-catalysis material and preparation method and application therefor
ActiveCN105406088AAvoid reunionUniform growthMaterial nanotechnologyCell electrodesAlcohol fuelAlloy catalyst
The invention discloses a small-molecular alcohol oxidation electro-catalysis material and a preparation method and an application therefor. The small-molecular alcohol oxidation electro-catalysis material is a noble metal-metal hydroxide-carbon material ternary composite system and prepared by two steps; firstly, a carbon material and a metal salt solution are used as the raw materials to be subjected to a first time of reaction to grow metal hydroxide particles on the surface of the carbon material; then, the noble metal salt is taken as the raw material to be subjected to a second time of reaction to grow noble metal particles on the surface of the carbon material loaded with the metal hydroxide particles to obtain the noble metal-metal hydroxide-carbon material ternary composite system. When the small-molecular alcohol oxidation electro-catalysis material is used as the positive electrode catalyst of a direct alcohol fuel cell, efficient and stable alcohol electro-catalysis oxidation is realized, and an outstanding anti-CO-poisoning capability is achieved as well; and in addition, the small-molecular alcohol oxidation electro-catalysis material is lower in cost compared with the commercialized Pt-Ru alloy catalyst.
Owner:SUZHOU UNIV
Cobalt-based catalyst for generating ester by alcohol oxidation, and preparation method and application of cobalt-based catalyst
ActiveCN104069883AMagneticEasy to operatePhysical/chemical process catalystsOrganic compound preparationNitrogenCobalt
The invention belongs to the technical field of liquid phase oxidation, and discloses a cobalt-based catalyst for generating ester by alcohol oxidation, and a preparation method and application of the cobalt-based catalyst. The cobalt-based catalyst mainly comprises the following elements in percentage by weight: 27.0-50.0 percent of cobalt, 45.0-60.0 percent of carbon and 0.5-20.0 percent of nitrogen, wherein the particle diameter of cobalt nano particles in the cobalt-based catalyst is 5-40 nm. The cobalt-based catalyst is prepared in a pyrolysis mode in an inert atmosphere with ZIF-67 as a precursor. In the presence of the cobalt-based catalyst, alkaline aids are not added in the normal-temperature and normal-pressure atmospheric environment, and two alcohols react to prepare ester compounds. The cobalt-based catalyst is easy to synthesize in quantity, and is magnetic and recyclable. A method for catalyzing alcohol oxidation to generate ester in the presence of the cobalt-based catalyst has the advantages of low cost, simplicity in operation, environment friendliness, mild reaction condition, easiness in product separation and the like.
Owner:SOUTH CHINA UNIV OF TECH
Preparation of loaded bimetallic nano-catalyst
InactiveCN102773097AHigh catalytic activityStrong alkalineOrganic compound preparationCarbonyl compound preparationNano catalystNanoparticle
