Preparation method of multifunctional integrated porous solid material for catalytic oxidation
A technology of catalytic oxidation and solid materials, applied in the field of composite materials, can solve problems such as waste and pollution, and achieve the effect of avoiding difficult recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
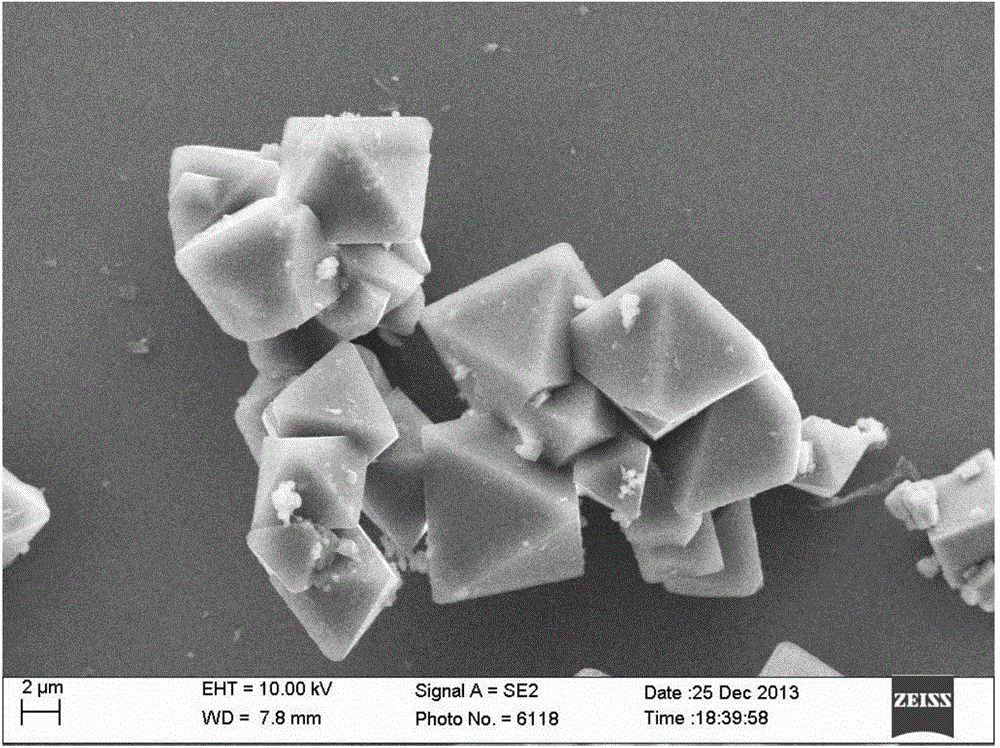
Image
Examples
Embodiment example 1
[0023] 1) Add 8mmol of chromium nitrate nonahydrate, 8mmol of 2-aminoterephthalic acid, and 20mmol of NaOH into 60mL of water, stir for 5 minutes until completely dispersed, then transfer the above mixed solution to a 100mL reaction kettle, place In an oven at 150°C, keep warm for 12 hours, wash twice with deionized water, soak in 10 mL of N,N-dimethylformamide for half a day, centrifuge, and wash with methanol three times to obtain Cr-MIL-101 ( NH 2 ).
[0024] 2) At room temperature, 0.6 mmol of Cr-MIL-101 (NH 2 ) with 6mL of CHCl 3 The solvent was added to a 25mL round-bottomed flask, stirred until it was completely dispersed, and then 0.9mmol of aziridine was added to it, and the round-bottomed flask was placed in an oil bath at 45°C. After continuous heating for 6 hours, centrifuged, 10mL of CHCl 3 Add it to the obtained solid, soak for two days, centrifuge, and place the obtained solid in a vacuum oven at 80° C. for 12 hours to dry.
[0025] 3) Weigh 0.5mol% of the ...
Embodiment example 2
[0028] 1) Add 27mmol of 5,5'-dimethyl-2,2'-bipyridine to 125mL of 95% concentrated sulfuric acid and keep stirring, slowly add 82mmol of potassium dichromate, control the adding speed in 20 minutes Complete, stir until the temperature drops to 35°C, pour the above solution into 800mL of ice water to cool, filter, wash with deionized water 5 times, then wash with acetone 3 times, and vacuum dry at 60°C for 12h to obtain 2,2'- Bipyridine-5,5'-dicarboxylic acid ligand. Mix and stir 2,2'-bipyridine-5,5'-dicarboxylic acid ligand with hydrochloric acid aqueous solution to obtain 2,2'-bipyridine-5,5'-dicarboxylic acid hydrochloride.
[0029]2) At room temperature, dissolve 0.2 mmol of ferric nitrate nonahydrate in 20 mL of N,N-dimethylformamide, stir until completely dissolved, and then add 0.2 mmol of 2,2'-bipyridine-5, 5'-dicarboxylic acid hydrochloride until completely dissolved, then add 6mmol of benzoic acid, ultrasonic the above mixed solution for 10 minutes, transfer it to a ...
Embodiment example 3
[0033] 1) Dilute 58g of 65% concentrated nitric acid in 131mL of water, add 35g of 2,4,6-tri(4-methylphenyl)-4,4',4"-triazine to it, dissolve and mix The solution was transferred to a polytetrafluoro reactor, heated to 220°C, kept for 5 hours, and washed 5 times with ether to obtain 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine ligand stand-by.
[0034] 2) At room temperature, dissolve 0.2 mmol of ferric nitrate nonahydrate in 20 mL of N,N-dimethylformamide, stir until completely dissolved, and then add 0.2 mmol of 2,4,6-tris(4- Carboxyphenyl)-1,3,5-triazine ligand until it is completely dissolved, then add 6 mmol of acetic acid, transfer the above mixed solution to a 100 mL polytetrafluoro reactor after ultrasonication for 10 minutes, and then place it in a 120°C Keep warm in the oven for 3 days. After filtering and washing with DMF for 3 times, the precipitate was soaked in ethanol for half a day, filtered and then soaked in ethanol, repeated 3 times, and dried at 40°C for 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com