Electrocatalyst for alcohol oxidation in fuel cells
a fuel cell and electrocatalyst technology, applied in the direction of metal/metal-oxide/metal-hydroxide catalysts, cell components, physical/chemical process catalysts, etc., can solve the problems of significant reduction of fuel cell performance, loss of electrochemical activity of even the best electrocatalyst, and serious drawbacks in the storage of hydrogen for use in fuel cells, etc., to achieve significant reduction of carbon monoxide poisoning, reduce cost, and reduce the weight of noble metal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
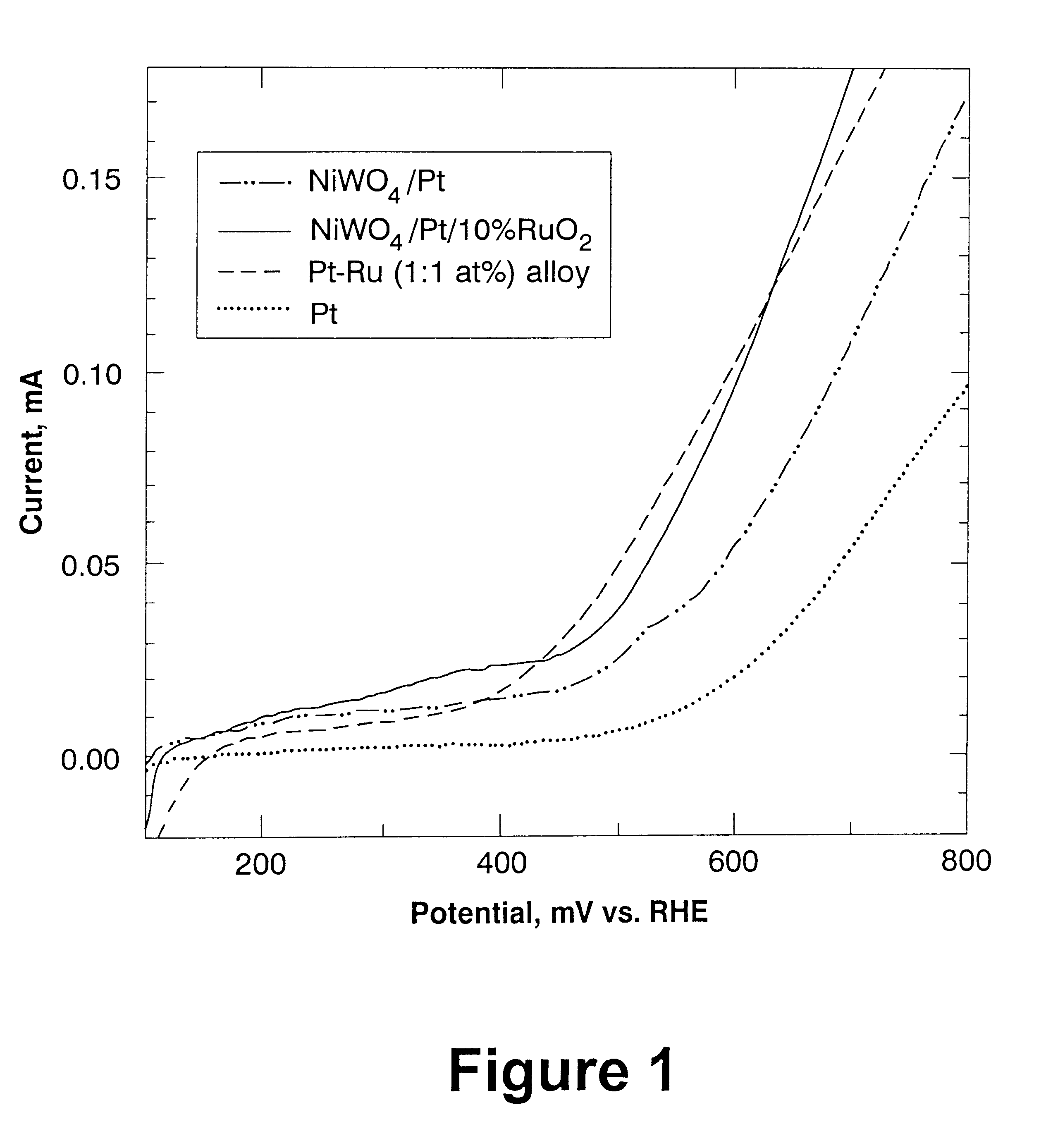
Current potential curves were obtained using various electrocatalysts in a methanol oxidation fuel cell. Electrocatalysts of the invention (i.e., NiWO.sub.4 / Pt and NiWO.sub.4 / Pt / 10% RuO.sub.2) and known electrocatalysts (i.e., Pt--Ru (1:1 wt %) and Pt) were all tested for performance in an anode of a methanol fuel cell.
Measurements were performed using a solution of 0.5M H.sub.2 SO.sub.4 and 0.5M CH.sub.3 OH at room temperature (approximately 21.degree. C.) in an all-glass electrochemical cell with three electrode arrangement. An equal amount of Pt (5 mg / cm.sup.2) was used for the preparation of all catalysts. The approximate surface area of the catalyst was 1.42 cm.sup.2. All of the catalysts were in the form of a powder and were supported by a 63% carbon (Vulcan.TM.) / 37% PTFE mixture. The reference electrode was connected to the solution through a Luggin capillary.
The current potential curves were obtained using quasi-stationary potentiodynamic regime, with the sweep rate of 1 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com