The invention relates to a kind of Mg-Al hydrotalcite loaded bimetallic nano-catalyst and its preparation method, and relates to application of the catalyst system in oxidation of alcohol into corresponding carbonyl compounds. The catalyst preparation method consists of: 1) preparing an Mg-Al hydrotalcite carrier through a urea decomposition method; 2) loading the precursors of metal Pd and Au on the Mg-Al hydrotalcite surface; and 3) then reducing Pd (II) and Au (III) through NaBH4, thus obtaining the bimetallic nanometer particle catalyst. The catalyst provided in the invention has the advantages of high catalytic activity, good stability, separability, and easy recovery, thus being applicable to alcohol oxidation reactions in aqueous phases.
Owner:EAST CHINA UNIV OF SCI & TECH
Alcohol oxidation catalyst and its preparation process
InactiveUS20080221331A1Higher catalytic turnoverLow costOrganic compound preparationCarbonyl compound preparationNonaneNitrogen
Owner:NISSAN CHEM IND LTD
Alcohol Oxidation Catalyst and Method of Synthesizing the Same
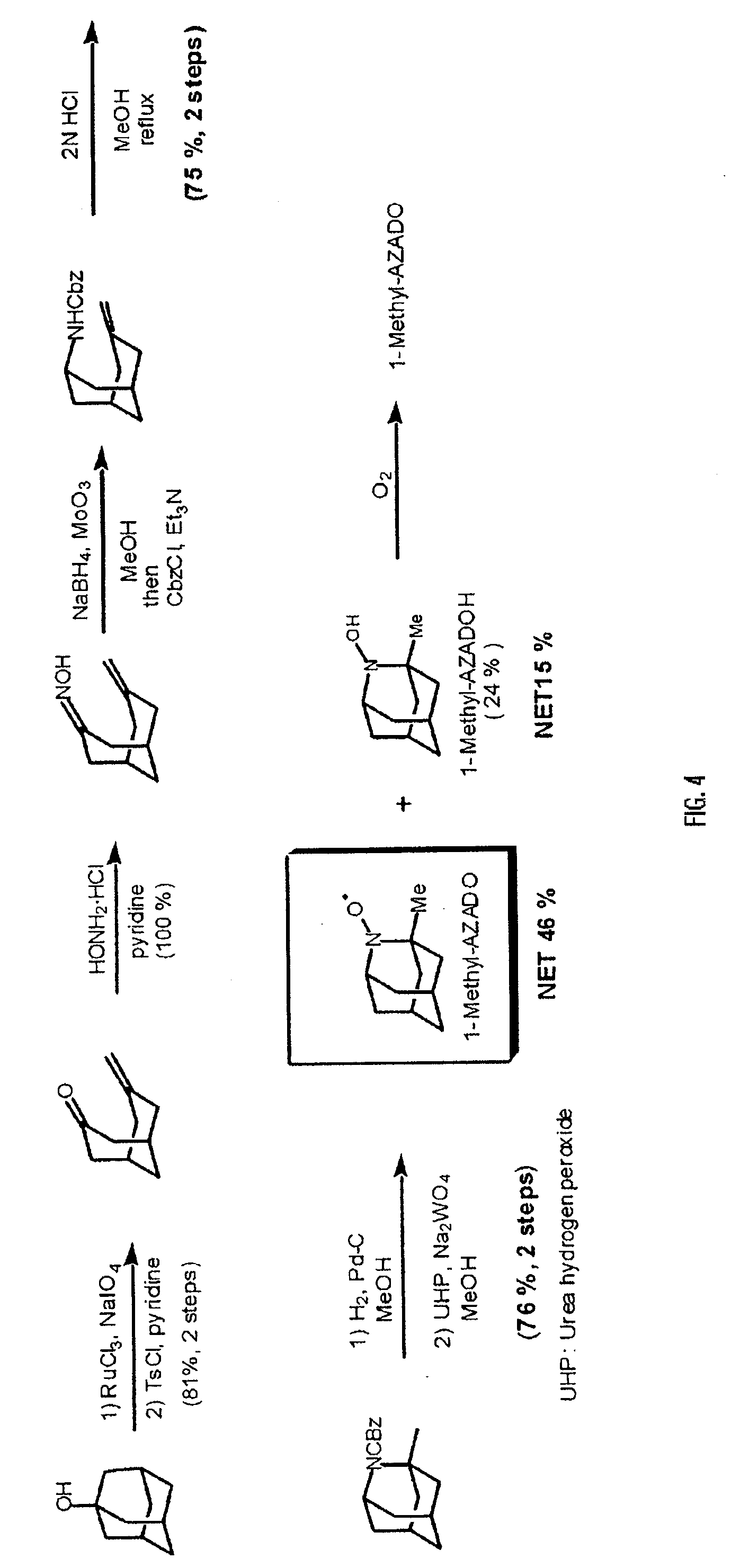
InactiveUS20070232838A1Small loadSimple structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsReaction fieldHydrogen
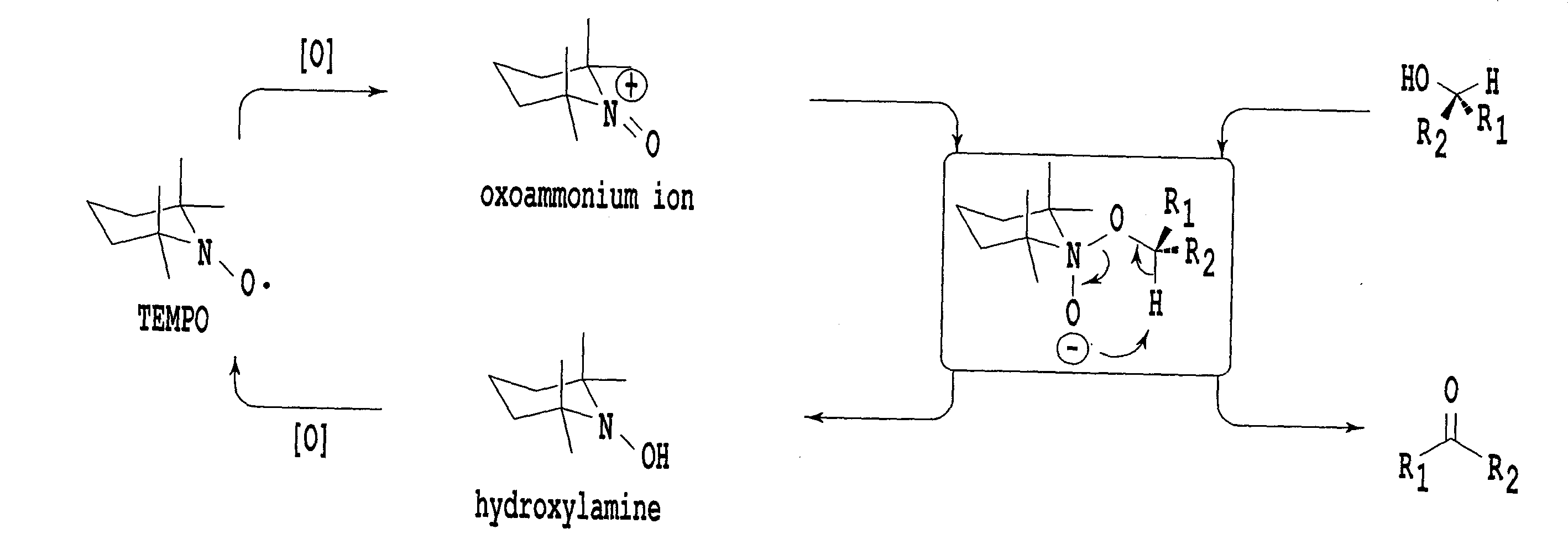
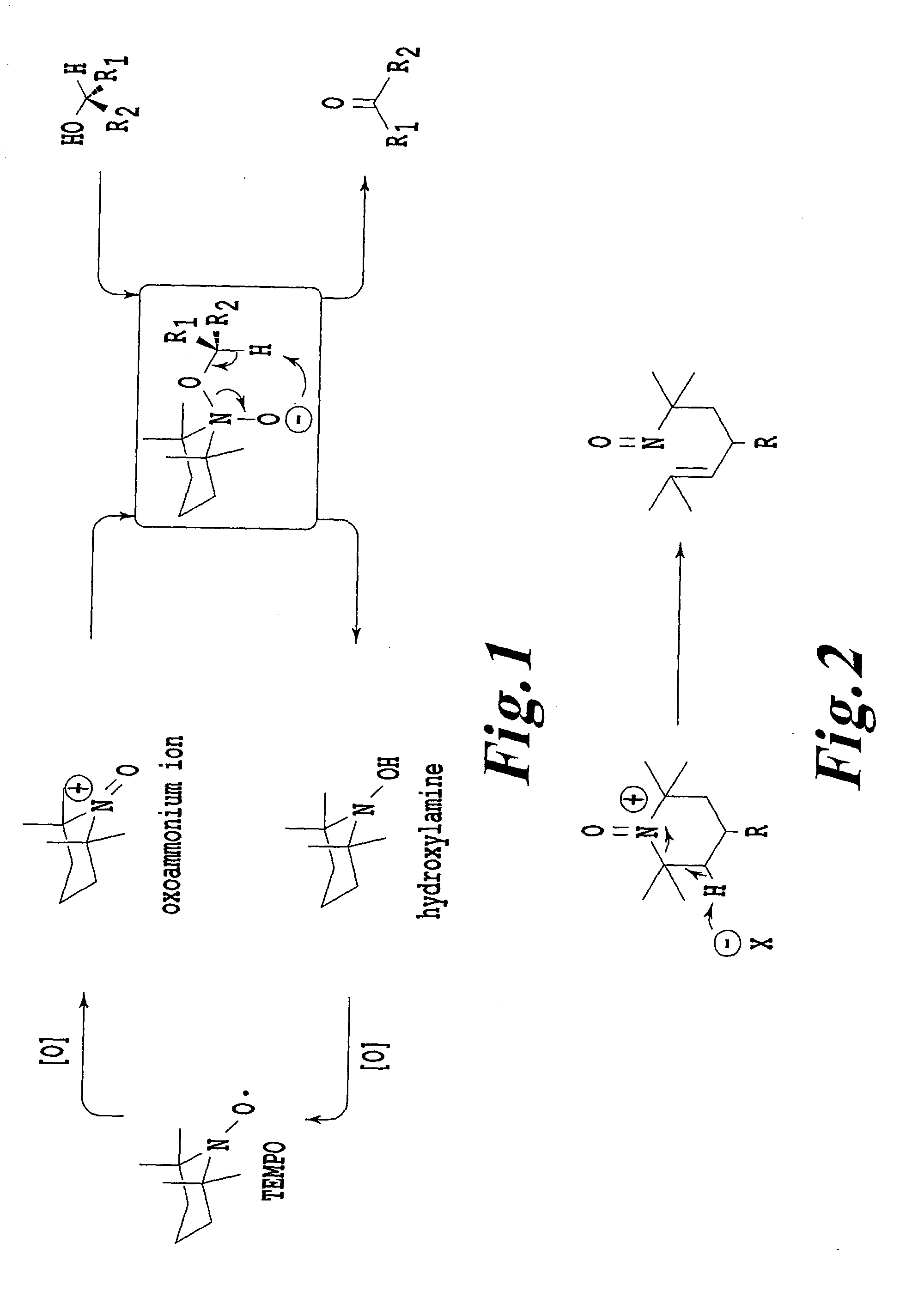
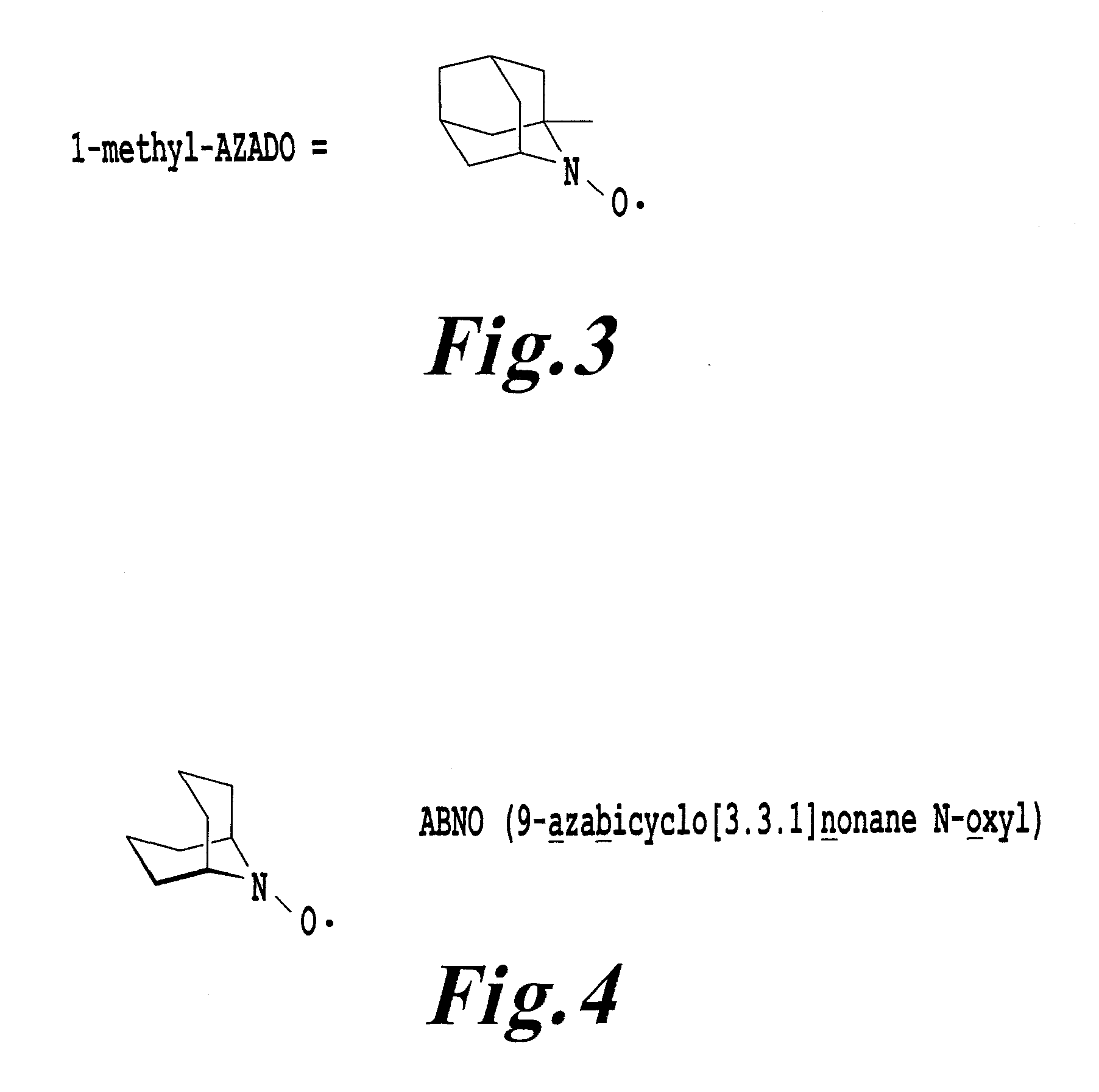
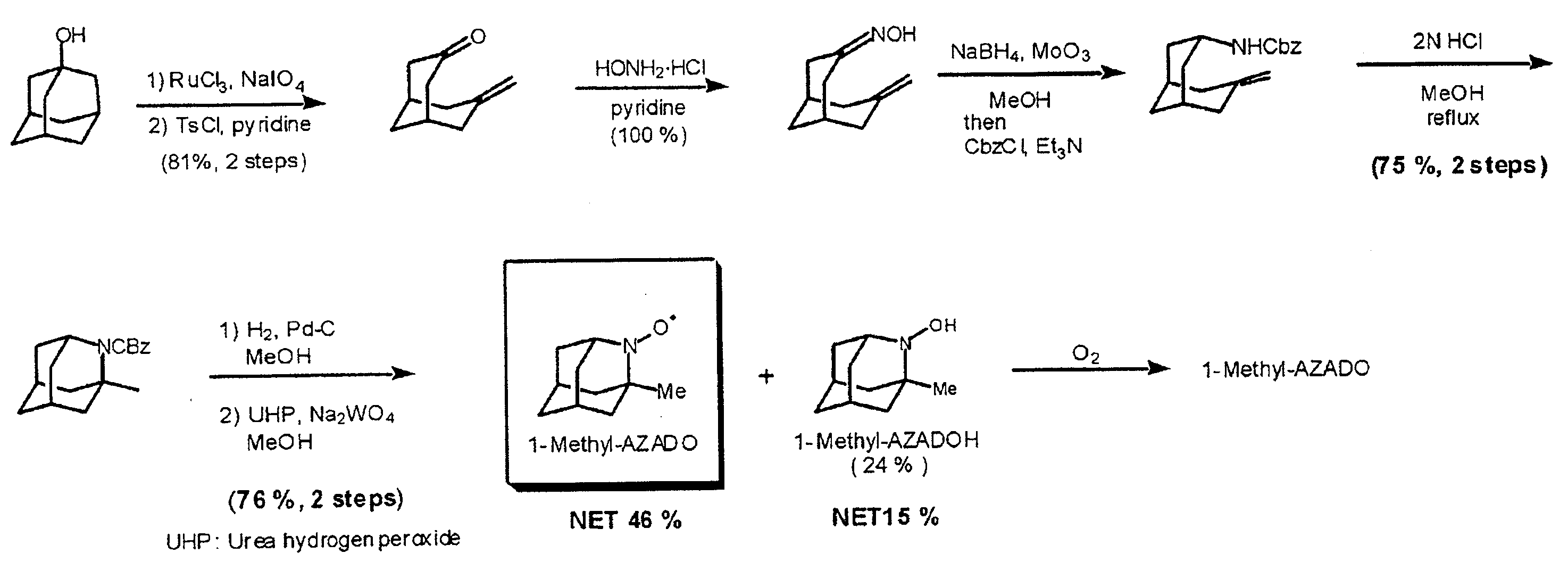
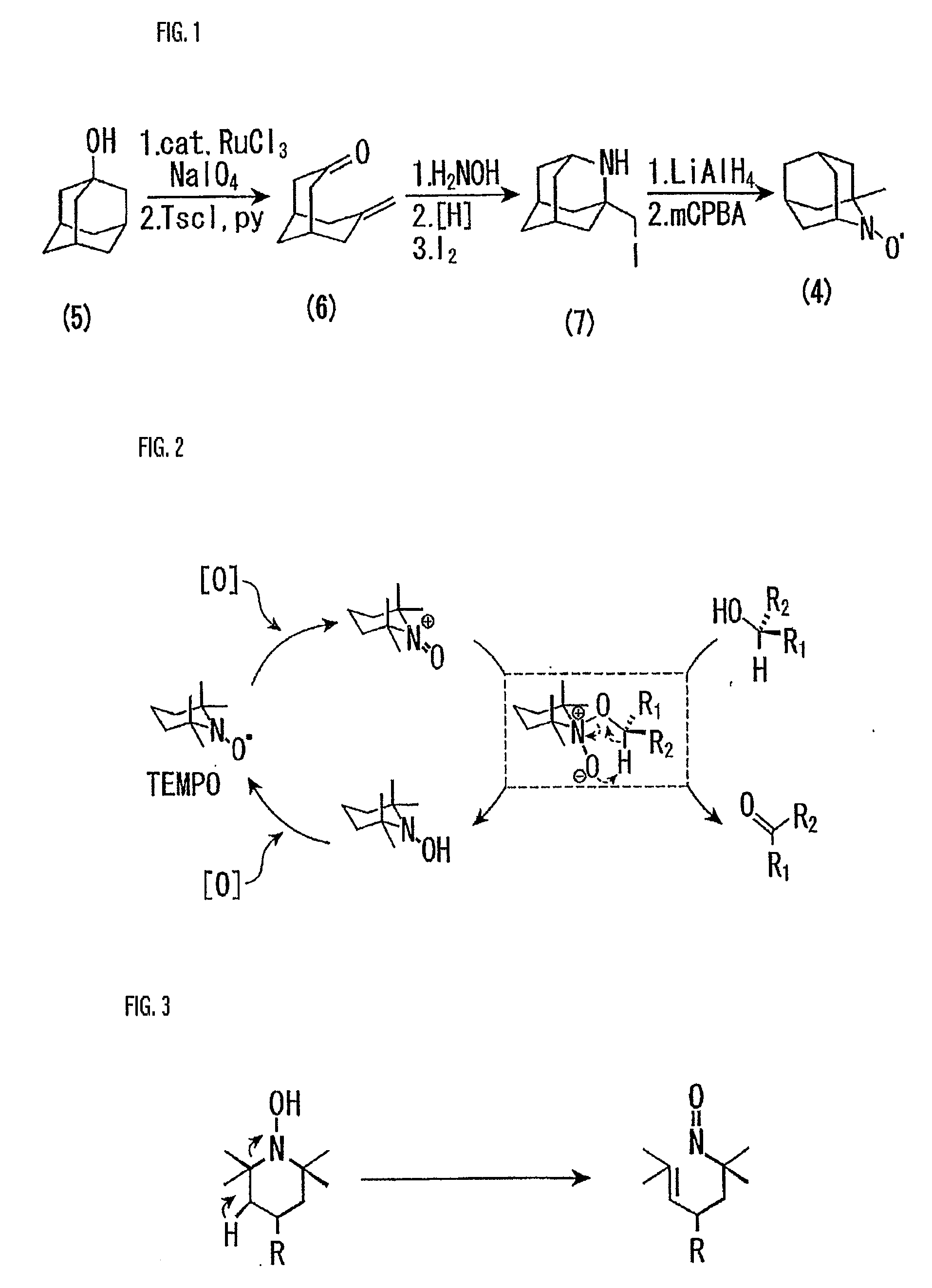
An organic oxidation catalyst for alcohols which is environmentally less harmful and with which efficient oxidation can be conducted. The oxidation catalyst for alcohols is a 1-alkyl-2-azadamantan-N-oxyl which has a nitroxyl group incorporated in the adamantane skeleton and was synthesized from as a base material a bicyclic compound obtained by the Grob-type ring-opening reaction of 1,3-adamantanediol. Due to the nitroxyl group on the adamantane skeleton, the α-position hydrogen is stabilized based on Bredt's rule and the stability of the oxoammonium group generated by the oxidation thereof is ensured. Compared to TEMPO, which is a conventional oxidation catalyst, this catalyst is reduced in steric hindrance and is usable in a wide range of reaction fields. Because of this, not only a primary alcohol but a secondary alcohol having a sterically complicated structure, which has been difficult to oxidize with TEMPO, can be oxidized at a high efficiency.
Owner:NISSAN CHEM IND LTD
Non-noble metal homogeneous catalysis system for alcohol oxidation carbonylation and using method thereof
InactiveCN101856625AHigh activityHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical industryPtru catalyst
The invention belongs to the field of chemical industry and discloses a non-noble metal homogeneous catalysis system for alcohol oxidation carbonylation and a using method thereof. The system consists of a composite catalyst MNC and a solvent NS (IV) which dissolves the composite catalyst, wherein the composite catalyst MNC is compounded by a metal complex MXn-Lm (I), a metal salt synergistic aid N (II) and / or an alkaline aid C (III). The general formula of the system is I+II+III+IV, I+II+IV, or I+III+IV. The catalyst has the advantages of simple process, mild reaction, high catalytic activity, good selectivity and stability, and low corrosion on production equipment. Meanwhile, the homogeneous system formed by the composite catalyst and the solvent can perform liquid phase circulation and has industrial application prospects.
