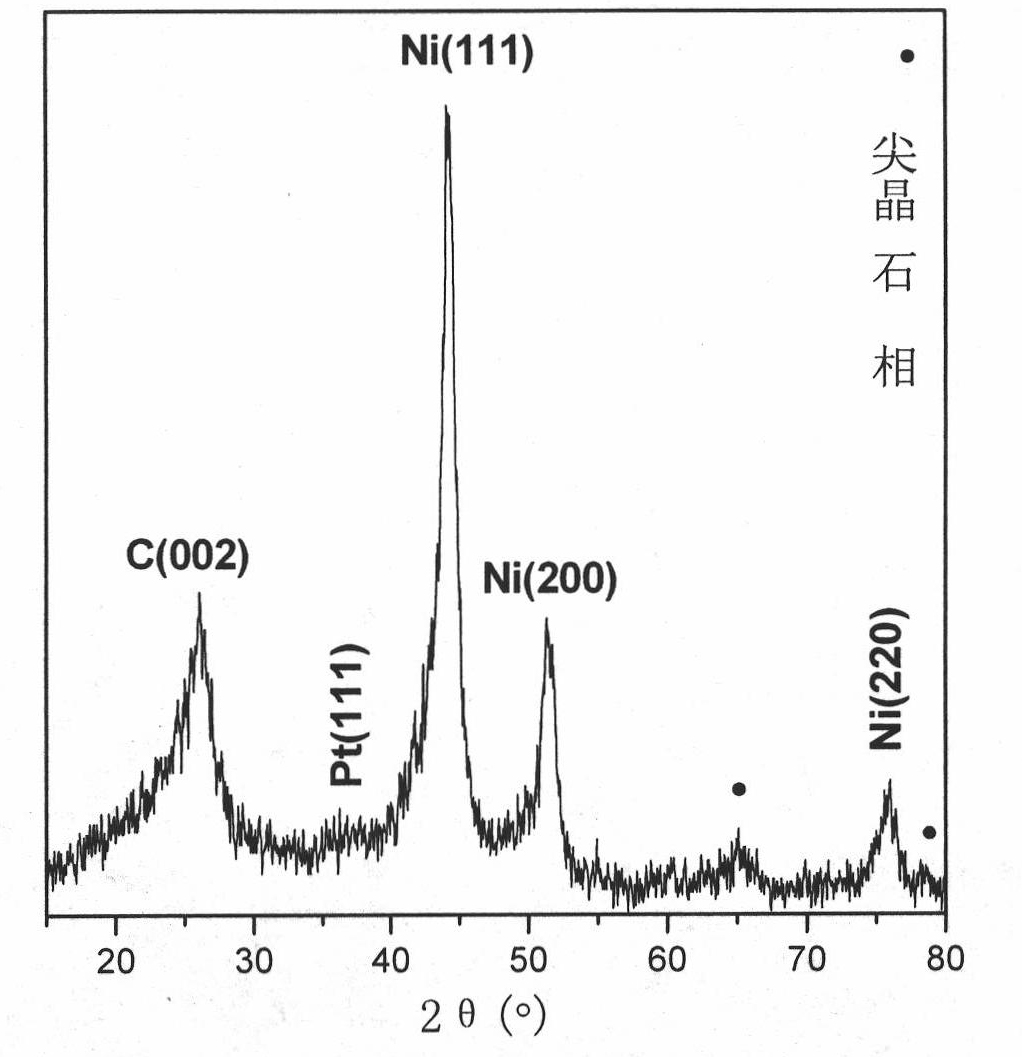
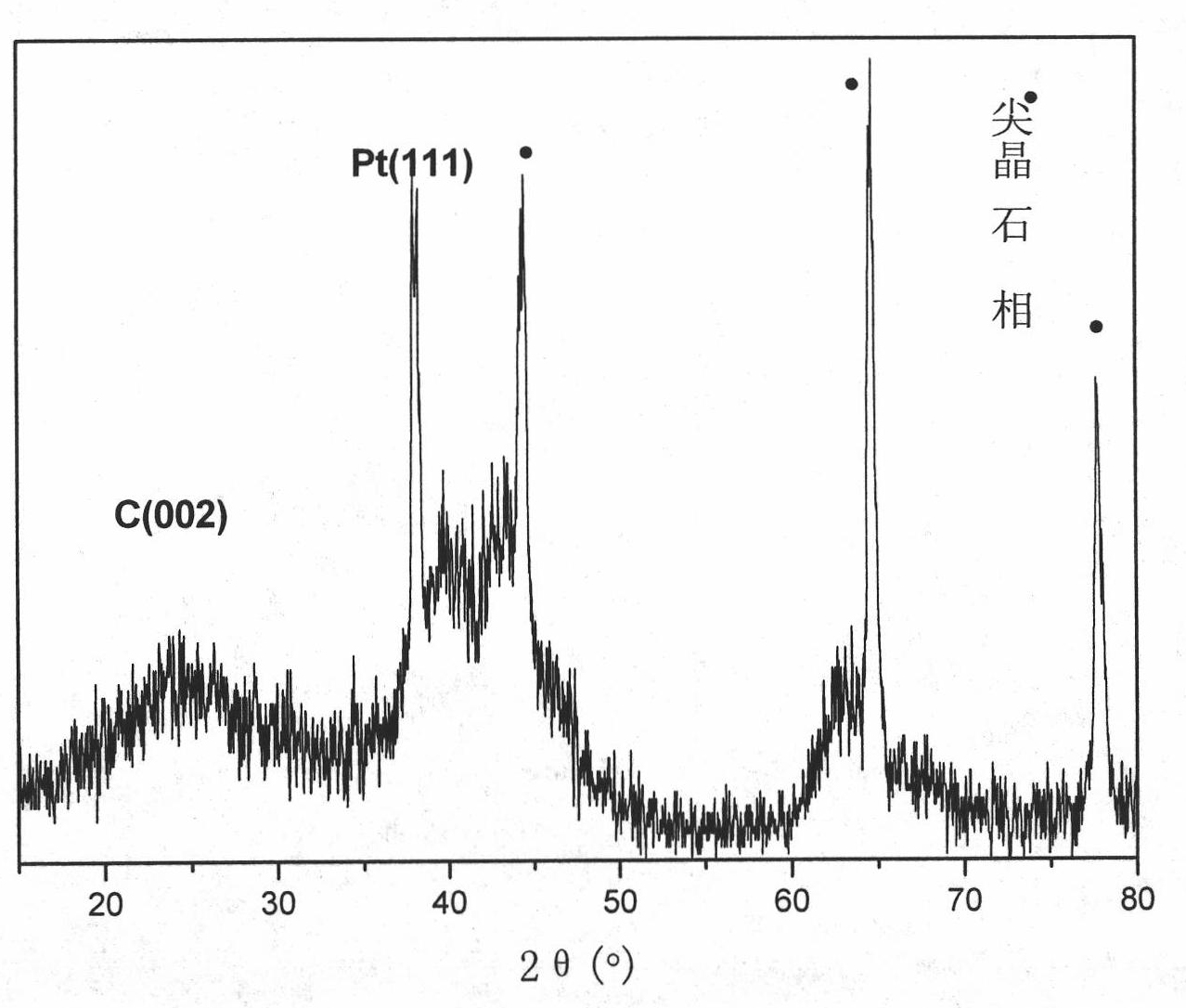
Noble-metal/composite metal oxide/ carbon nanometer tubular electro-catalyst and preparation method and application
A carbon nanotube and composite metal technology is applied in the field of noble metal/composite metal oxide/carbon nanotube type electrocatalyst and its preparation, and achieves the effects of uniform particle size, good crystallinity, and overcoming the inability to uniformly disperse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Will K 2 PtCl 4 2H 2 O, NiSO 4 ·6H 2 O, MgCl 2 ·6H 2 O and Al(NO 3 ) 3 ·7H 2 O was dissolved in 80ml deionized water to prepare a salt solution, in which Pt 2+ The molar concentration is 0.08mol / L, Ni 2+ The molar concentration is 0.3mol / L, Mg 2+ The molar concentration of Al is 0.6mol / L, Al 3+ The molar concentration is 0.3mol / L;
[0030] Add urea to the salt solution to make a mixed solution, so that the molar concentration ratio of urea and all metal cations is 5:1.
[0031] Put this mixed solution into a 100ml polytetrafluoroethylene resin liner, place it in a reactor to seal it, put it into an oven at 150° C. to crystallize for 36 hours, and finally take out the liner. After the reaction was completed, it was suction filtered, washed twice with deionized water, and dried at 60° C. for 10 hours.
[0032] Put 150 mg of the layered double metal hydroxide prepared above into a porcelain boat, put it into a tubular heating furnace, pass in nitrogen gas (70...
Embodiment 2
[0035] Will K 2 PtCl 4 2H 2 O, Mg(NO 3 ) 2 ·6H 2 O and Al(NO 3 ) 3 ·7H 2 O was dissolved in 80ml deionized water to prepare a salt solution, in which Pt 2+ The molar concentration is 0.04mol / L, Mg 2+ The molar concentration is 0.3mol / L, Al 3+ The molar concentration is 0.1mol / L;
