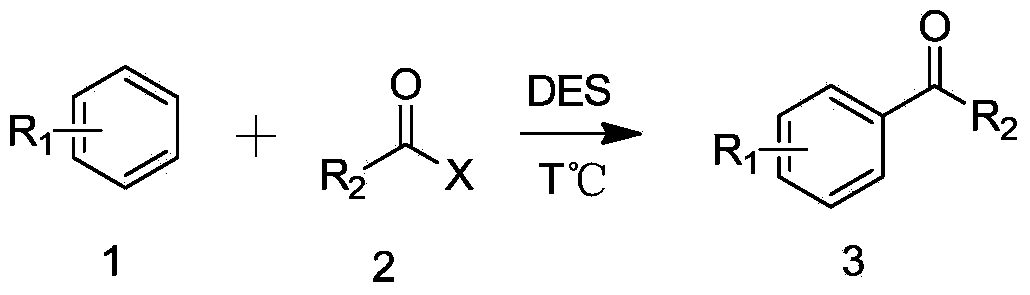
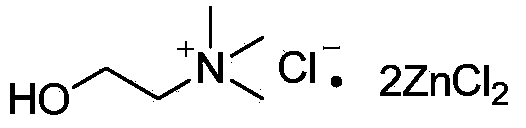Method for synthesizing aromatic ketone compound
A technology for compounds and aromatic ketones, which is applied in the field of catalytic preparation of aromatic ketone compounds, can solve the problems of poor catalyst stability, serious environmental pollution and high corrosiveness, and achieves the effects of strong stability, high catalytic activity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
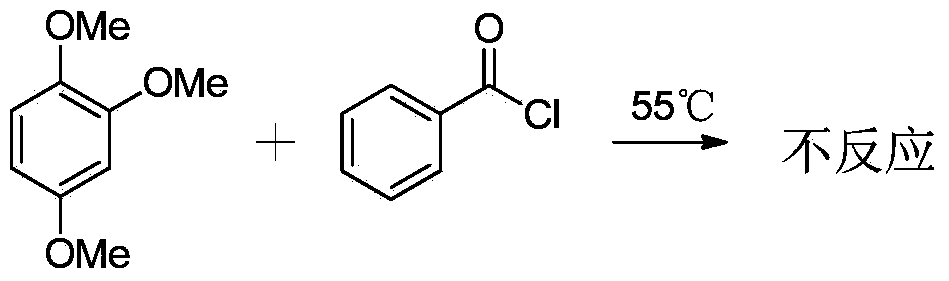
[0032] Experimental method: Add 2mmol of 1,2,4-trimethoxybenzene into a 15mL round bottom flask, raise the temperature to 55°C, slowly add 2.2mmol of benzoyl chloride, and monitor the progress of the reaction by thin-layer chromatography. It was found that no product was formed, that is, in the absence of DES(ZnCl 2 / ChCl=2:1) the reaction does not proceed in the presence of conditions.
[0033] Reaction equation:
[0034]
Embodiment 2
[0036] Experimental method: 1 equivalent (2mmol) of DES (ZnCl 2 / ChCl=2:1) was added to a 15mL round bottom flask, then 1 equivalent (2 mmol) of 1,2,4-trimethoxybenzene was added, the temperature was raised to 55°C, and 1.1 equivalent (2.2 mmol) of benzene was slowly added Formyl chloride, the reaction was stopped after stirring for 10 minutes. After adding 5 mL of water and 5 mL of ethyl acetate to extract twice, the ethyl acetate layer was washed with saturated NaHCO 3 Wash once, then wash with anhydrous MgSO 4 After drying and distillation under reduced pressure, the obtained substance was subjected to column separation with a developing solvent of petroleum ether: ethyl acetate = 6:1. The yield of 2,4,5-trimethoxyphenylbenzophenone was 89.7%.
[0037] Reaction equation:
[0038]
Embodiment 3
[0040] Experimental method: the water layer in example 2 is distilled under reduced pressure, recyclable obtains DES (ZnCl 2 / ChCl=2:1). Using recovered DES(ZnCl 2 / ChCl=2:1) Repeat the steps of Example 2. So repeated 4 times, it was found that DES(ZnCl 2 / ChCl=2:1) the catalytic effect remains basically unchanged. The yields of the obtained products were: 89.4%, 89.2%, 88.5%, 87.7%.
[0041] Reaction equation:
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com