Method for preparing esters through primary alcohol oxidation
A primary alcohol and oxygen technology, applied in the field of catalysts, can solve problems affecting the adsorption of substrates and intermediates, reduce selectivity, etc., and achieve the effects of easy separation, less by-products, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
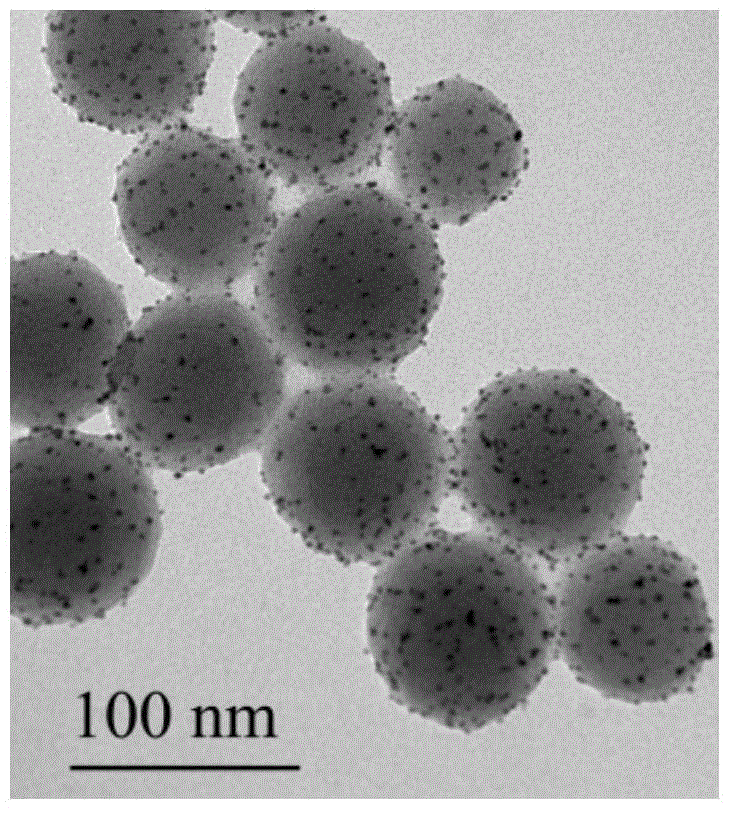
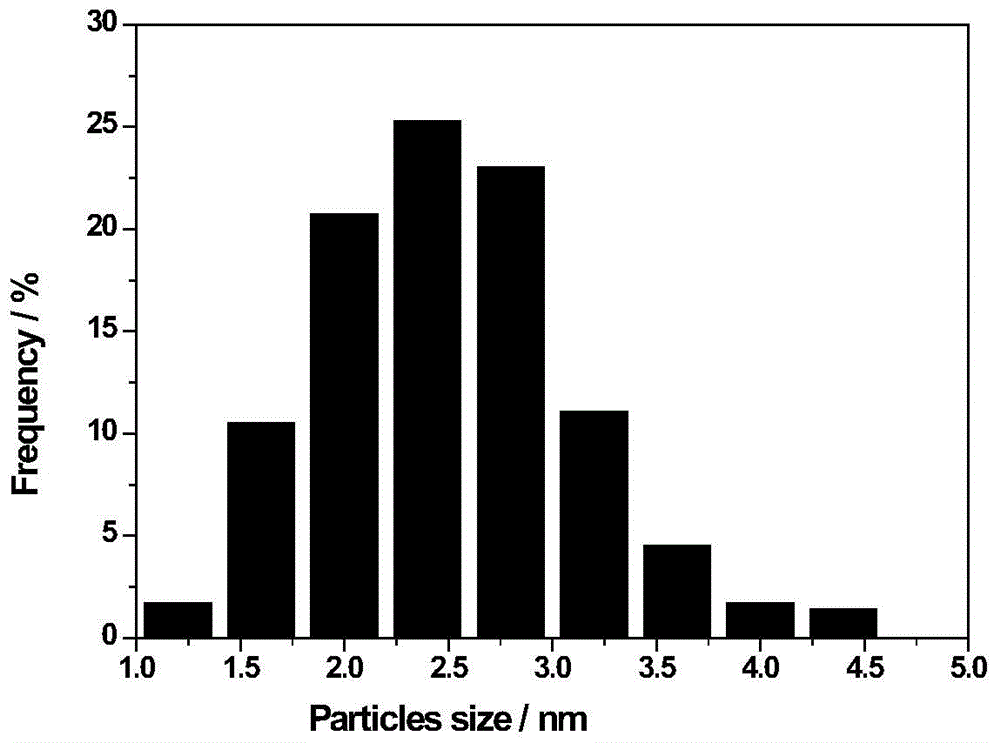
[0026] Embodiment 1: Take 1.05g1wt%H by weighing 2 PtCl 6 ·H 2 O aqueous solution and 0.1 g of PVPK10 were dissolved in 40 mL of deionized water, then, 5 mL of 0.4 wt% NaBH was added under stirring condition 4 . Then 20mL was dispersed with 0.2g of hydrophobic carrier Ph-SiO 2 The ethanol solution was added to the above solution, stirred at room temperature for 12 hours, evaporated to dryness, washed with water for 3-5 times, and dried at 80°C for 12 hours. Catalyst named: 2wt%-Pt / Ph-SiO 2 (Based on Pt, the loading amount is 2wt%), the average size of Pt ions is 2.5nm, and the catalyst number is 1.