Owner:NANJING UNIV OF TECH
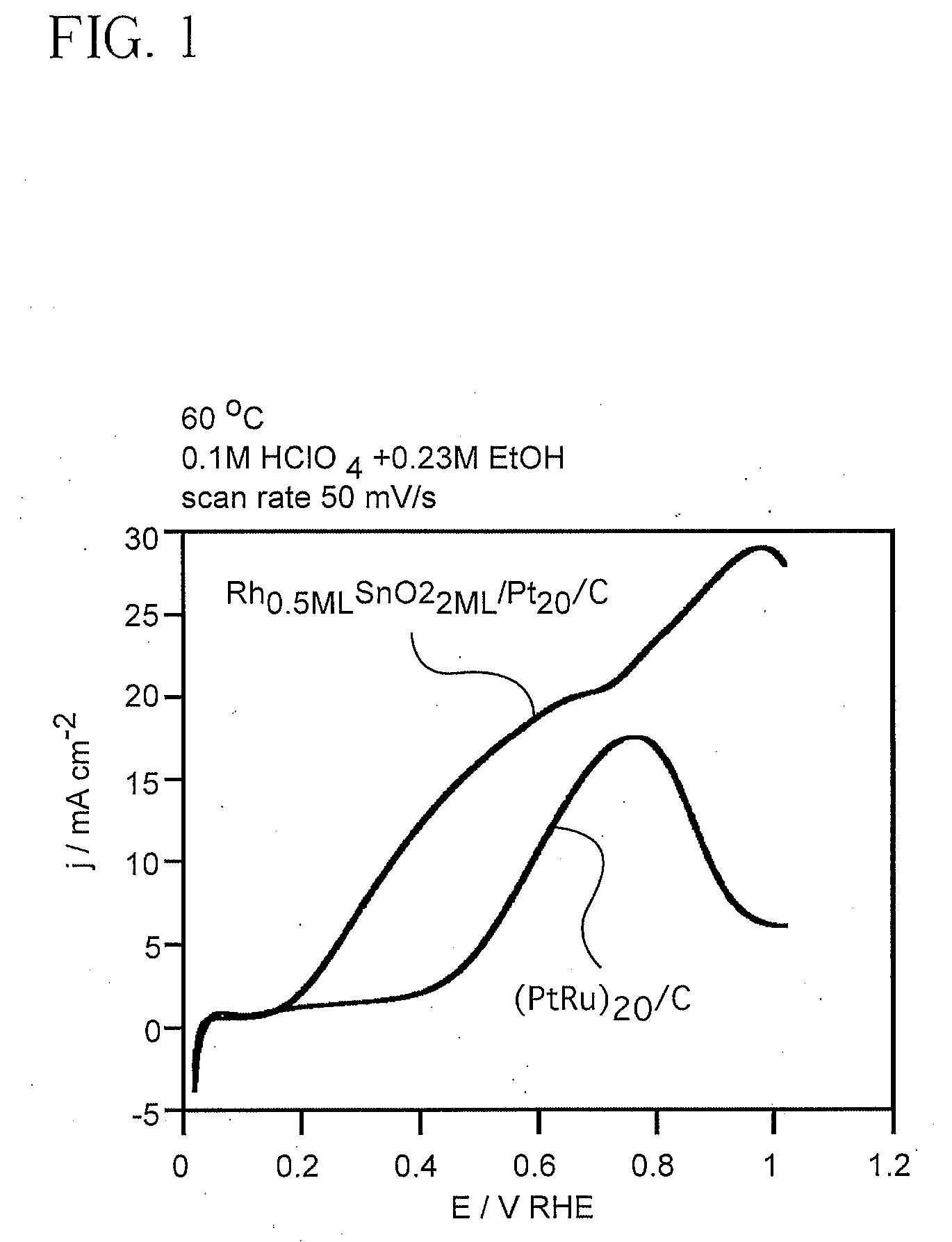
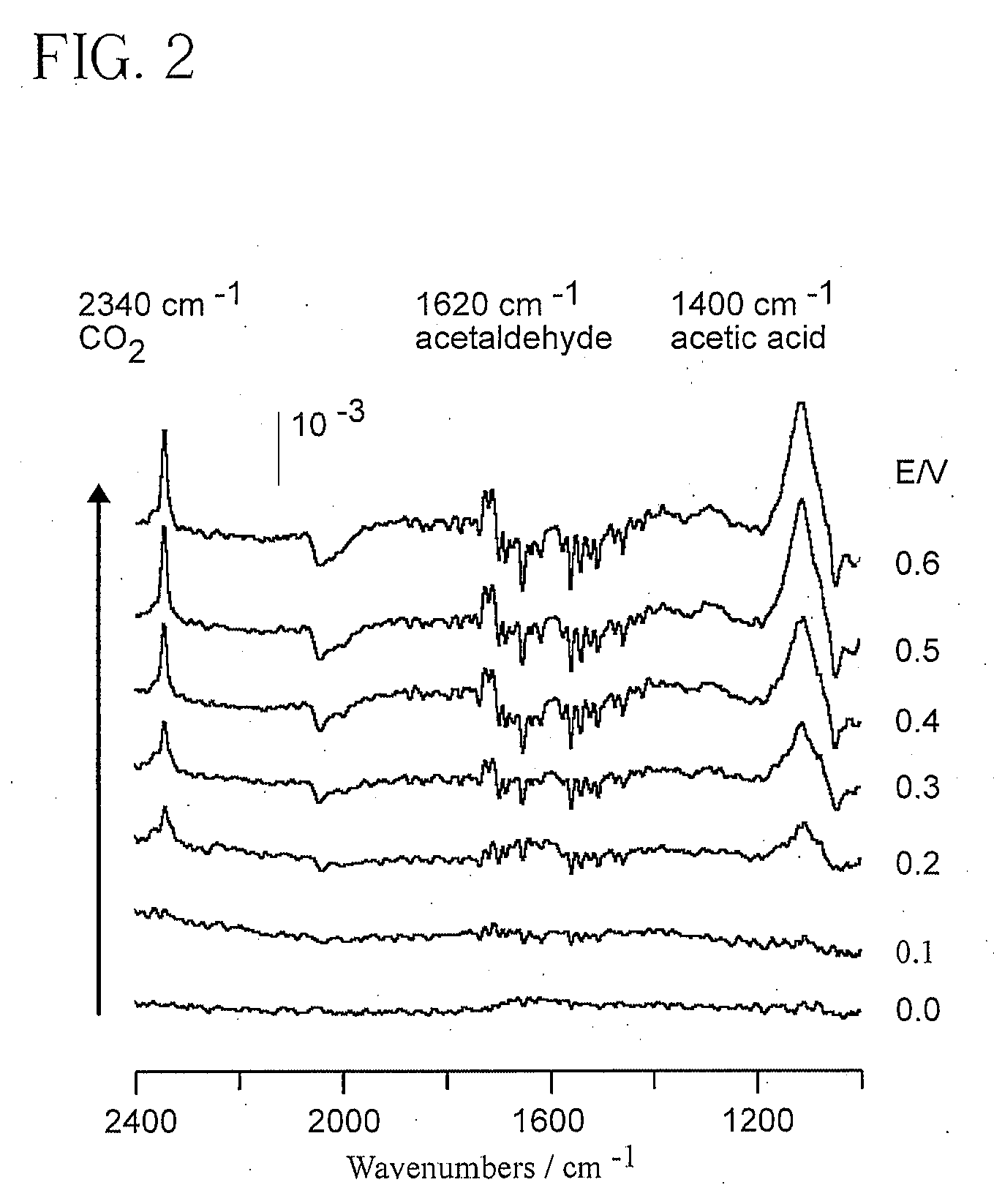
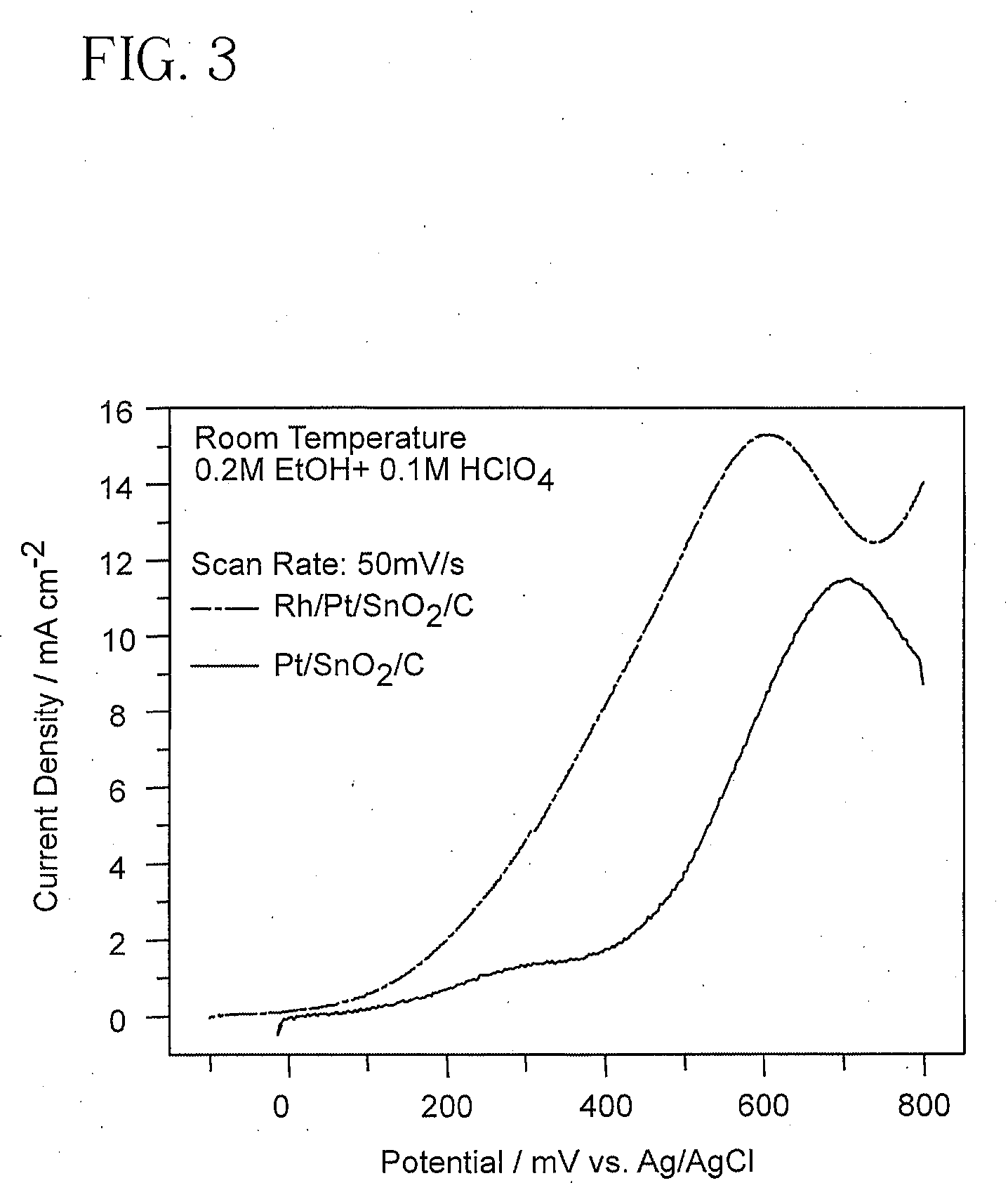
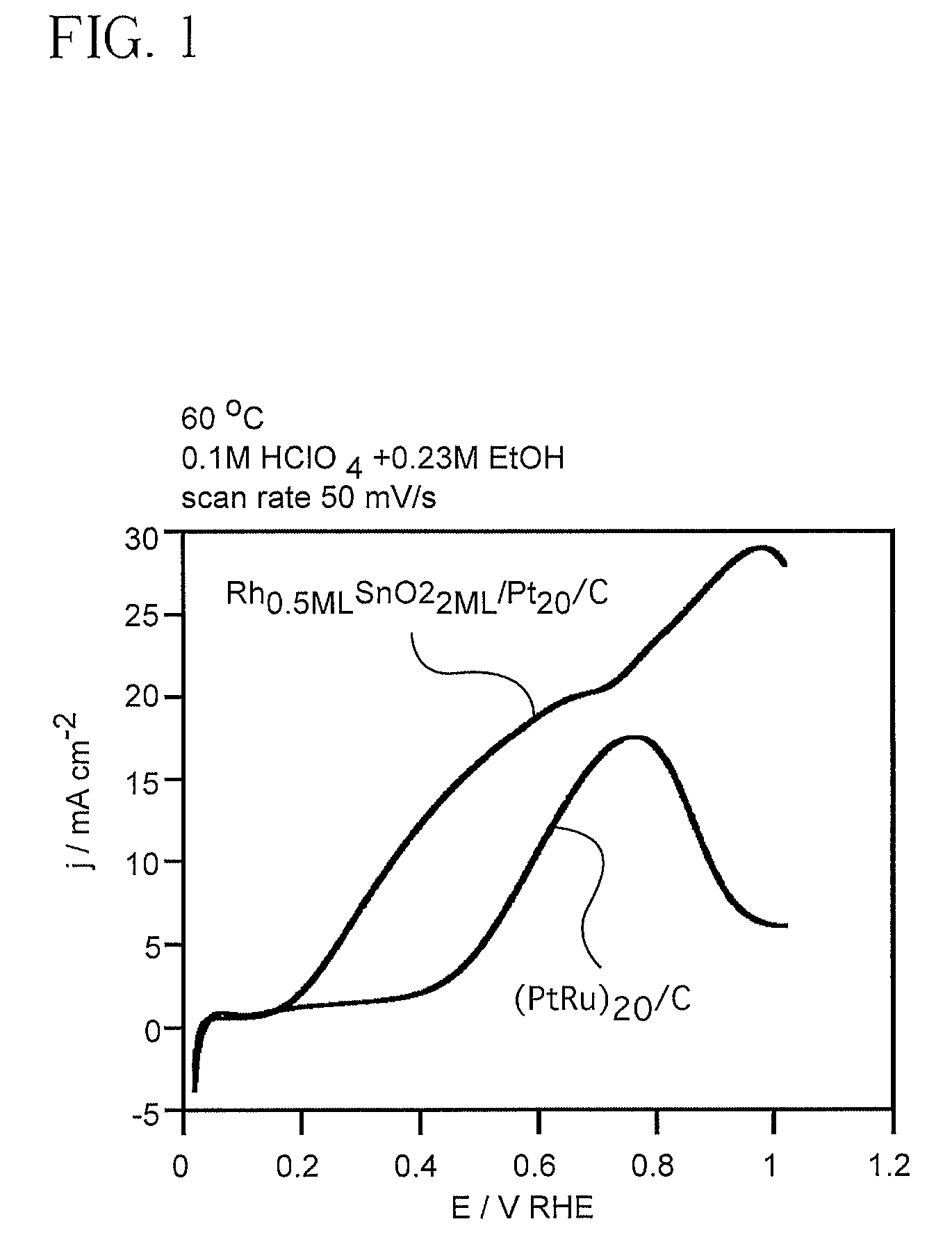
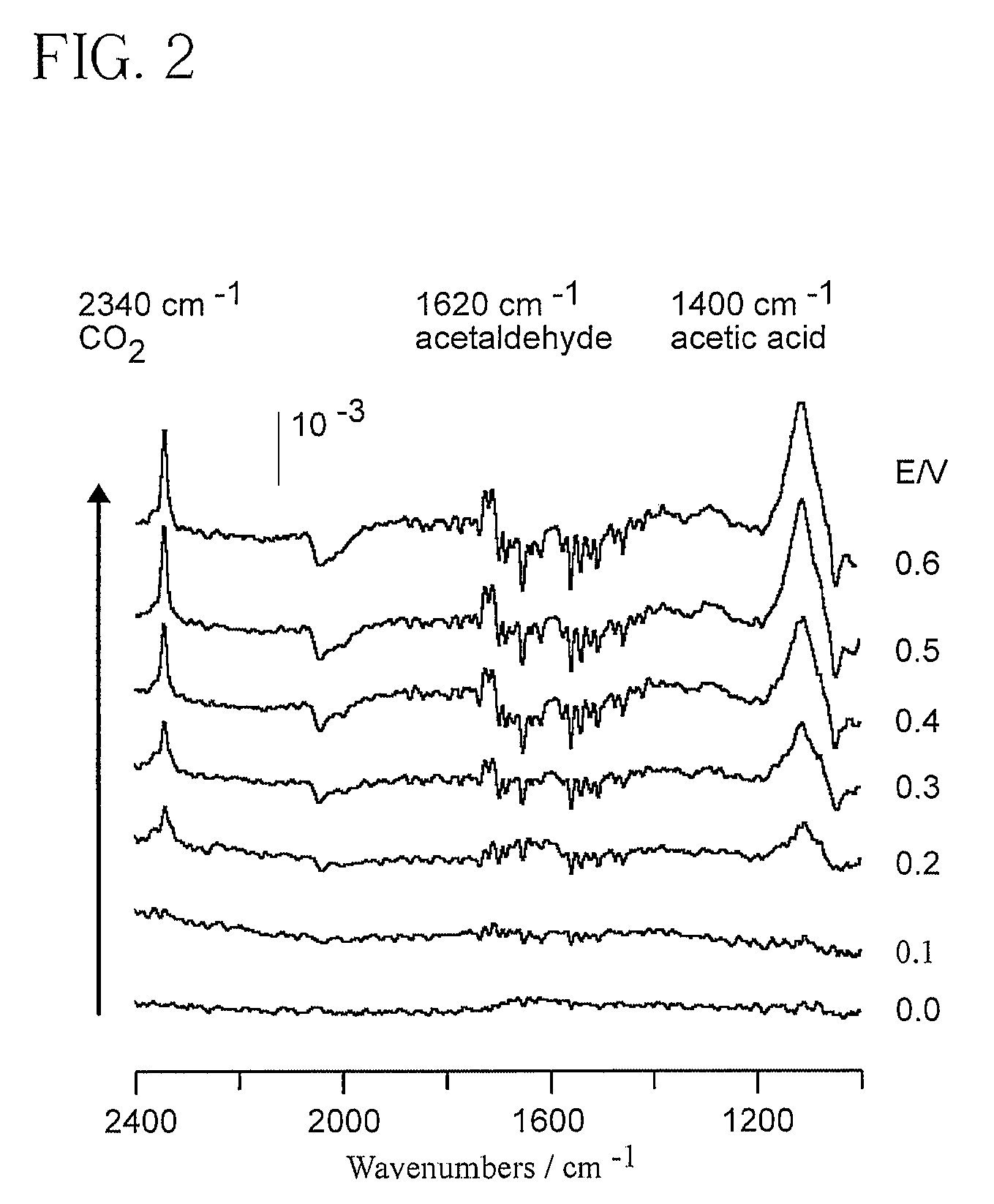
Electrocatalyst for Alcohol Oxidation at Fuel Cell Anodes
InactiveUS20090068505A1Promote oxidationImprove abilitiesPhysical/chemical process catalystsActive material electrodesPlatinumIridium
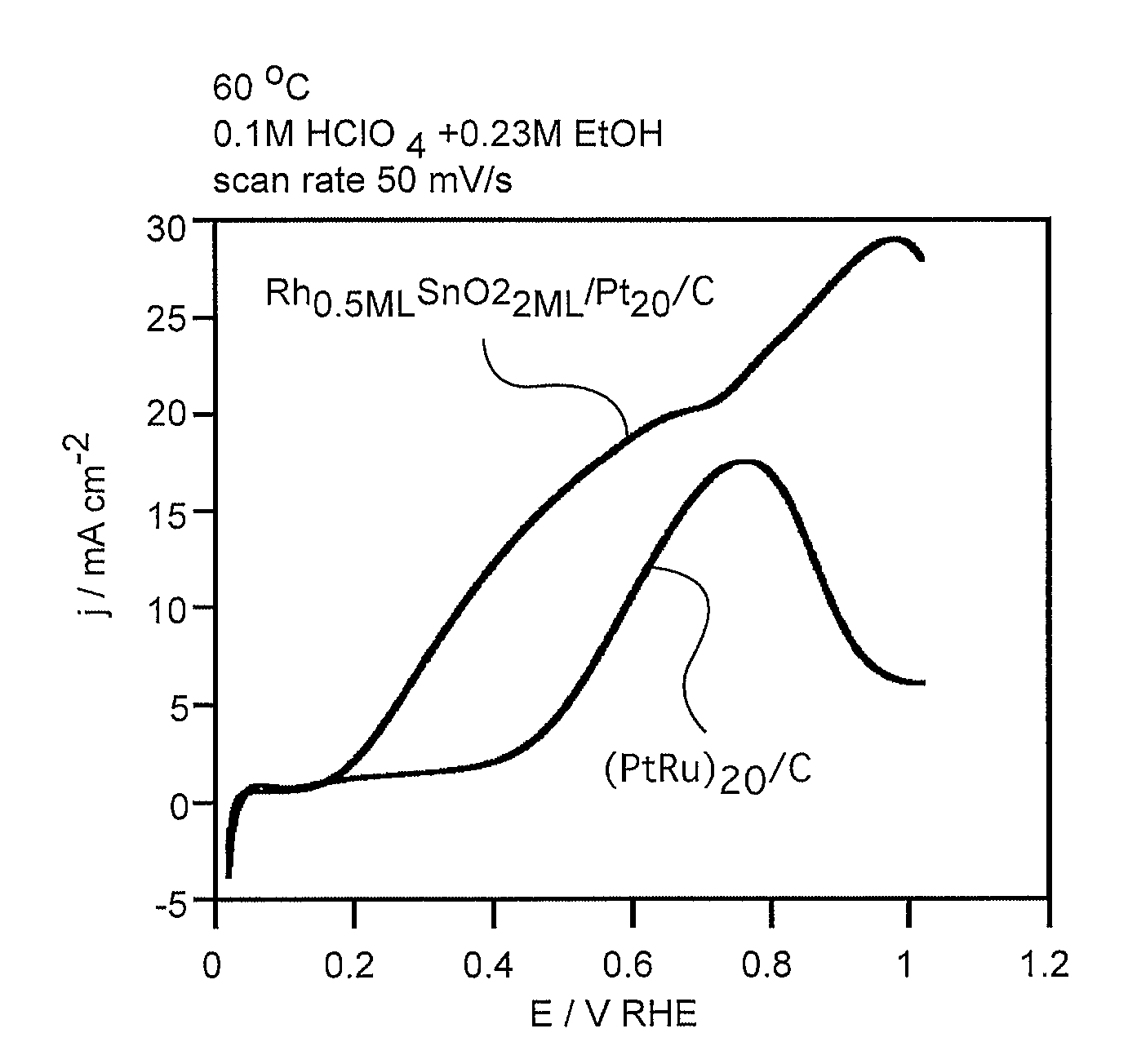
In some embodiments a ternary electrocatalyst is provided. The electrocatalyst can be used in an anode for oxidizing alcohol in a fuel cell. In some embodiments, the ternary electrocatalyst may include a noble metal particle having a surface decorated with clusters of SnO2 and Rh. The noble metal particles may include platinum, palladium, ruthenium, iridium, gold, and combinations thereof. In some embodiments, the ternary electrocatalyst includes SnO2 particles having a surface decorated with clusters of a noble metal and Rh. Some ternary electrocatalysts include noble metal particles with clusters of SnO2 and Rh at their surfaces. In some embodiments the electrocatalyst particle cores are nanoparticles. Some embodiments of the invention provide a fuel cell including an anode incorporating the ternary electrocatalyst. In some aspects a method of using ternary electrocatalysts of Pt, Rh, and SnO2 to oxidize an alcohol in a fuel cell is described.
