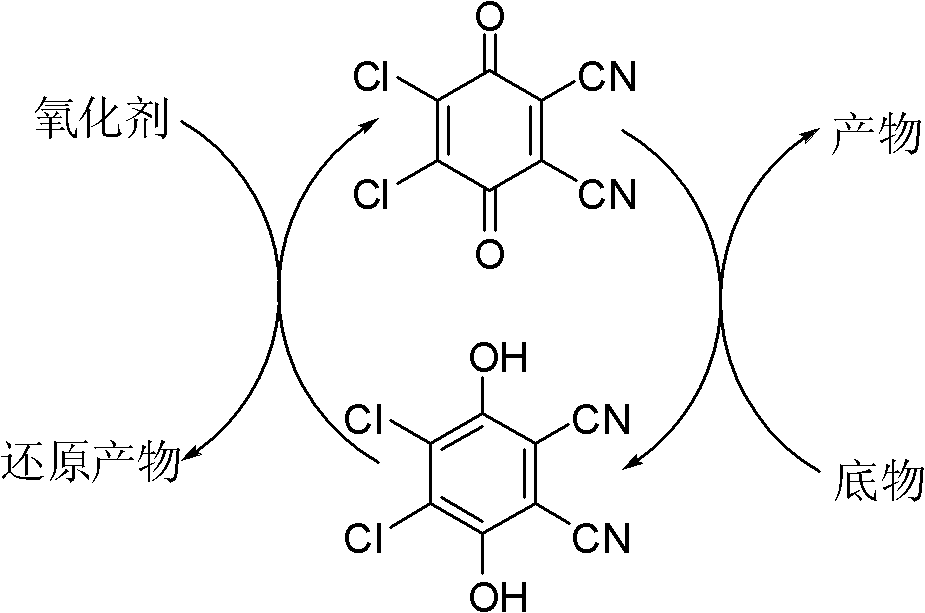
Method for preparing aldehyde and ketone by alcohol oxidation
An alcohol oxidation, -cnh2n technology, applied in the oxidation preparation of carbonyl compounds, organic chemistry, carbonyl formation/introduction, etc., can solve problems such as troublesome post-processing, pollution, etc., and achieve the effects of easy product, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] The investigation of example 1 reaction condition
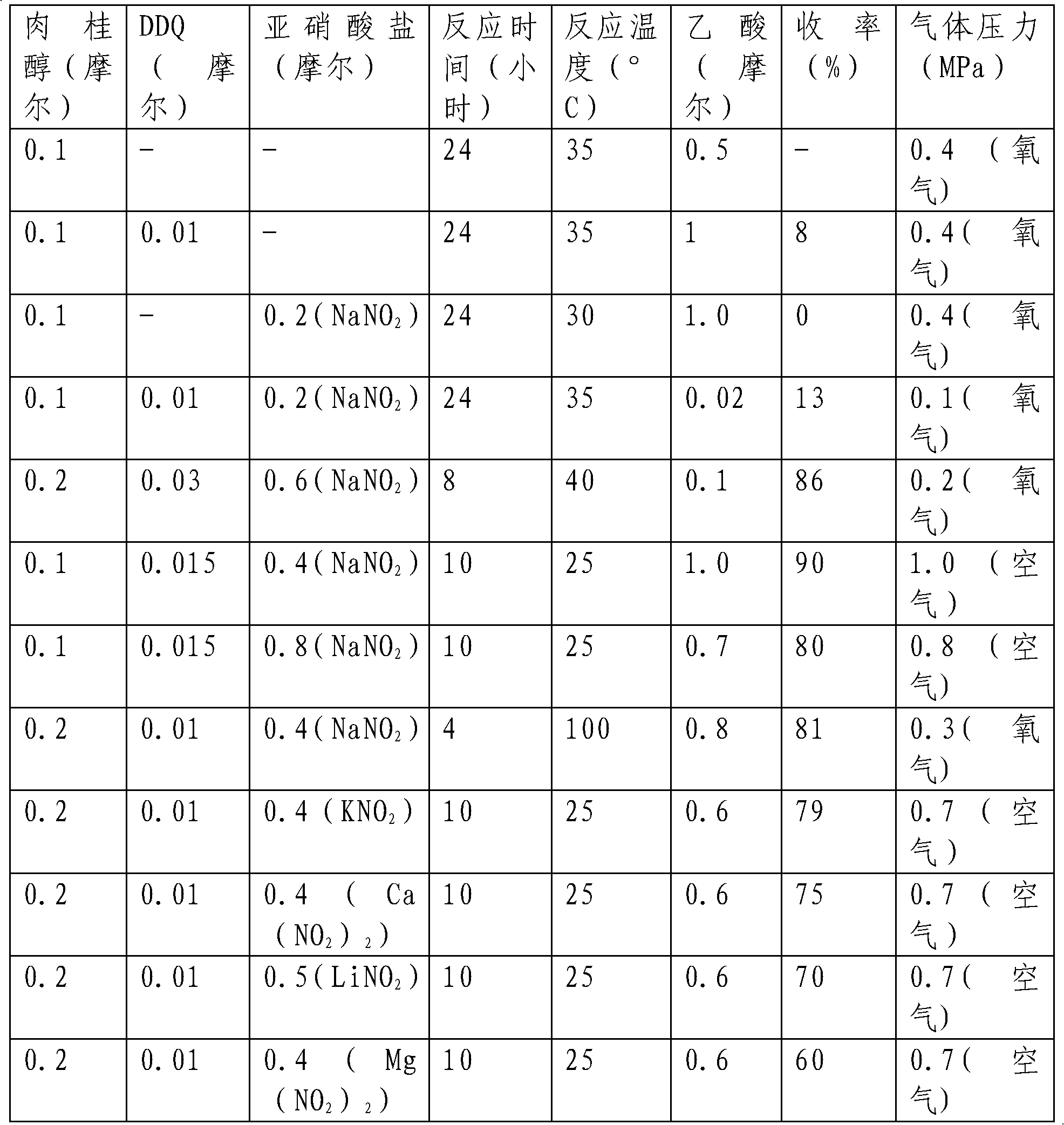
[0018] First, we investigated the reaction conditions using cinnamyl alcohol as a model substrate. The results are shown in Table 1
[0019] The investigation of table 1 reaction conditions
[0020]
[0021] It can be seen from Table 1 that DDQ, nitrite, and oxygen play a vital role in the oxidation reaction and are indispensable. At the same time, acetic acid also plays a very important role in the reaction. If it is not added or the amount added is too small, the reaction will proceed. It's not good. Among the nitrites, sodium nitrite and potassium nitrite gave better results than the other nitrites. Considering that sodium nitrite is an easy-to-get chemical, the price is relatively cheap, and the following examples all use sodium nitrite as a cocatalyst for the reaction. The higher the reaction temperature of the system, the greater the pressure, and the faster the reaction.
[0022] 2. Oxidation of cinnamyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com