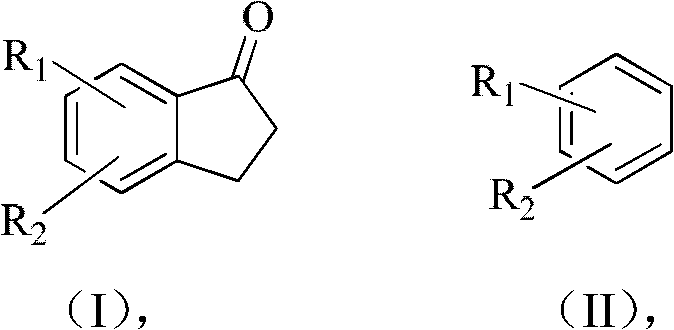
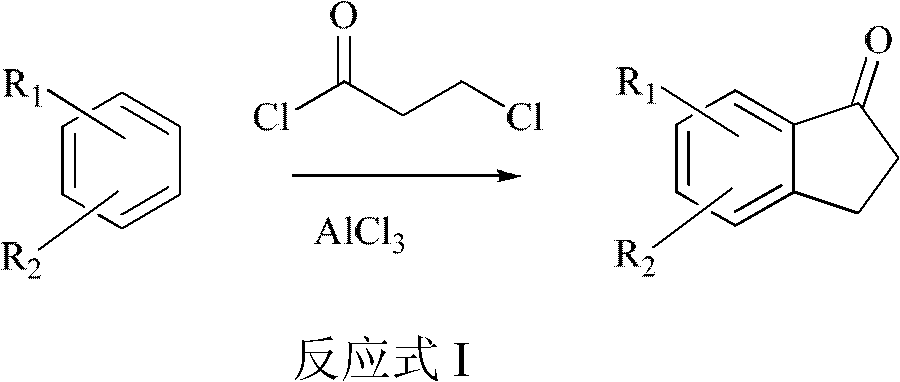
Method for preparing 2,3-dihydro-1-indanone and derivative thereof
A derivative, indanone technology, applied in the field of preparation 2, can solve the problems of easy fire, large amount, easy to block the pipeline of the reactor, and achieve the effects of low equipment occupancy rate, reduced pollution, and reduced sublimation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
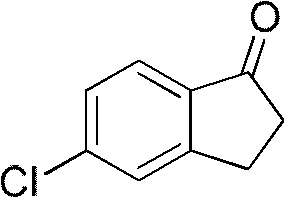
[0028] The preparation of embodiment 1.5-chloro-2,3-dihydro-1-indanone
[0029]
[0030] Add chlorobenzene (200g, 1.78mol), aluminum chloride (670g, 5mol), NaCl (110g, 1.88mol) in a 1-liter three-necked flask, stir, and add dropwise 3-chloropropionyl chloride ( 237g, 1.87mol), the mol ratio of chlorobenzene and 3-chloropropionyl chloride is 1: 1.1, and the mol ratio between chlorobenzene, aluminum trichloride and NaCl is 1: 2.8: 1.1, and the dropping temperature is room temperature, in Stir at 45-65°C for 1 hour, raise the temperature to 150-165°C and react for 1 hour, cool to 80-100°C, pour into 2kg of ice water hydrochloric acid, filter with suction to obtain 335g of crude product, and obtain 219.5g of solid after recrystallization. The yield is 74% (purity > 98%), and the melting point is 97-98°C.
[0031] HNMR (ppm, CDCl 3) 7.68-7.71 (m, 1H), 7.45-7.49 (m, 1H), 7.36-7.38 (m, 1H), 3.13-3.16 (t, 2H), 2.71-2.74 (t, 2H).
Embodiment 2
[0032] The preparation of embodiment 2.5-bromo-2,3-dihydro-1-indanone
[0033]
[0034] Add bromobenzene (100g, 0.64mol), aluminum trichloride (212.5g, 1.6mol), NaCl (37g, 0.64mol) into a 1-liter three-necked flask, stir, and add 3-chloropropane dropwise at 20-30°C Acyl chloride (83g, 0.65mol), the molar ratio of bromobenzene and 3-chloropropionyl chloride is 1:1, the molar ratio between bromobenzene, aluminum trichloride and NaCl is 1:2.5:1, at 50~70℃ Stir for 30 minutes at 160-165° C. for 1 hour, cool to 70-80° C., pour into 1 kg of ice-water hydrochloric acid, and suction-filter to obtain 107 g of crude product. After recrystallization, 85 g of solid is obtained, with a yield of 63% (purity> 98%), melting point 127 ~ 128 ℃.
[0035] HNMR (ppm, CDCl 3 ) 7.59-7.64 (m, 2H), 7.48-7.51 (m, 1H), 3.09-3.12 (t, 2H), 2.66-2.69 (t, 2H).
Embodiment 3
[0036] The preparation of embodiment 3.5-fluoro-2,3-dihydro-1-indanone
[0037]
[0038] Add fluorobenzene (96g, 1mol), aluminum chloride (333g, 2.5mol), NaCl (65g, 1.1mol) in a 1-liter three-necked flask, stir, and add dropwise 3-chloropropionyl chloride ( 133g, 1.05mol), the molar ratio of fluorobenzene and 3-chloropropionyl chloride is 1: 1.1, the molar ratio between fluorobenzene, aluminum trichloride and NaCl is 1: 2.5: 1.1, stirring at 65~70°C 2 hours, stirred at 165-175°C for 3 hours, cooled to 75-80°C, poured into 1kg of ice-water hydrochloric acid, filtered with suction to obtain 134g of crude product, and obtained 84g of solid after recrystallization, with a yield of 56% (purity>98%) ), with a melting point of 38-39°C.
[0039] HNMR (ppm, CDCl 3 ) 7.75-7.78 (m, 1H), 7.57-7.59 (m, 1H), 7.42-7.45 (m, 1H), 3.17-3.20 (t, 2H), 2.85-2.87 (t, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com