Owner:BROOKHAVEN SCI ASSOCS
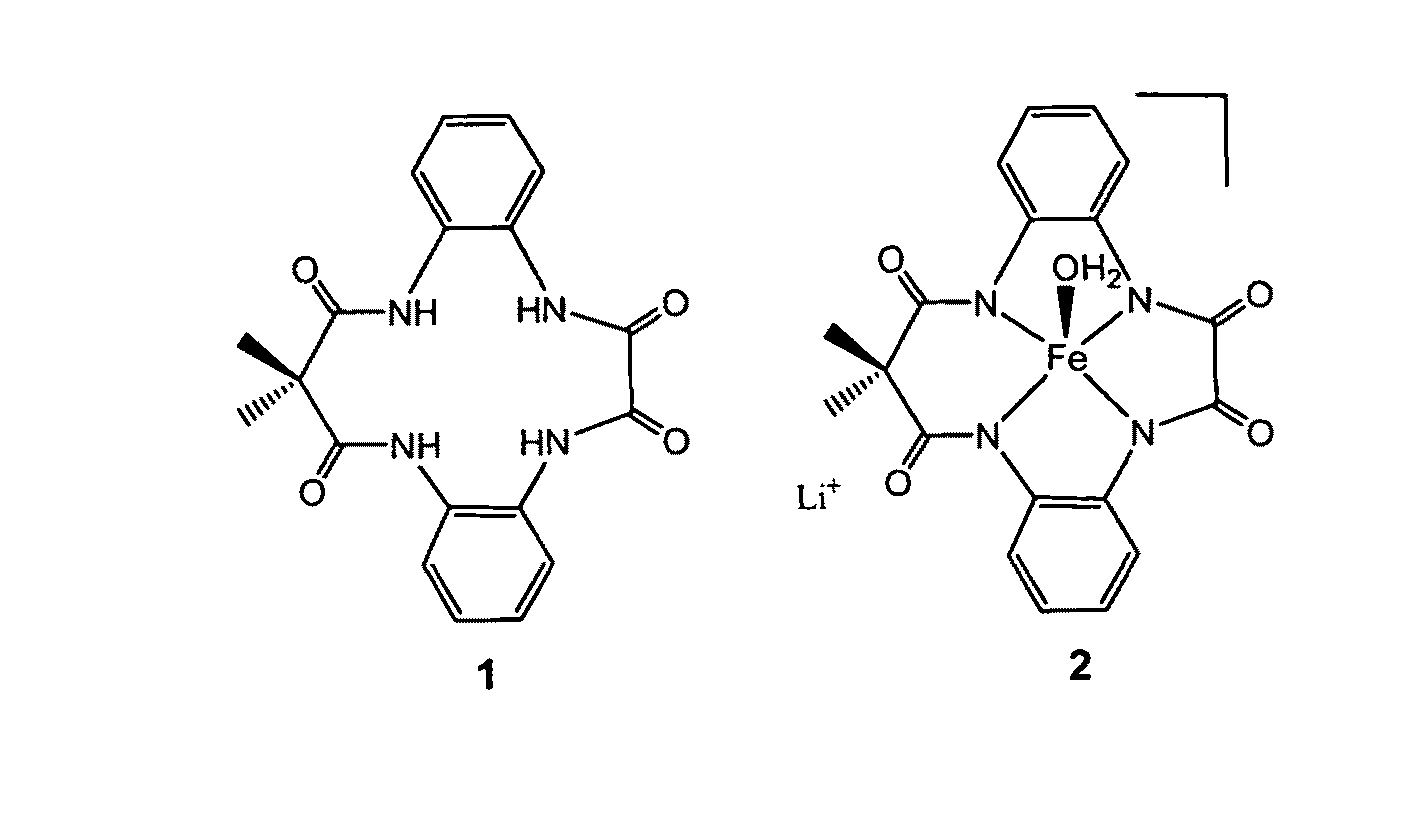
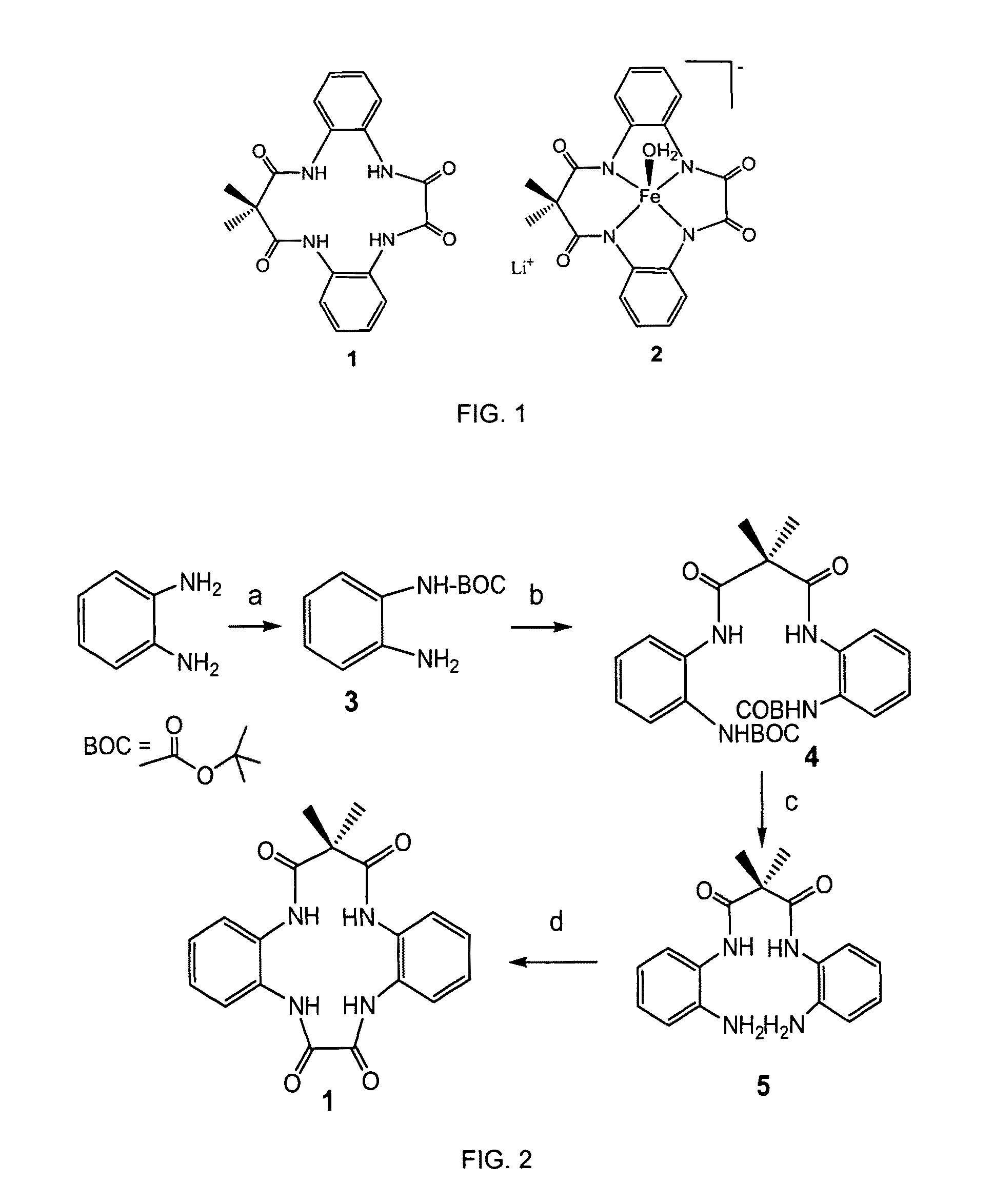
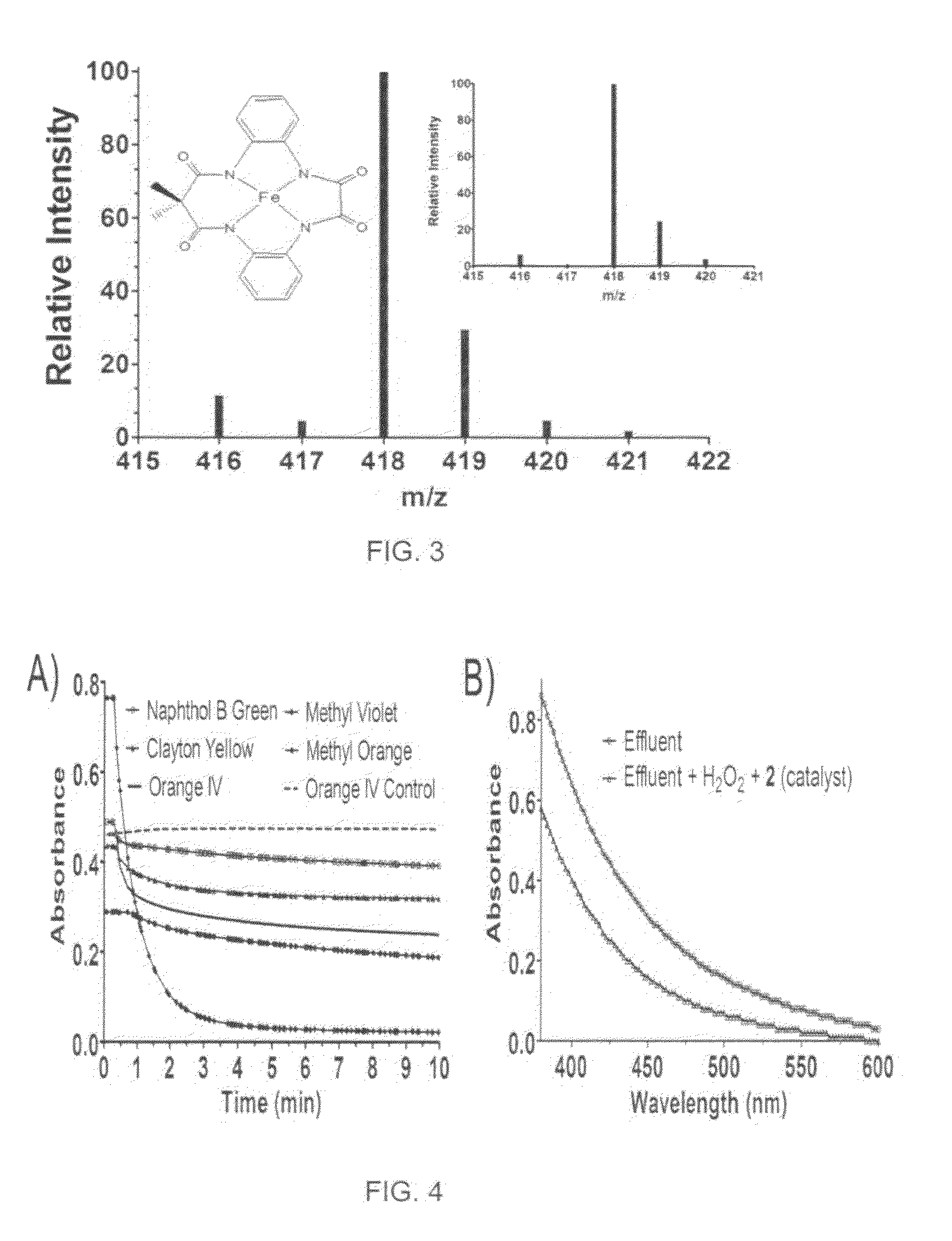
Method of synthesis of tetradentate amide macrocycle ligand and its metal-complex
ActiveUS8722881B2Efficient executionHigh yieldCellulosic pulp after-treatmentPulp liquor regenerationOrganic dyeMacrocyclic ligand
A tetradendate amide based macrocyclic ligand and its Fe(III) complex which act as activators of hydrogen peroxide. The synthetic methodology to develop the ligands is new, simple and provides better yield for each step of the ligand synthesis. The Fe(III)-complexes and hydrogen peroxide together are can perform several environmentally benign oxidation reactions. Organic dye bleaching, bleaching of pulp and paper effluent and N-oxide synthesis may be performed using the newly developed catalyst and hydrogen peroxide. Alcohol oxidation and alkene epoxidation may also be performed using the catalysts and hydrogen peroxide.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Alcohol oxidation aldehyde ketone production applied novel catalyst and preparation method thereof
ActiveCN106964404AHigh activityRich sourcesOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationHydrotalcite
The invention discloses an alcohol oxidation aldehyde ketone production applied novel catalyst and a preparation method thereof. According to the alcohol oxidation aldehyde ketone production applied novel catalyst, hydrotalcite-like compounds serve as the carrier, metal complexes which is one or two of V, Cr, Mn, Fe, Co, Ni, Cu, Au, Pd, and Pt serve as a catalytic oxidative activity center, and the content of carried metal elements is 0.03-5.00% of the total mass of the alcohol oxidation aldehyde ketone production applied novel catalyst. The invention also discloses the preparation method of the alcohol oxidation aldehyde ketone production applied novel catalyst. According to the alcohol oxidation aldehyde ketone production applied novel catalyst and the preparation method thereof, alcohol and oxidants serve as the raw materials, under the action of the prepared alcohol oxidation aldehyde ketone production applied novel catalyst, high ketone conversion rate and high aldehyde ketone absorbing rate can be achieved; meanwhile, no acid or alkali or organic additives exist in a reaction system, so that the alcohol oxidation aldehyde ketone production applied novel catalyst has the advantages of being high in yield rate, low in cost, free from environmental pollution, easy to separate, high in repeatability and the like. Meanwhile, the preparation method of the alcohol oxidation aldehyde ketone production applied novel catalyst has the advantages of being rich in raw material resource, free from environmental pollution and the like.
Owner:NANJING INST OF TECH
Loaded composite metal catalyst used for unsaturated alcohol oxidation and preparation method thereof
ActiveCN103769162AImprove responseImprove structural stabilityOrganic compound preparationCarbonyl compound preparationAlkaline earth metalMetal catalyst
Owner:WANHUA CHEM GRP CO LTD +1
Preparation method of multifunctional integrated porous solid material for catalytic oxidation
ActiveCN104907095ASolve wasteSolve pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkali freeCatalytic oxidation
The invention relates to a preparation method of a multifunctional integrated porous solid material for catalytic oxidation, and belongs to the field of composite materials. The method comprises the following steps: preparing an organic ligand having a Bronsted alkaline function or capable of modifying Bronsted alkalinity, and carrying out a hydrothermal / co-precipitating reaction on the organic ligand and a transition metal source with catalytic activity to obtain the metal organic skeleton catalysis material integrating multiple catalysis functions. The material is applied in benzyl oxidation and alcohol oxidation systems with molecular oxygen as an oxygen source for the first time. The catalysis material prepared through the preparation method of the multifunctional integrated catalysis material effectively integrates a catalytic activity site with the Bronsted alkaline function, solves the problem of difficult activation of the green molecular oxygen source, and avoids the problems of difficult recovery and severe pollution of a homogeneous organic alkali needed by a catalysis system for realizing H removal of C-H bonds; and the multifunctional integrated catalysis material is used in the catalytic oxidation systems with the molecular oxygen as an oxygen source for the first time to realize establishment of high efficiency catalytic oxidation systems under alkali-free conditions.