Embodiment 2
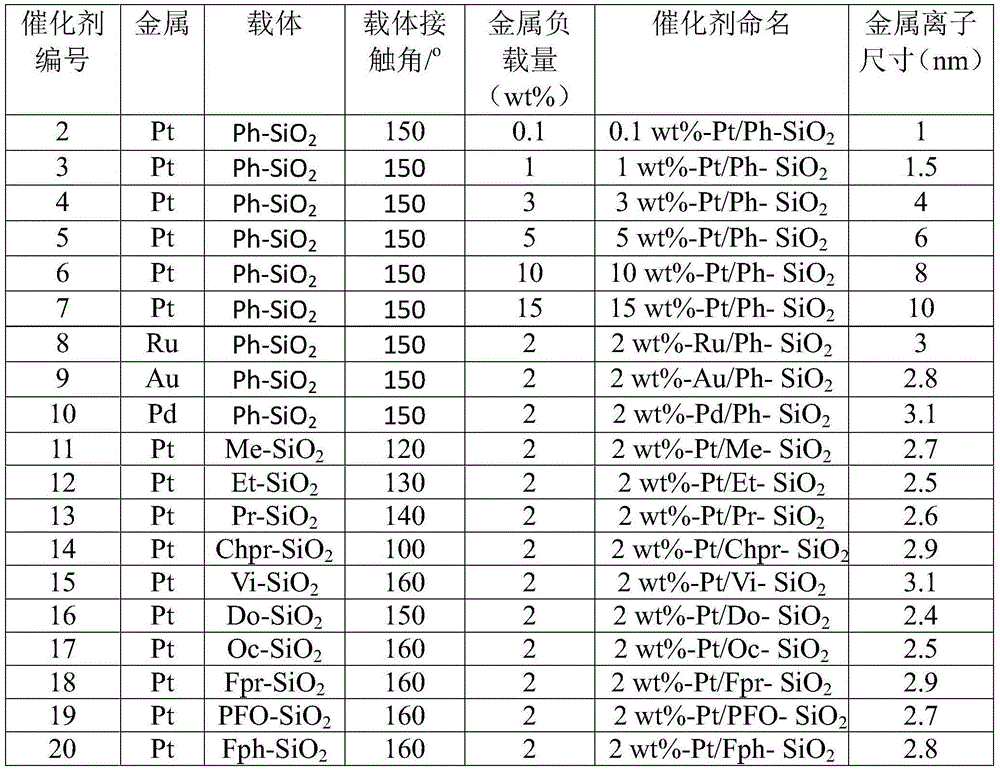
[0027] Embodiment 2: The preparation method of catalyst 2-20 adopts the method described in embodiment 1, just change different hydrophobic type supports, different metals and loading capacity, the metal source of Ru, Au, Pd is respectively RuCl 3 ·3H 2 O, HAuCl 4 4H 2 O, PdCl 2 , see Table 1 for details.
[0028] The preparation method of table 1 catalyst 2-20 and its properties
[0029]
Embodiment 3-20
[0030] Example 3-20: 0.2-2.0mmol 1-butanol, 0.002mmol (calculated as Pt) 2wt%-Pt / Ph-Ph-SiO 2 , 0.05mmol mesitylene (internal standard), 2mL solvent was added to a 30mL reaction kettle, the reaction kettle was sealed, air or oxygen at a certain pressure was introduced, and the temperature was raised to a certain temperature under stirring. After a certain period of reaction, cooled and centrifuged to separate the catalyst . The qualitative analysis of the obtained samples was performed by gas chromatography-mass spectrometry, and the quantitative analysis was realized by gas chromatography. The results are shown in Table 2.
[0031] Table 2 Oxidation of butanol to prepare butyl butyrate
[0032]
[0033] Analysis of the results in Table 2 shows that the hydrophobic carrier phenyl-modified silica (Ph-SiO 2 ) supported Pt catalyst 2wt%-Pt / Ph-SiO 2 , the best solvent for catalyzing molecular oxygen oxidation of n-butanol to butyl butyrate is toluene, the optimal condition is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com