Organic heteropoly acid hybrid catalyst for alcohol oxidation reaction and preparation method thereof
A solid acid catalyst and heteropoly acid salt technology, which is applied in the direction of organic compound/hydride/coordination complex catalyst, oxidation to carbonyl compound, physical/chemical process catalyst, etc., can solve the problem of low alcohol oxidation activity and unstable stability Ideal and other problems, to achieve the effect of simple synthesis process, high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
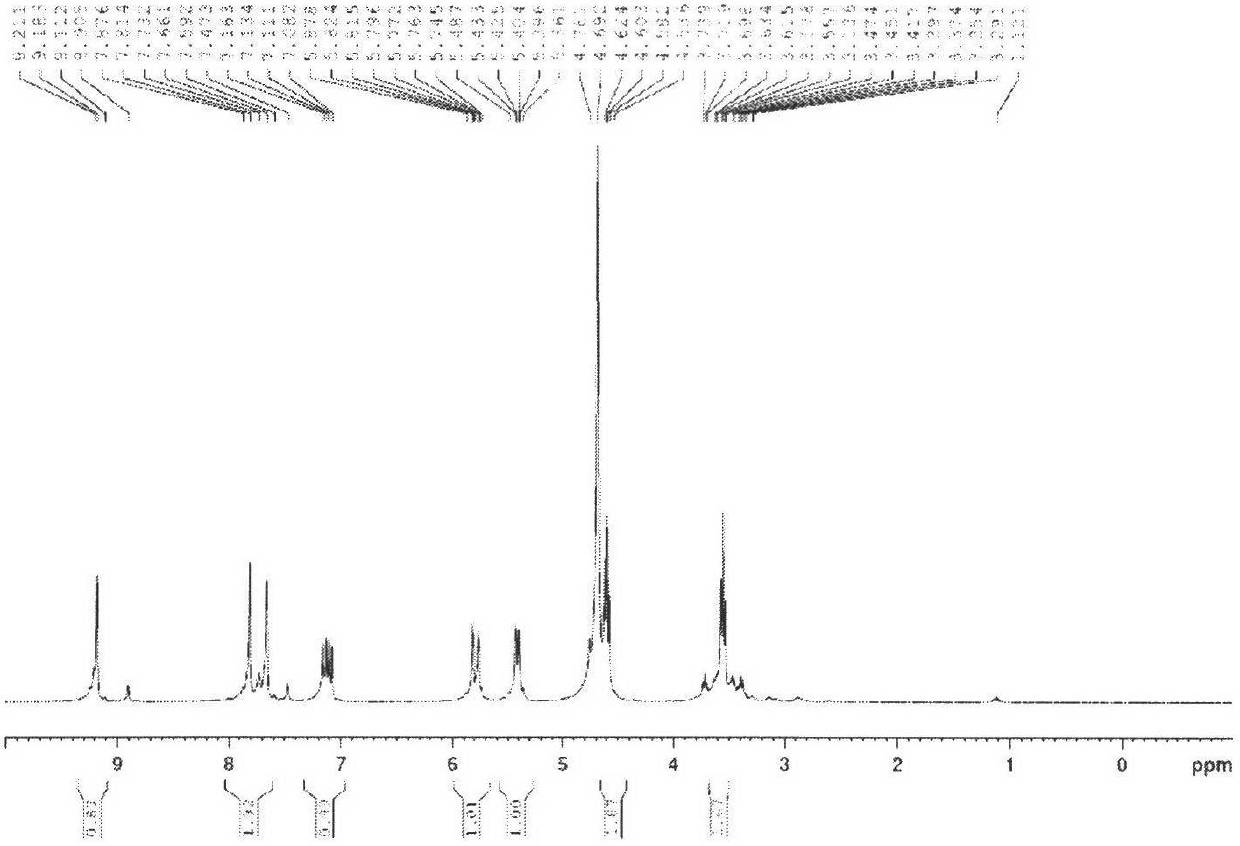
[0020] 1) In a 100mL three-necked flask equipped with a magnetic stirrer, a constant pressure dropping funnel and a spherical condenser, add 9.4g N-vinylimidazole, 20.5g 2-bromoethylamine hydrobromide and 50mL ethanol, 70 Stir at °C for 48 h, cool and filter, wash repeatedly with ethanol, and dry at 80 °C for 12 h to obtain an amine functionalized ionic liquid [VmimAM]Br·HBr with a yield of 90%. 1 H NMR (300MHz, D 2 O, TMS) characterization results are ( figure 1 ) δ (ppm) = 2.00 (m, 2H, -CH 2 ), 4.34 (s, 2H, -CH 2 ), 5.44 (d, 1H, -CH), 5.86 (d, 1H, -CH), 7.18 (m, 1H, -CH), 7.63 (s, 1H, -CH), 7.81 (s, 1H, -CH ), 9.11(s, 1H, -CH).
[0021] 2) Add 1g of amine-functionalized ionic liquid [VmimAM]Br·HBr, 2g of divinylbenzene and 10mL of methanol into a 100mL round-bottomed flask, protect it with nitrogen, stir and dissolve at 60°C, then add 0.05g of azobis Isobutyronitrile (AIBN), continue stirring at 60°C for 10-24h. Centrifuge, wash repeatedly with ethanol, adjust the pH v...
Embodiment 2
[0025] 1) In a 100mL three-necked flask equipped with a magnetic stirrer, a constant pressure dropping funnel and a spherical condenser, add 9.4g of N-vinylimidazole, 18.5g of N-chloroethylpiperidine hydrochloride and 50mL of acetonitrile , stirred at 80°C for 48h, cooled and filtered, washed repeatedly with ethanol, and dried at 80°C for 12h to obtain piperidine functionalized ionic liquid [VmimPD]Cl·HCl with a yield of 85%.
[0026] 2) Add 1g of piperidine functionalized ionic liquid [VmimPD]Cl·HCl, 2g of divinylbenzene and 10mL of methanol into a 100mL round-bottomed flask, pass through nitrogen protection, stir and dissolve at 60°C, add 0.05g of AIBN, continue Stir at 60°C for 10-24h. Centrifuge, wash repeatedly with ethanol, adjust the pH value to neutral with NaOH aqueous solution, centrifuge, and dry at 100°C for 12 hours to obtain 2.0 g of cross-linked ionic liquid polymer (PD-DVB).
[0027] 3) Disperse the above-mentioned ionic liquid polymer PD-DVB in the aqueous so...
Embodiment 3
[0030] 1) In a 100mL three-necked flask equipped with a magnetic stirrer, a constant pressure dropping funnel and a spherical condenser, add 9.4g N-vinylimidazole, 20.5g 2-bromoethylamine hydrobromide and 50mL ethanol, Stir at 70°C for 48h, cool and filter, wash repeatedly with ethanol, and dry at 80°C for 12h to obtain amine-functionalized ionic liquid [VmimAM]Br·HBr with a yield of 90%.
[0031]2) Add 1g of amine-functionalized ionic liquid [VmimAM]Br HBr, 2g of styrene and 10mL of a mixed solvent of ethanol and water (v:v=1:1) into a 100mL round-bottomed flask, nitrogen protection, 60°C After stirring and dissolving under the condition, add 0.05g AIBN, and continue stirring at 60°C for 10-24h. The solvent was removed by rotary evaporation, washed repeatedly with ethanol, adjusted to neutral pH with NaOH aqueous solution, filtered, and dried at 100°C for 12 hours to obtain 2.4g of ionic liquid polymer (AM-St).
[0032] 3) Disperse the above-mentioned ionic liquid polymer AM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com