Owner:UNIV OF SCI & TECH BEIJING
Greening method for preparing aldehydes and ketones through alcohol oxidation of copper catalyst
InactiveCN103420748ALow toxicityReduce pollutionOrganic compound preparationCarbonyl group formation/introductionOrganic solventDouble teeth
The invention provides a greening method for preparing aldehydes and ketones through the alcohol oxidation of a copper catalyst. The greening method comprises the following steps: taking copper salt as the catalyst, a single-tooth or double-teeth N ligand as the ligand and an organic nitric oxide compound as a co-catalyst, and performing the alcohol aerobic oxidation reaction in water-phase or organic solvent under the existence of alkali and air to prepare an aldehyde and Ketone compound. A copper catalyst system, which is low in cost, easy to obtain, low in toxicity and high in activity, is adopted in the greening method, air is used as an economic, safe and green oxidant, and under the mild condition of the indoor temperature air, the aldehyde and ketone compound is prepared through effective alcohol oxidation. The reaction condition is simple and mild, the operation is easy, the reaction conversion rate is high, the product separation and purification are simple, and the recovery rate is high. The requirement on the reaction condition is relatively low, and the greening method has a good research and industrial application prospect.
Owner:WENZHOU UNIVERSITY
Electrocatalyst for alcohol oxidation at fuel cell anodes
InactiveUS8048548B2Promote oxidationImprove abilitiesPhysical/chemical process catalystsActive material electrodesPlatinumIridium
In some embodiments a ternary electrocatalyst is provided. The electrocatalyst can be used in an anode for oxidizing alcohol in a fuel cell. In some embodiments, the ternary electrocatalyst may include a noble metal particle having a surface decorated with clusters of SnO2 and Rh. The noble metal particles may include platinum, palladium, ruthenium, iridium, gold, and combinations thereof. In some embodiments, the ternary electrocatalyst includes SnO2 particles having a surface decorated with clusters of a noble metal and Rh. Some ternary electrocatalysts include noble metal particles with clusters of SnO2 and Rh at their surfaces. In some embodiments the electrocatalyst particle cores are nanoparticles. Some embodiments of the invention provide a fuel cell including an anode incorporating the ternary electrocatalyst. In some aspects a method of using ternary electrocatalysts of Pt, Rh, and SnO2 to oxidize an alcohol in a fuel cell is described.
Owner:BROOKHAVEN SCI ASSOCS
Organic heteropoly acid hybrid catalyst for alcohol oxidation reaction and preparation method thereof
InactiveCN102671700AThe synthesis process is simpleHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationHeteropoly acidIon exchange
An organic heteropoly acid hybrid catalyst is prepared with ionic liquid polymer and heteropoly acid. A preparation method for the organic heteropoly acid hybrid catalyst is divided into the following three steps: firstly, ionic liquid is amino-functionalized; the unsaturated functional ionic liquid and unsaturated aromatic compound are initiated by initiator to polymerize, so that the ionic liquid polymer is produced; and finally, after the anion exchange between the ionic liquid polymer and the heteropoly acid, the ionic liquid polymer-heteropoly acid hybrid catalyst is produced. The catalyst is applicable to a variety of alcohol oxidation reactions with H2O2 as oxidizer, solvent is not used in the process of alcohol oxidation, the environment cannot be polluted, and the catalyst also has the characteristics of high activity and selectivity and stable reusability, and is a environment-friendly novel organic heteropoly acid hybrid catalyst.
Owner:JIANGNAN UNIV
Method for preparing aldehyde and ketone by alcohol oxidation
InactiveCN102964191ALow costMild reaction conditionsCarbonyl group formation/introductionCarbonyl compound preparation by oxidationAcetic acidNitrite
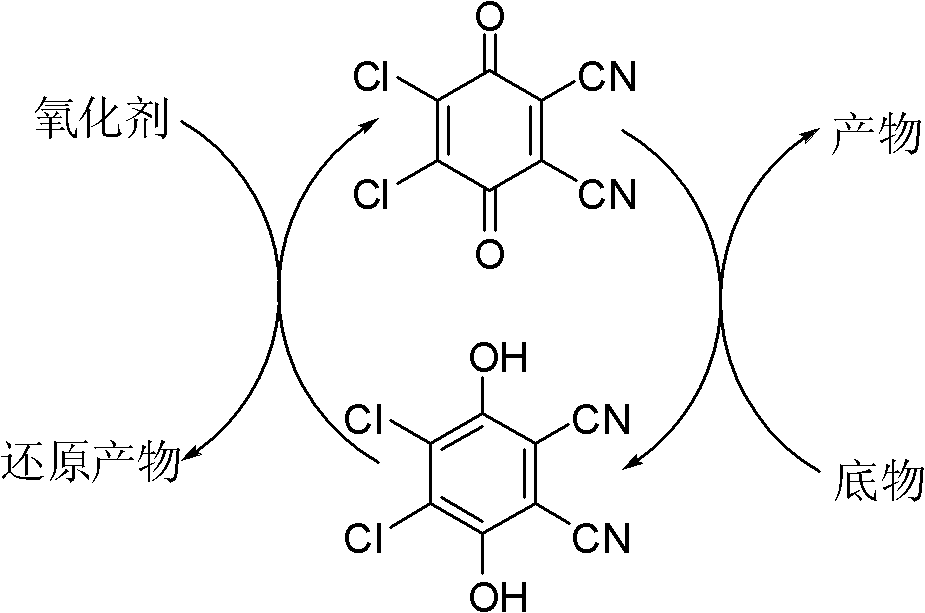
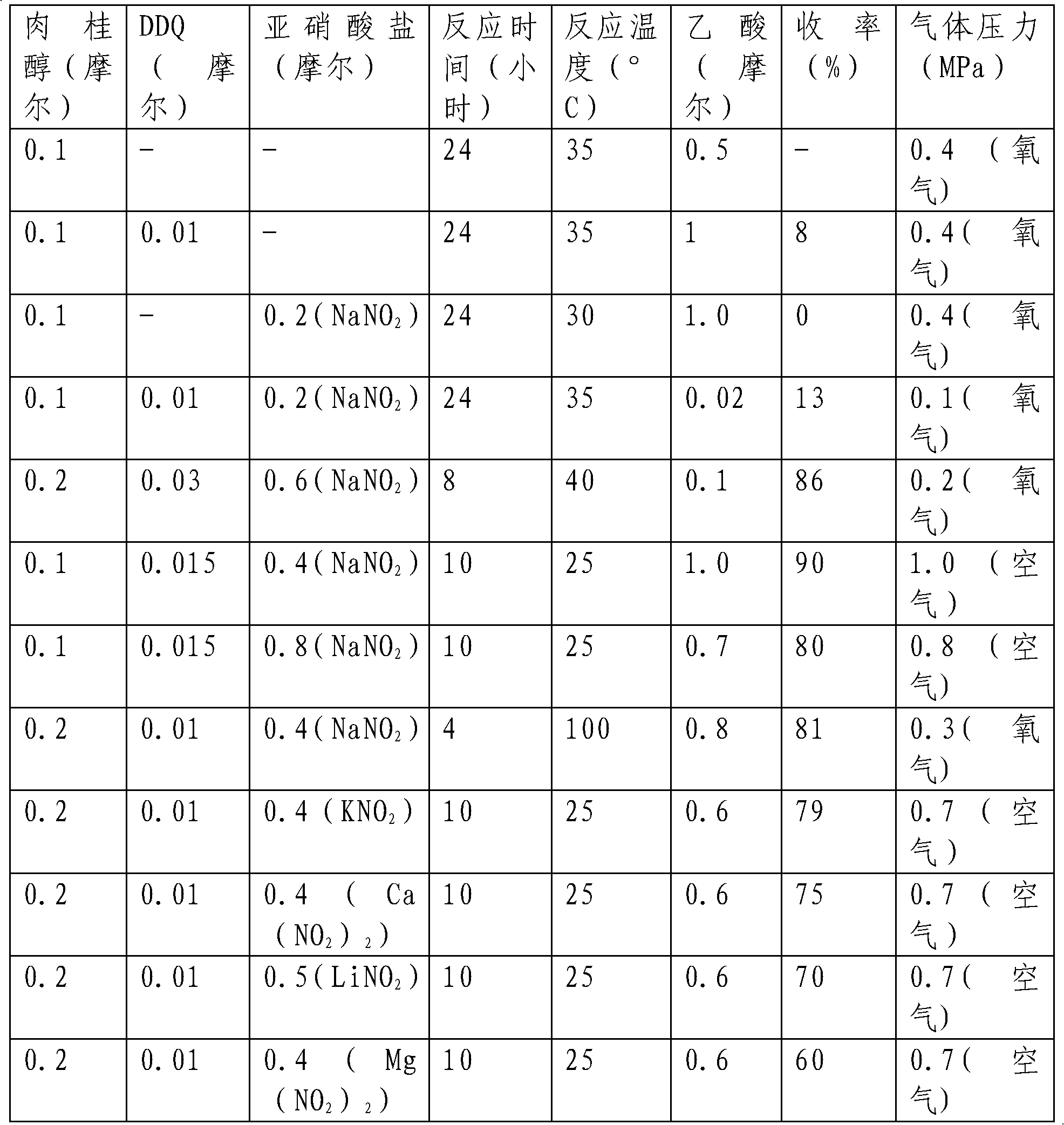
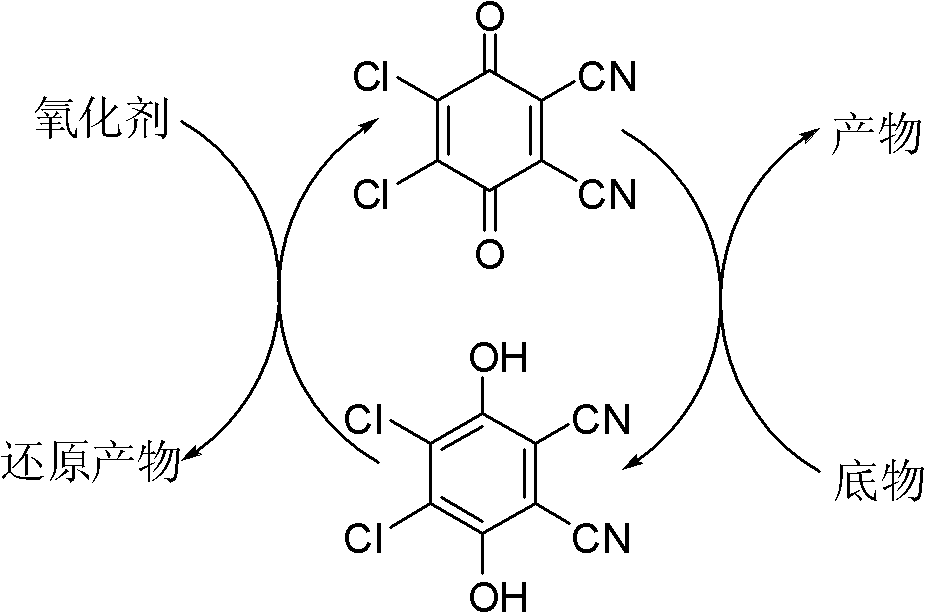
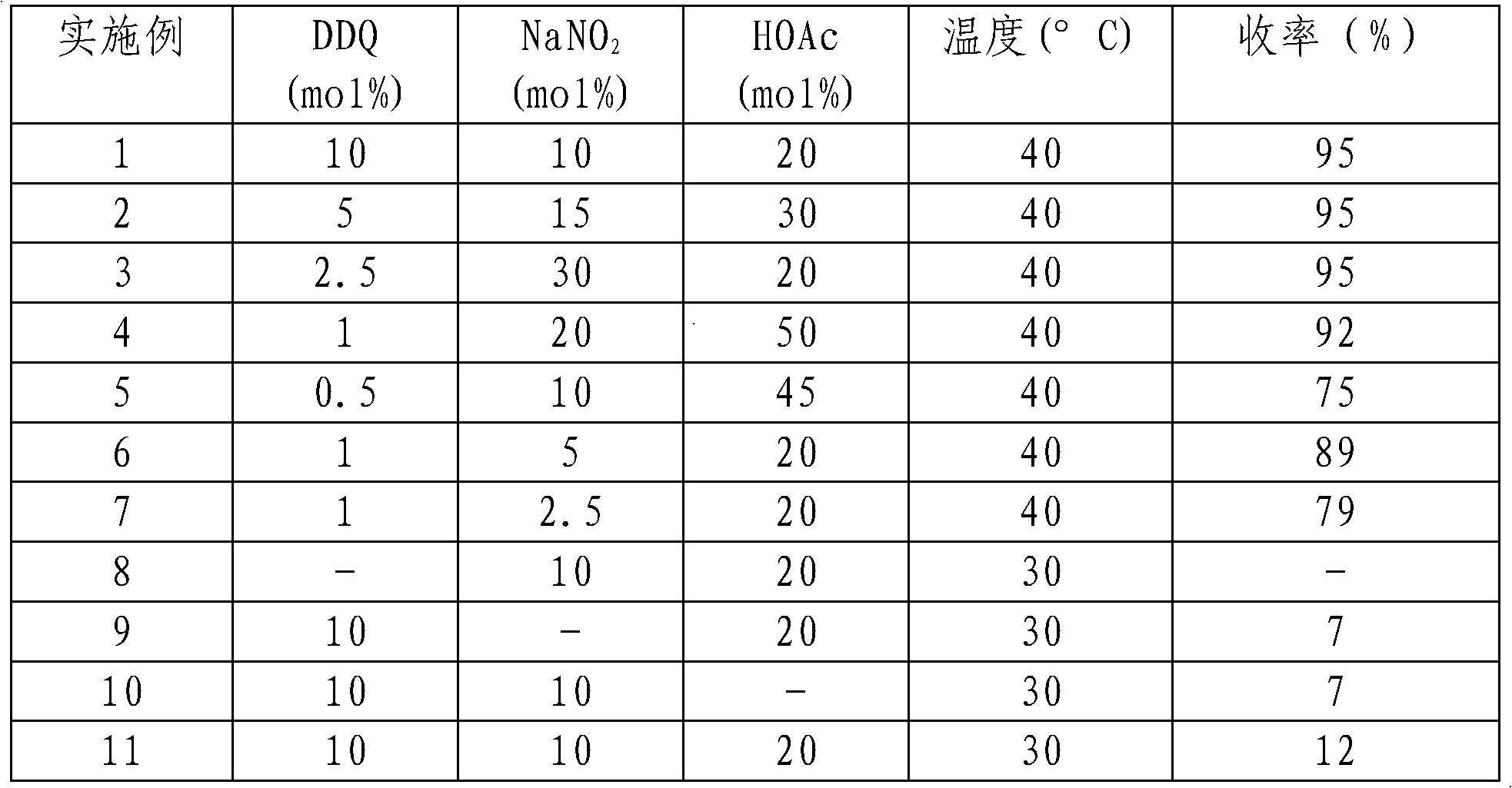
The invention provides a method for preparing aldehyde and ketone by alcohol oxidation, which adopts 2,3-dichloro-5,6-dicyan p-benzoquinone as a catalyst, nitrite as a cocatalyst, and oxygen (or air) as an oxidant, and performs oxidation of alcohol in an acetic acid solvent to generate aldehyde and ketone. The method is mild in reaction condition, simple in operation, free of metal, and less in pollution, and is a green, environment-friendly new method for preparing aldehyde and ketone by alcohol oxidation with nonmetal catalysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Sulfur alcohol oxidation catalyst, its preparing and use
InactiveCN1978058AHigh activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsRefining with oxygen compoundsAlkaline earth metalPhthalocyanine
The present invention provides a mercaptan oxidation catalyst. Said catalyst uses multiporous material as carrier, and uses metallophthalocyanine with positive ion group and metallophthalocyanine with negative ion group as active component. Said invention also provides a method for mercaptan oxidation, said method is characterized by that in the presence of oxygen gas and alkaline solution medium the hydrocarbon flow is contacted with the invented catalyst. Besides, said invention also provides the preparation method of said catalyst and its concrete steps.