[0036] Add appropriate urea to the salt solution to make a mixed solution, the molar concentration ratio of urea and all metal cations is 3.
[0037] Put this mixed solution into a 100ml polytetrafluoroethylene resin liner, place it in a reactor to seal it, put it into an oven at 180° C. for crystallization for 36 hours, and finally take out the liner. After the reaction was completed, it was filtered with suction, washed twice with deionized water, and dried at 75° C. for 15 hours.
[0038] Put 150 mg of the layered double metal hydroxide prepared above into a porcelain boat, put it into a tubular heating furnace, pass in nitrogen gas (70ml / min), raise the temperature to 600°C at a rate ...
Embodiment 3
[0041] Na 2 PdCl 4 2H 2 O, Co(NO 3 ) 3 ·6H 2 O and Fe(NO 3 ) 3 ·7H 2 O was dissolved in 80ml deionized water to prepare a salt solution, in which Pd 2+ The molar concentration is 0.04mol / L, Co 2+ The molar concentration of Fe is 0.4mol / L, Fe 3+ The molar concentration is 0.1mol / L;
[0042] Add appropriate urea to the salt solution to make a mixed solution, the molar concentration ratio of urea and all metal cations is 4.
[0043] Put this mixed solution into a 100ml polytetrafluoroethylene resin liner, place it in a reactor to seal it, put it into an oven at 150° C. to crystallize for 36 hours, and finally take out the liner. After the reaction was completed, it was suction filtered, washed twice with deionized water, and dried at 70° C. for 10 hours.
[0044] Put 100 mg of the layered double metal hydroxide prepared above into a porcelain boat and put it into a tubular heating furnace, feed nitrogen gas (60ml / min), raise the temperature to 700°C at a rate of 10°C / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com