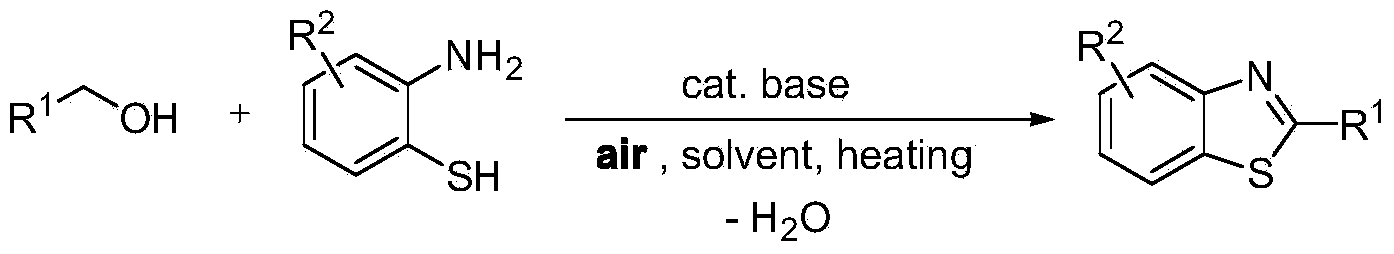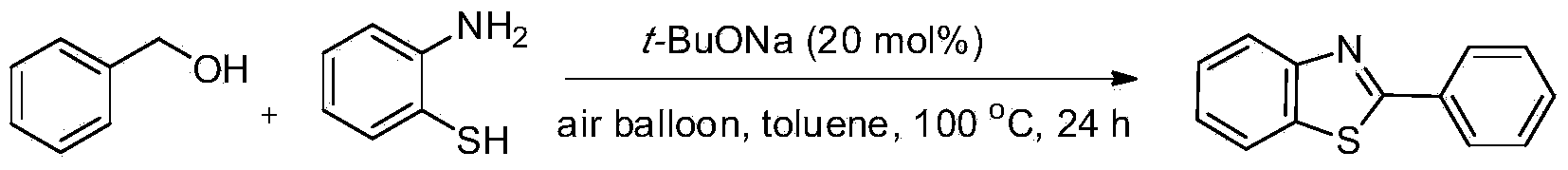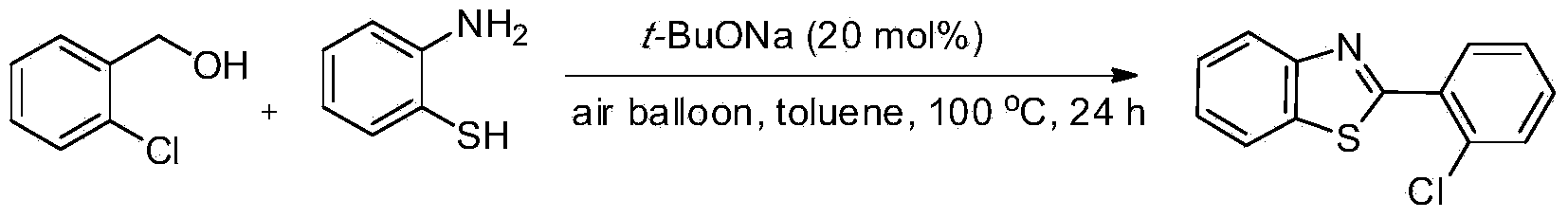Green synthesis method of benzothiazole heterocyclic compound
A heterocyclic compound, benzothiazole technology, applied in the field of chemical synthesis, to achieve the effect of low requirements, wide application range and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2-phenylbenzothiazole from benzyl alcohol and o-aminothiophenol
[0020]
[0021] Add t-BuONa (0.0192g, 20mol%), benzyl alcohol (1.5mmol, 1.5equiv.) and o-aminothiophenol (0.1252g, 1.0mmol,) to the reaction tube in sequence, then add toluene (1.0mL) as solvent , Add an air balloon at normal pressure, heat to 100°C and react for 24h. After the completion of the reaction monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 82%. 1 HNMR (500MHz, CDCl 3 ):δ8.11-8.07(m,3H),7.91-7.90(m,1H),7.51-7.48(m,4H),7.40-7.37(m,1H). 13 CNMR (125.4MHz, CDCl 3 ): δ168.1, 154.1, 135.0, 133.6, 131.0, 129.0, 127.5, 126.3, 125.2, 123.2, 121.6. MS (EI): m / z (%) 212 (17), 211 (100), 210 (22), 184(6), 108(24), 106(6), 92(5), 82(8), 69(13), 63(6), 58(4).
Embodiment 2
[0023] Preparation of 2-(2-chloro)phenylbenzothiazole from 2-chlorobenzyl alcohol and o-aminothiophenol
[0024]
[0025] In the reaction tube, add t-BuONa (0.0192g, 20mol%), 2-chlorobenzyl alcohol (1.5mmol, 1.5equiv.) and o-aminothiophenol (0.1252g, 1.0mmol.), then add toluene (1.0mL ) as the solvent, plus atmospheric pressure air balloons, heated to 100 ° C for 24 hours. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 89%. 1 HNMR (500MHz, CDCl 3 ):δ8.22-8.20(m,1H),8.13(d,J=8.5Hz,1H),7.94(d,J=8.0Hz,1H),7.53-7.50(m,2H),7.44-7.39( m,3H). 13 CNMR (125.4MHz, CDCl 3 ): δ164.1, 152.4, 136.1, 132.7, 132.2, 131.7, 131.1, 130.8, 127.1, 126.2, 125.4, 123.4, 121.3. MS (EI): m / z (%) 246 (19), 245 (100), 244 (9), 210(20), 209(8), 183(6), 108(32), 105(8), 82(11), 69(18), 63(7), 58(6).
Embodiment 3
[0027] Preparation of 2-(3-chloro)phenylbenzothiazole from m-chlorobenzyl alcohol and o-aminothiophenol
[0028]
[0029] Add t-BuONa (0.0192g, 20mol%), m-chlorobenzyl alcohol (1.5mmol, 1.5equiv.) and o-aminothiophenol (0.1252g, 1.0mmol.) in turn to the reaction tube, then add toluene (1.0mL) As a solvent, add an air balloon at atmospheric pressure, and heat to 100 ° C for 24 hours. After the reaction was complete as monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 81%. 1 HNMR (500MHz, CDCl 3 ):δ8.12(t,J=1.5Hz,1H),8.08(d,J=8.0Hz,1H),7.95-7.90(m,2H),7.53-7.49(m,1H),7.47-7.39( m,3H). 13 CNMR (125.4MHz, CDCl 3 ): δ166.3, 153.9, 135.2, 135.1, 135.0, 130.8, 130.2, 127.4, 126.5, 125.7, 125.5, 123.4, 121.7. MS (EI): m / z (%) 246 (18), 245 (100), 244 (9), 210(14), 209(5), 123(5), 108(28), 105(8), 69(17), 63(6), 58(5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com