Owner:CHINA PETROLEUM & CHEM CORP +1
M1 + M2/C catalyst and its preparation method
The invention discloses a M1+M2 / C catalyst; M1 is any one of Pb, Bi, Sn and Ru, M2 / C is any one of Pt / C, Pd / C and Au / C, the M2 / C comprises, by mass, 0.5-85% of M2, and the M1+M2 / C catalyst comprises, by mass, 1-30% of M1. The catalyst is prepared by impregnation method, and the preparation comprises three steps of (1) M2 / C dispersion solution preparation, (2) M1 salt impregnation solution preparation and (3) M1+M2 / C catalyst preparation. In the preparation process of the catalyst, a protective agent is not needed to add to control the particle size of the catalyst, the catalytic activity of an alkaline medium on small molecule alcohol oxidation is high, the stability is good, the preparation process is simple, the production can be enlarged, and the catalyst can be used as an electrochemical oxidation catalyst of small molecule alcohol in the alkaline medium, and is especially suitable for using as an oxidation catalyst of a small molecule alcohol fuel in an alkaline direct alcohol fuel cell.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Alcohol oxidation catalyst, method of manufacturing the same, and fuel cell using the alcohol oxidation catalyst
InactiveUS20100081036A1Improve oxidation activityMolecular sieve catalystsCell electrodesFuel cellsAlloy
Owner:SAMSUNG ELECTRONICS CO LTD +1
Multilevel core-shell structure magnetic nano gold catalyst and preparation method thereof
InactiveCN102513126AStrong superparamagnetic propertiesImprove oxidation activityMaterial nanotechnologyNanomagnetismPhenethyl alcoholSuperparamagnetism
The invention relates to a multilevel core-shell structure magnetic nano gold catalyst and a preparation method thereof, belonging to the field of magnetic nano catalytic material. A core-shell structure composite which is formed by taking a lamellar double-hydroxyl composite metal oxide (M2+)(M3+)-LDHs as a shell and Fe3O4 magnetic nano particles as a core is taken as a carrier to load nano goldparticles, the shell (M2+)(M3+)-LDHs nano slice is vertically intersected and epitaxially grow and has a honeycomb appearance, the gold nano particles are carried and loaded on the surface of the (M2+)(M3+)-LDHs nano slice, and H2 is reduced, thus the multilevel core-shell structure magnetic nano gold catalyst Fe3O4@(M2+)(M3+)-LDHs@Au is obtained. The invention has the advantages that the multilevel core-shell structure magnetic nano gold catalyst has stronger superparamagnetism and also has excellent catalytic alcohol oxidation activity and selectivity, 1-phenethyl alcohol oxidation is takenas a probe reaction, the air is taken as an oxidant for reaction at the temperature of 80 DEG C, reaction is carried out for 3 hours, and yield of phenylacetaldehyde is 99%; meanwhile, the preparation method is simple, the catalyst is conveniently enriched and recycled by adding an external magnetic field after reaction is finished, and catalytic activity of the catalyst is not reduced after the catalyst is reused for five times.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing aldehyde or ketone by alcohol oxidation
InactiveCN102964192ALow costMild reaction conditionsCarbonyl group formation/introductionCarbonyl compound preparation by oxidationOrganic solventKetone
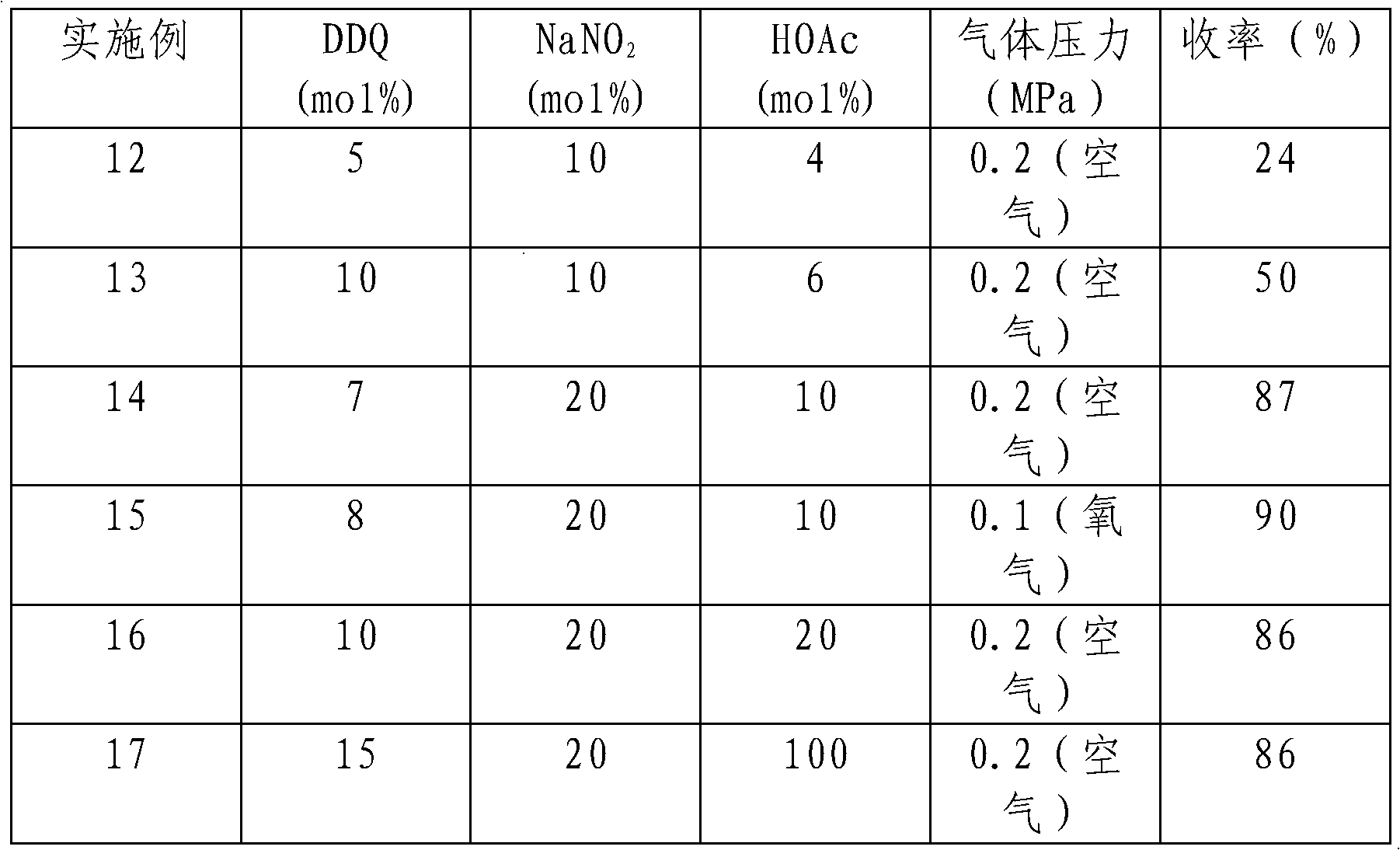
The invention provides a method for preparing aldehyde and ketone by alcohol oxidation, which adopts 2,3-dichloro-5,6-dicyan p-benzoquinone as a catalyst, nitrite as a cocatalyst, and oxygen (or air) as an oxidant, and performs oxidation of alcohol in an organic solvent in the presence of an acid additive and under a liquid phase condition to generate aldehyde and ketone. The method is mild in reaction condition, simple in operation, and less in metal pollution, and is a green, environment-friendly new method for preparing aldehyde and ketone by alcohol oxidation with nonmetal catalysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
TiO2@C supported RuNi direct methanol fuel cell anode catalyst and preparation method thereof
The invention discloses a multi-hole hollow TiO2@C supported RuNi direct methanol fuel cell anode catalyst and a preparation method thereof, and the catalyst is composed of a TiO2@C carrier and a RuNi nano-alloy. The multi-hole hollow TiO2@C nano-carrier with high ratio surface and the RuNi nano-alloy can form the multi-element catalyst in a recombination manner. With the recombination of C and the deposition of the RuNi alloy on the surface of the carrier, the electrical conductivity of the TiO2 is improved; a catalytic oxidation of TiO2 on methyl alcohol is greatly improved through the synergistic effect on TiO2 of the recombination of C and the deposition of the RuNi alloy; meanwhile, intermediate products, such as CO, generated by methyl alcohol oxidation are adsorbed and transferred to the surface of the composite catalyst and are directly deeply oxidized to obtain a final product CO2; in addition, the TiO2@C nano-carrier is stable, and is less prone to oxidation. The price of RuNi is less than that of a precious metal Pt, and the dosage of RuNi in the catalyst is small, thus, the cost of the catalyst is greatly reduced, a CO toxicity resisting capacity of the catalyst is improved, the cost of the catalyst in a direct methanol fuel cell is greatly reduced, and the property of the direct methanol fuel cell is improved.
