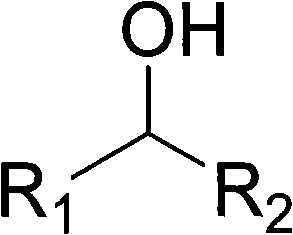
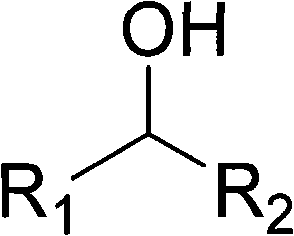
Method for preparing aldehyde or alkone by oxygen catalysis and alcohol oxidation under mild condition
A technology for oxidizing alcohol and oxygen, which is applied in the fields of organic chemistry, carbon-based compound preparation, chemical instruments and methods, etc., can solve problems such as inability to recycle and apply, and achieve the effects of reducing raw material cost, easy operation control, and convenient product separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
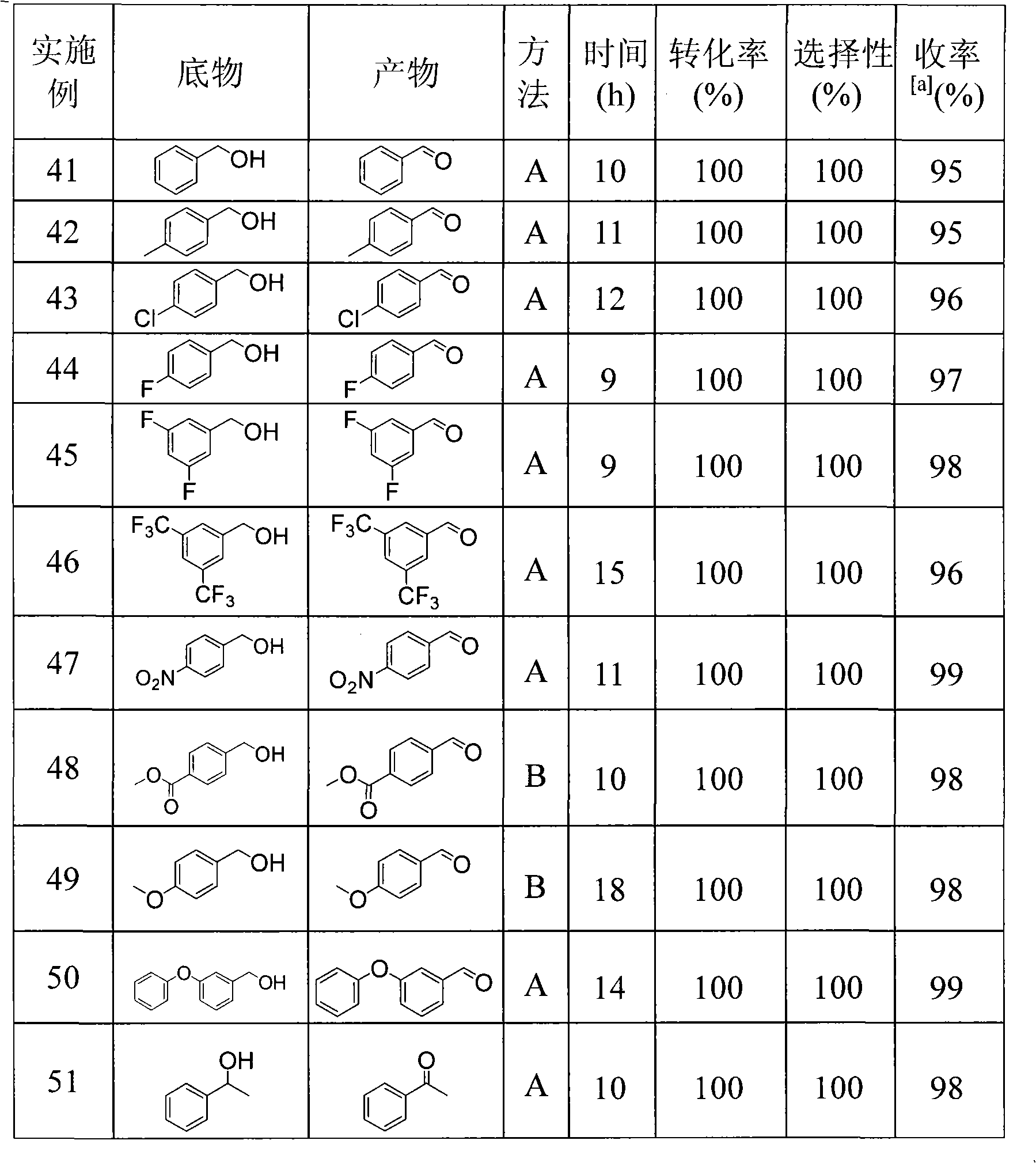
[0032] The oxidation reaction was carried out in a long-neck single-necked round bottom flask (50 mL) equipped with a magnet. First add 5.0mmol benzyl alcohol and 0.05mmol TEMPO into the round bottom flask, then add 8mL dichloromethane as the reaction solvent, then add 0.50mmol hydrochloric acid (HCl), and finally add 0.5mmol nitric acid (HNO 3 ), airtight and make the top of the flask communicate directly with a balloon filled with oxygen. After 10 hours of reaction at room temperature, the stirring was stopped. Samples were taken for gas chromatography analysis. After the reaction was complete, the reaction liquid was transferred to a separatory funnel, and then the flask was carefully washed with dichloromethane, and the organic solutions were combined. followed by saturated Na 2 S 2 o 3 Aqueous solution and NaHCO 3 The organic phase was washed with aqueous solution to remove TEMPO and inorganic salts, the organic layer was dried with anhydrous sodium sulfate, and then...
Embodiment 2
[0034] The test method and steps are the same as in Example 1, but the catalyst used is 4-OH-TEMPO, the reaction time is 15 hours, the yield of benzaldehyde is 95%, and the content is more than or equal to 99%.
Embodiment 3
[0036] Test method and step are the same as embodiment 1, but the catalyst used is still TEMPO, but its consumption increases to 0.10mmol. In addition, the top of the flask was open, directly communicated with the atmosphere of the environment, and the reaction was stirred and reacted for a certain period of time. The conversion rate and selectivity of the content of benzyl alcohol were analyzed by GC. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com