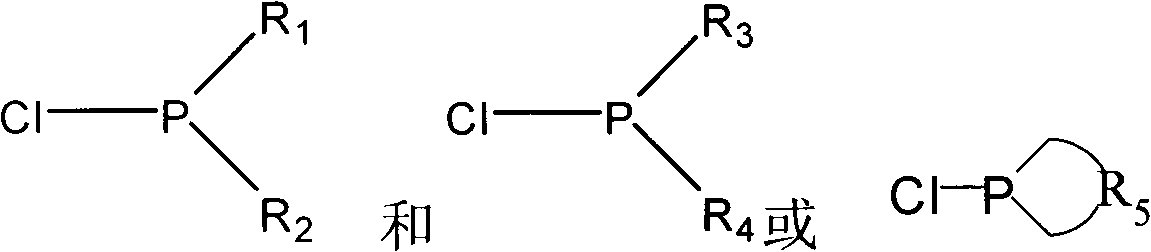
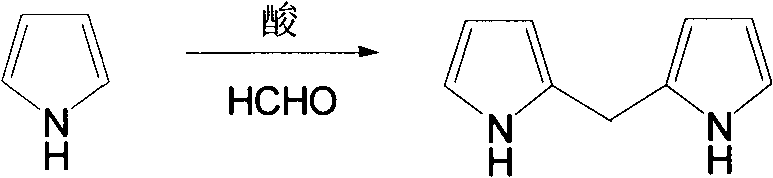
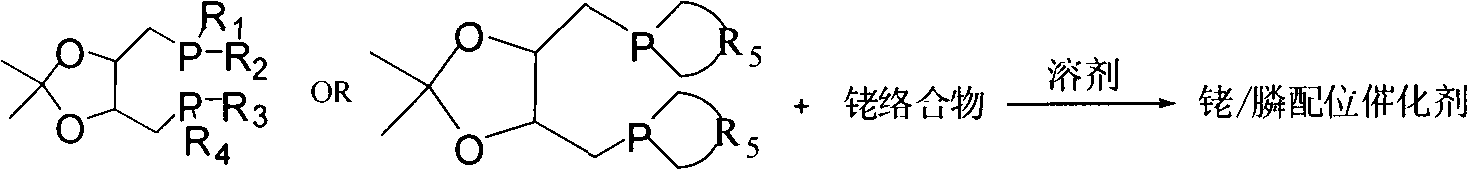Catalyst for hydroformylation reaction and preparation method of catalyst
A catalyst, chemical reaction technology, applied in the preparation of carbon monoxide reaction, preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve the problems of high price of rhodium metal, harshness, poor stability of phosphite, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0039] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS: In order to better understand the present invention, the content of the present invention will be further clarified below in conjunction with the examples, but the content of the present invention is not limited to the following examples.
[0040] In the embodiment, the instrument used for quantitative analysis of various hydroformylation reaction products is gas chromatography, and the analysis conditions are:
[0041]
[0042] The internal standard method was selected for accurate quantification, and the standard substance was methyl acrylate.
[0043] Melting point determination using: MPA100 automatic melting point apparatus
[0044] NMR analysis use: Bruck 400MHz NMR instrument
[0045] Elemental analysis using: Heraeus CHN-O-RAPID
[0046] The rhodium complex used in the catalyst system is selected from H(CO)(PPh 3 ) 3 Rh[tris(triphenylphosphine)hydrocarbonyl rhodium] or Rh(acac)(CO) 2 One of [rhodium acet...
Embodiment
[0051] The preparation process of the phosphoramidite ligand of the present invention is as follows:
[0052] Synthesis of Ligand A:
[0053] a) Synthesis of phosphine-chlorine compounds:
[0054] 5.2g PCl at -65°C under argon protection 3 (38mmol) and 5.1g of pyrrole and 200ml of dry tetrahydrofuran were mixed in a container, 11.5g of triethylamine was added to the mixture, stirred overnight at room temperature, filtered under reduced pressure, and the filtrate was distilled out of tetrahydrofuran and triethylamine at 60°C , the residue was dissolved in 50ml of toluene, and then the remaining precipitated triethylamine hydrochloride was filtered out, and the resulting solution was isolated from air and stored at low temperature. That is, dipyrrole phosphine chloride dissolved in toluene with a yield of 87%. The pure dipyrrolophosphine chloride was obtained after removing the toluene solvent under reduced pressure.
[0055] b) Ligand A synthesis:
[0056] 3.8g (13.3mmol) ...
Embodiment 1
[0116] Hydroformylation of Allyl Alcohol
[0117] Use respectively above-mentioned synthesized catalyst A-A, A-B, A-C, B-D, B-L, A-W, B-O to hydroformylate allyl alcohol, and the reaction process is as follows:
[0118] Add 15ml of the catalyst solution into a 100ml autoclave, and use CO / H 2 = 1:1 syngas to flush the solvent three times, then pressurize to 10bar, heat the autoclave to 65°C under stirring, then add 3.5ml of allyl alcohol, continue to pressurize to 12bar with syngas, under this constant pressure Gas uptake was monitored and when there was no more gas uptake, the autoclave was cooled and depressurized, and the resulting solution was analyzed by gas chromatography to determine the products, the reaction yielded 4-hydroxybutyraldehyde (HBA), 2-methyl-3-hydroxypropanal (HMPA), and propionaldehyde, propanol, propane, etc. C 3 by-product.
[0119] The comparison of catalytic results, such as the results in Table 3, shows that the catalysts A-A to A-C, B-D, and B-L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com