Owner:NANTONG UNIVERSITY
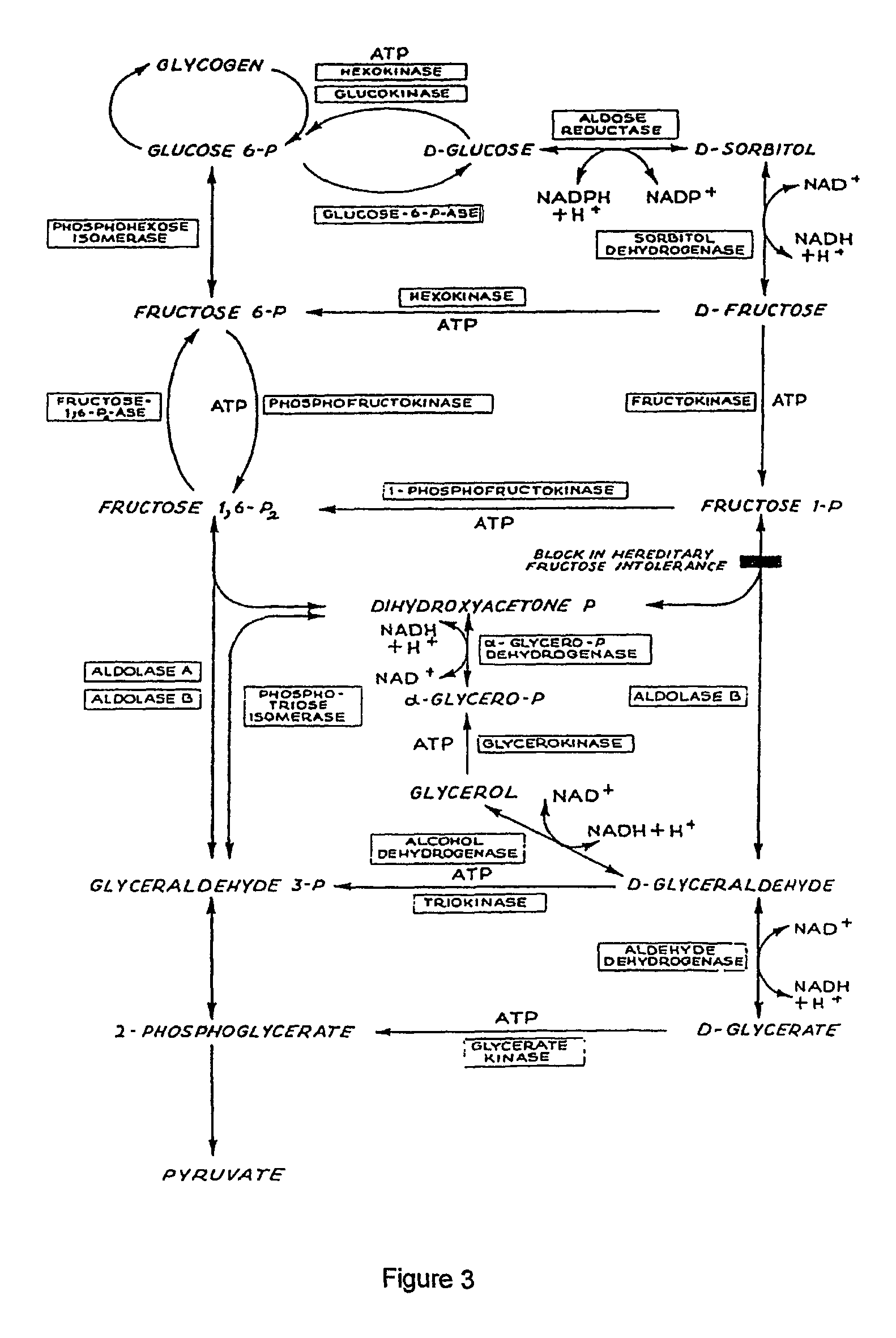
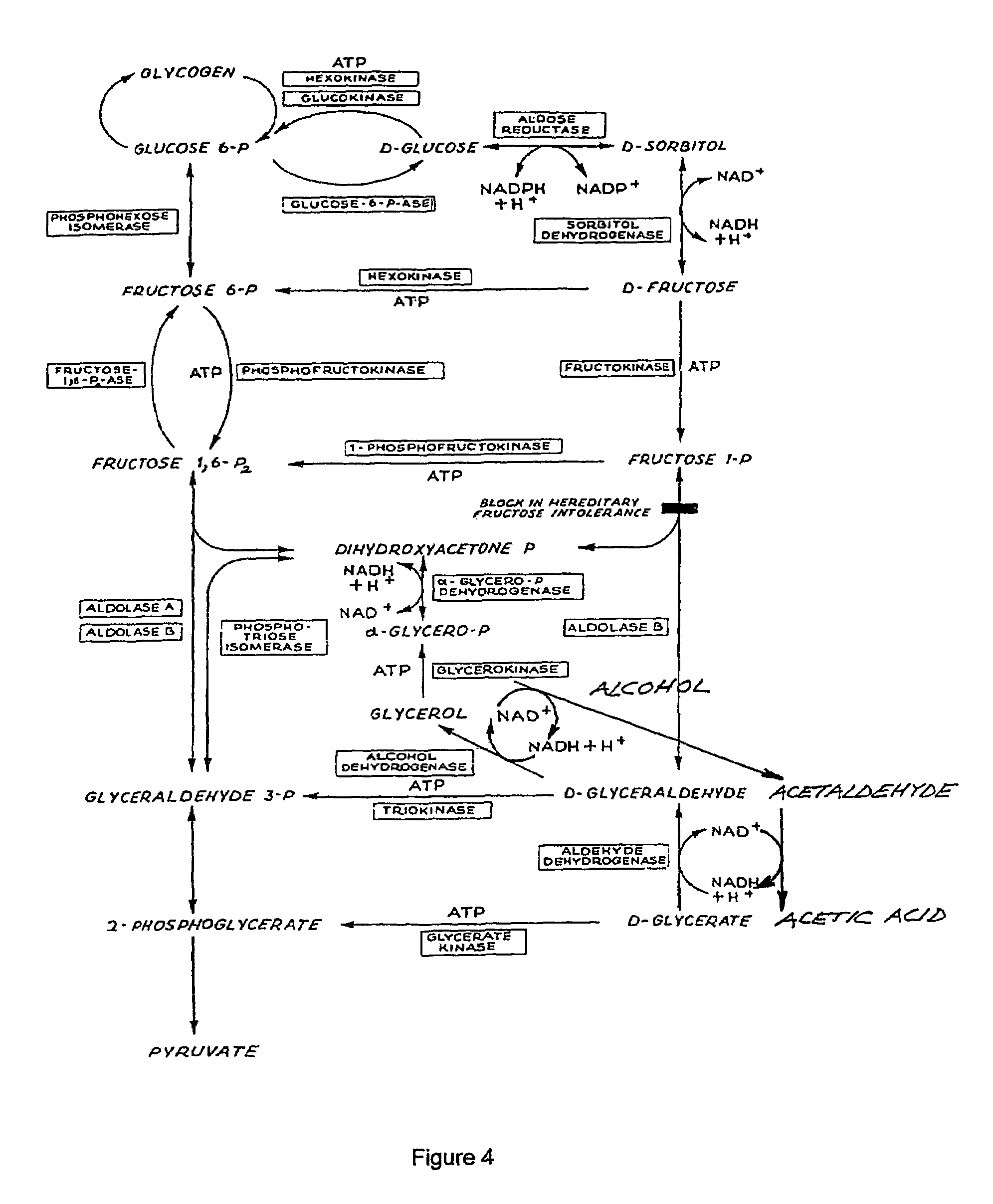
Enhancement of alcohol metabolism
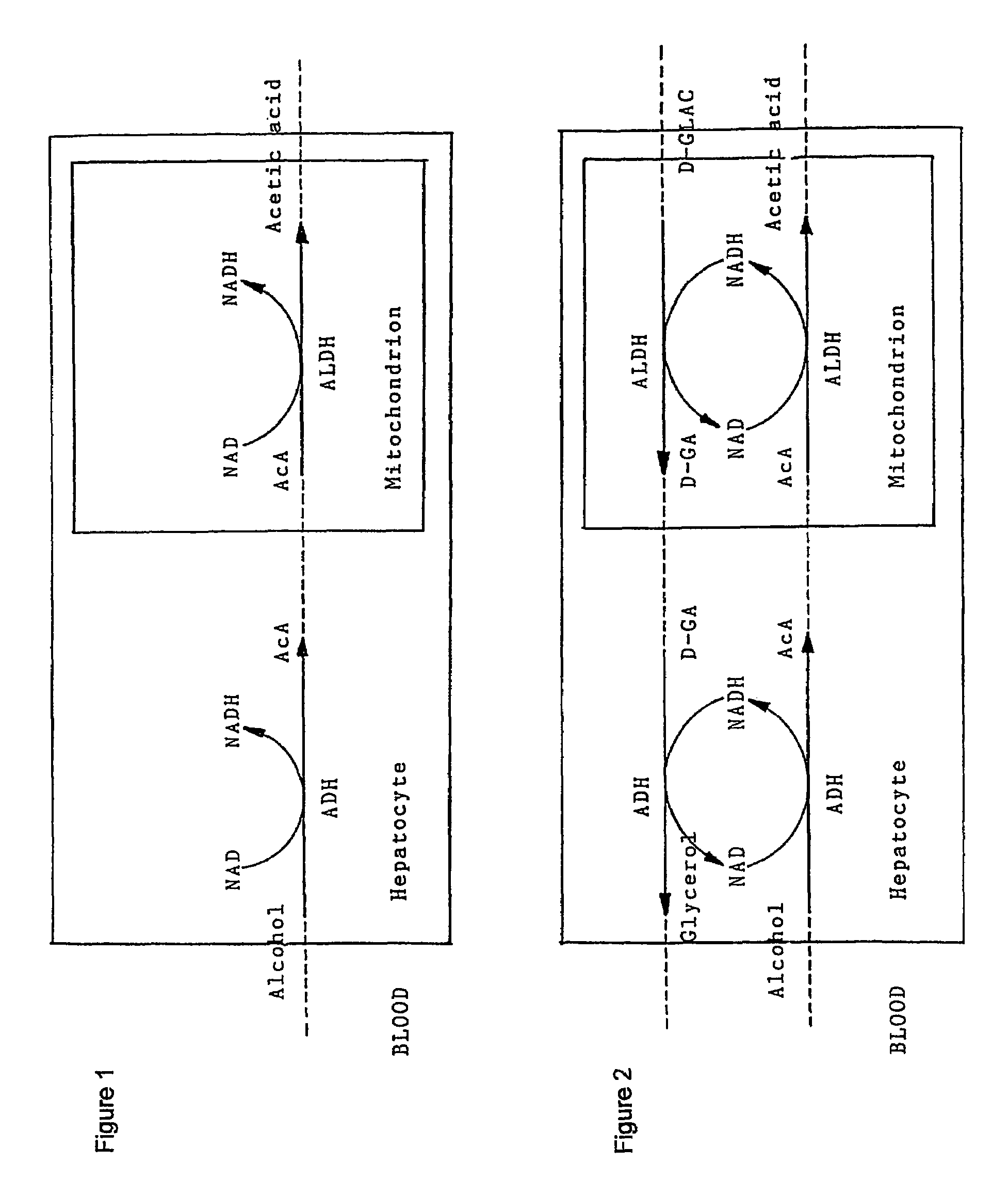
D-glyceric acid has been found to enhance alcohol metabolism and thereby prevent adverse effects of alcohol consumption. D-glyceric acid is administered concurrently with alcohol, to accelerate the elimination of the alcohol from the body. D-glyceric acid is converted into D-glyceraldehyde and further into glycerol in reactions catalysed by NADH-aldehyde dehydrogenase and NADH-alcohol dehydrogenase complexes, which are produced in excess during alcohol oxidation, in the cells of alcohol-metabolising tissues. In these reactions, the NADH complexes become NAD-aldehyde dehydrogenase and NAD-alcohol dehydrogenase complexes. These complexes in turn accelerate the oxidation of alcohol, which is paralleled by enhancement of acetaldehyde oxidation to metabolically harmless acetic acid. D-glyceric acid or its salt or ester is used for the manufacture of a pharmaceutical preparation for enhancing the metabolism of alcohol. A method of enhancing the metabolism of alcohol in a subject by administering said compounds an effective amount of D-glyceric acid or its salt or ester is disclosed. An oral or parenteral preparation comprising said compounds is also disclosed.
Owner:HEINO PEKKA
Green synthesis method of benzothiazole heterocyclic compound
InactiveCN103450111AWide variety of sourcesLow reaction conditionsOrganic chemistrySynthesis methodsBy-product
The invention provides a green synthesis method of a benzothiazole heterocyclic compound. Alcohol and aminothiophenol compounds are directly subjected to an oxidative condensation cyclization reaction to synthetize the benzothiazole heterocyclic compound in air under the condition of taking a water-soluble inorganic base as a catalyst and air as an oxidizing agent. By adopting the method, alcohols which are cheap and available, wide in source, stable, and low in toxicity are taken as raw materials; a water-soluble non-transition metal catalyst which is low in price is used; air which is convenient to use, economic and safe is directly utilized as the oxidizing agent, so as to obtain a target heterocyclic compound by air alcohol oxidation and cyclic condensation synthesis. The method is simple and mild in reaction condition, free of protection of inert gases, and easy to operate, and is carried out in air; the only by-product is water; separation and purification of the product are simple and easy; the recovery rate is high. The method is low in demands on reaction conditions, and wide in application range, and has good research and industrial application prospects.
Owner:WENZHOU UNIVERSITY
Method for preparing esters through primary alcohol oxidation
InactiveCN104945210AReduce dosageEasy to separateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAfter treatmentOxygen
The invention relates to a method for preparing esters through primary alcohol oxidation. The method comprises the following steps: taking one or two of air and oxygen as an oxygen source, and oxidizing alcohols into esters under the catalytic action of hydrophobic carrier loaded metal nanoparticles. According to the method disclosed by the invention, the oxidation efficiency is high, and the product yield is high; the air or oxygen serves as an oxygen source, and the method is economic and environment-friendly; the product is separated from the catalyst, and the after-treatment is simple; and the catalyst is easily repeatedly used and has excellent application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com